Abstract
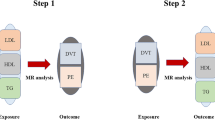
In view of the current debate about the relationship between lipids and deep venous thrombosis (DVT) in clinical studies, a two-sample Mendelian randomization (MR) study was conducted to clarify the effects of five circulating lipids (apolipoprotein A1, apolipoprotein B, low-density lipoprotein, high-density lipoprotein and triglycerides) on DVT from the perspective of genetic inheritance. Five lipids (exposure) were analysed by MR with DVT (outcome) from two different data sources. For the analysis, we used inverse variance weighting and a weighted mode, weighted median, simple mode and MR–Egger regression to analyse the effect of circulating lipids on DVT. In addition, we used the MR–Egger intercept test, Cochran’s Q test and “leave-one-out” sensitivity analysis to evaluate horizontal multiplicity, heterogeneity and stability, respectively, in the analysis. In the analysis, the two-sample Mendelian randomization analysis of five common circulating lipids and DVT showed that common circulating lipids had no causal effect on DVT, which is somewhat inconsistent with the findings of many published observational studies. Based on our results, our two-sample MR analysis failed to detect a statistically significant causal relationship between five common circulating lipids and DVT.
Similar content being viewed by others
Introduction
Deep venous thrombosis (DVT) is a common venous thromboembolism that usually affects the lower extremities. The annual incidence rate is close to 1.6 per 1000 people1. Thrombosis usually begins in the deep vein of the leg and spreads proximally. The main risk factors for DVT include trauma, cancer and gene mutations that promote blood hypercoagulability2. The formation mechanism of DVT can be explained by Virchow’s triad, i.e., a blood flow disorder, a hypercoagulable state of the blood and a procoagulant state of the vascular wall3.
At present, some studies suggest that lipid metabolism disorders can affect DVT; although there are many clinical studies on the effect of lipids on venous thrombosis (VT), current conclusions are still controversial. Some studies have suggested that the levels of low-density lipoprotein (LDL), high-density lipoprotein (HDL) and triglycerides (TGs) are not associated with an increased risk of VT, whereas a decrease in apolipoprotein B (APOB) and apolipoprotein A1 (APOA1) levels is associated with an increased risk of VT, but the specific mechanism has not been clarified4,5,6. However, Schouwenburg et al.7 found no association between apolipoproteins and the risk of venous thromboembolism. Because lipid levels can be regulated by lifestyle and medications, the relationship between lipid levels and VT and its pathophysiology is a clinical issue worth exploring8. A previous study showed that lipid-lowering drugs are associated with a reduced risk of VT, which may indicate the possible role of lipids in the pathophysiology of VT9. The ambiguous relationship between blood lipids and DVT requires further clarification.
Mendelian randomization (MR) is a new strategy based on Mendelian inheritance and uses genetic variation as an instrumental variable (IV) to study the causal relationship between different traits10. MR provides a valuable tool, especially when randomized controlled trials to check causality are not feasible and observational studies have biased associations due to confounding or reverse causality11. MR also enables the use of published results from large genome-wide association studies (GWASs) to study risk factors (exposure) and disease (outcome) and to avoid confounding factors and reverse causality deviations in observational studies12. Fernando et al. used MR to analyse the relationship between inflammatory pathways and suicide and found that IL-6 signalling was associated with suicide13. In view of the current debate on the relationship between lipids and DVT in clinical studies and the limitations of clinical studies, a two-sample MR study was conducted to clarify the effects of five kinds of circulating lipids (APOA1, APOB, LDL, HDL and TGs) on DVT from a genetic perspective.
Methods
We referred to Xu et al.14 two-sample Mendelian randomization study to test the causal relationship between DVT and circulating lipids.
Data sources
The DVT data used in this article all came from Neale lab analysis of UK Biobank phenotypes (excluding pulmonary embolism). The DVT data in the analysis included 6767 DVT patients and 330,392 control cases. Up to 10,894,596 SNPs were included in the analysis. Information on various phenotypes was collected from each participant, and blood samples were collected when the subjects visited the UK Biobank Assessment Centre. DNA extraction and genotyping were carried out in the Affymetrix Research Service Laboratory. Summary GWAS data on DVT can be downloaded from the UK Biobank (UKBB) database and MRC IEU OpenGWAS repositories (https://gwas.mrcieua.ac.uk/). UKBB details, including geographic areas, recruitment processes, and other features, have been described in previous articles15.
Data on circulating lipids came from two other non-UK biobank studies. APOA1 and APOB data came from the research of Kettunen et al. They conducted an extended genome-wide association study of as many as 24,925 individuals from 10 European studies. Up to 12,133,295 SNPs were included in the meta-analysis after applying quality control filters16. GWAS data on LDL, HDL and TGs came from the research of Willer et al. In the study, to identify new loci and extract known loci that affect these blood lipids, they examined 188,578 people using genome-wide and custom genotyping arrays17. More details on the sample treatment, determination details, genotyping quality control, staging, interpolation and association tests of circulating lipids (APOA1, APOB, LDL, HDL and TGs) included in this analysis can be obtained from previous reports16,17. Details of all the data and GWAS IDs used are shown in Supplementary File 1.
Selection of instrumental variables
When selecting instrumental variables (IVs), we followed the three basic hypotheses of MR: first, genetic variation should be closely related to the exposure; second, variation should not be affected by confounding factors of the relationship between the exposure and outcome; and third, the exposure should only affect the outcome (i.e., pleiotropy should be eliminated, and the exclusion limitation hypothesis should be satisfied). Therefore, we extracted genomic single-nucleotide polymorphisms (SNPs) associated with exposure (P < 5 × 10−8)14. In addition, none of the instrumental SNPs were in linkage disequilibrium (LD). We performed the clumping process (R2 < 0.001, Magna window size = 10,000 kb) to eliminate the LD between the SNPs14. Third, SNPs with a minor allele frequency (MAF) < 0.01 were removed. By default, if the SNP for a particular request did not exist in the resulting GWAS, the SNP (agent) with the requested SNP (target) in the LD was searched14. The LD agent was defined using 1000 genomes of European sample data. Then, we searched the human gene phenotypic association database (PhenoScanner V2) to evaluate possible pleiotropic associations between instrument variables and other phenotypes and excluded DVT-related IVs (such as weight and whole-body fat mass). In addition, to test whether there was a weak instrumental deviation in the IV, we used the F statistic (F = R2 (n − k − 1)/k (1 − R2), where R2 is the variance of exposure explained by selected instrumental variables (obtained from the MR Steiger directionality test), n is the sample size, and k is the total variables. If the F statistic of the IV is much greater than 10, it indicates that the possibility of weak instrument variable bias is very small14.
Study design and statistical analyses
We first conducted univariable MR analyses for each lipid-related trait. Because there are varying degrees of overlap between SNPs related to different lipid properties, it may not be accurate to test the effect of individual lipids on DVT. Multivariable MR allows associations of SNPs with multiple phenotypes to be included in the analysis so that the direct impact of each phenotype on the outcome can be estimated18,19. Therefore, we regarded multivariable MR as the primary analysis method. A multiple test correction with a p value < 0.01 (0.05/5 exposures) was regarded as a significant correlation.
When horizontal pleiotropy does not exist in the analysis, the inverse variance weighted mode can provide the most accurate causal estimation20. When there is heterogeneity in the analysis, random effects IVW can provide accurate causal estimation. We also used several other MR methods, including the MR–Egger method, weighted median method, simple mode method and weighted mode method, to test the robustness of the results21. We used the MR–Egger method22 to analyse the sensitivity and check the consistency and possible pleiotropy of the correlation. The weighted median method provides consistent causal estimates when the effective tool has more than 50% of the weight23. MR–Egger regression can detect multiplicity by intercept; however, it impairs statistical power22. The effect of horizontal heterogeneity was evaluated and tested by Cochrane’s Q value in the MR–Egger regression and IVW test, with a P value of 0.05 indicating considerable heterogeneity. The odds ratios (ORs) and corresponding 95% confidence intervals (CIs) of lipids were associated with the presence or absence of genetically predicted DVT. We then conducted a further sensitivity analysis using the “leave-one-out” test to evaluate whether the results were affected by individual SNPs. All statistical data analyses were conducted with R (version 4.2.0) software (TwoSampleMR and MendelianRandomization packages).
Ethical statement
All data were downloaded from the internet.
Results
Selection of instrumental variables
The details of all independent SNPs associated with exposure are shown in Supplementary File 2. In our study, the F statistics of the instrumental variables associated with exposure were all greater than 10, indicating that the possibility of variable deviation of weak instrumental variables was very small.
The causal relationship between APOA1 and DVT
In the MR analyses, we found that there was no causal relationship between APOA1 (exposure) and DVT (outcome) using the various MR analysis methods (IVW (random effects): Beta = − 3e−4, Pbeta = 0.870; weighted median: Beta = − 0.001, Pbeta = 0.480; weighted mode: Beta = − 0.001, Pbeta = 0.447; simple mode: Beta = − 4e−4, Pbeta = 0.847; MR‒Egger: Beta = − 0.006, Pbeta = 0.291) (Table 1, Fig. 1). In addition, multivariable MR analysis showed that there was no causal relationship between APOA1 and DVT (Beta = − 0.002, P = 0.701) (Table 3). Because the heterogeneity test in the analysis found that there was a certain level of heterogeneity (IVW: Q–p values = 0.002; MR–Egger: Q p values = 0.004) (Table 2), we performed an IVW (random effects) analysis, and the results were consistent with those of other models: both showed that there was no causal relationship (Table 1).
The MR–Egger intercept in the analysis showed that there was no horizontal multiplicity (MR–Egger intercept p value = 0.284) (Table 2). The scatter plot shows the estimated impact of SNPs on exposure (APOA1) and outcome (DVT) (Fig. 1). The results of the “leave-one-out” test in the analysis showed that there was no abnormal IV in this analysis affecting the overall results (Supplementary File 3, Fig. S11). The forest plots and funnel plots in the analysis are shown in Supplementary File 3, Figs. S1 and S6. The funnel plot shows the location of directional horizontal pleiotropy in each result.
The causal relationship between APOB and DVT
As shown in Table 1, based on the results of IVW (random effects), simple mode and MR–Egger methods, there was no evidence of a causal relationship between APOB and DVT (IVW (random effects): Beta = − 0.001, Pbeta = 0.715; simple mode: Beta = 0.001, Pbeta = 0.669; MR‒Egger: Beta = − 0.008, Pbeta = 0.165). However, the results of the weighted median and weighted mode methods showed that APOB reduced the incidence of DVT (weighted median: Beta = − 0.004, Pbeta = 0.005; weighted mode: Beta = − 0.006, Pbeta = 0.003) (Table 1). The multivariable MR analysis also showed that there was no causal relationship between APOB and DVT (Beta = 0.008, P = 0.052) (Table 3).
Because the heterogeneity test in the analysis indicated a certain level of heterogeneity (IVW: Q p values = 6.07e−54; MR–Egger: Q p values = 3.17e−47) (Table 2), we performed IVW (random effect) analysis, and the results showed that there was no causal relationship between APOB and DVT (Table 1). The MR–Egger intercept in the analysis showed that there was no horizontal multiplicity in the analysis (MR–Egger intercept p value = 0.151) (Table 2).
The scatter plot shows the estimated impact of SNPs on the exposure (APOB) and outcome (DVT) (Fig. 2). In addition, the results of the “leave-one-out” test in the analysis showed that there was no SNP affecting the overall results in this analysis (Supplementary File 3, Fig. S12). The forest plots and funnel plots in the analysis are shown in Supplementary File 3, Figs. S2 and S7.
The causal relationship between LDL and DVT
As shown in Table 1, based on the results of IVW (random effects), weighted median, weighted mode, simple mode and MR‒Egger methods, there was no causal relationship between LDL and DVT (IVW (random effects): Beta = − 0.002, Pbeta = 0.117; weighted median: Beta = − 0.001, Pbeta = 0.366; weighted mode: Beta = − 0.001, Pbeta = 0.637; simple mode: Beta = − 0.001, Pbeta = 0.767; MR‒Egger: Beta = − 3e−4, Pbeta = 0.881) (Table 1). The multivariable MR analysis also showed that there was no causal relationship between LDL and DVT (Beta = − 0.008, P = 0.012) (Table 3).
The heterogeneity analysis found some heterogeneity (IVW and MR–Egger Q p values < 0.05) (Table 2). The MR–Egger intercept in the analysis showed that there was no horizontal multiplicity (MR–Egger intercept p value = 0.309) (Table 2).
The scatter plot shows the estimated impact of SNPs on the exposure (LDL) and outcome (DVT) (Fig. 3). The results of the “leave-one-out” analysis showed that the results of the analyses were robust (Supplementary File 3, Fig. S13). The forest plots and funnel plots in the analysis are shown in Supplementary File 3, Figs. S3 and S8.
The causal relationship between HDL and DVT
As shown in Table 1, in the analysis, we found that there was no causal relationship between HDL and DVT using various MR analysis methods (IVW (random effects): Beta = − 5e−4, Pbeta = 0.670; weighted median: Beta = − 0.001, Pbeta = 0.259; weighted mode: Beta = − 0.001, Pbeta = 0.484; simple mode: Beta = 0.003, Pbeta = 0.384; MR‒Egger: Beta = − 3.54–4, Pbeta = 0.870). The multivariable MR analysis also showed that there was no causal relationship between HDL and DVT (Beta = 7e−4, P = 0.881) (Table 3).
The heterogeneity test in the analysis found that there was some heterogeneity (the Q p values of IVW and MR–Egger methods were both less than 0.05). The MR–Egger intercept in the analysis showed that there was no horizontal multiplicity (MR–Egger intercept p value = 0.901) (Table 2).
The scatter plot shows the estimated impact of SNPs on the exposure (HDL) and outcome (DVT) (Fig. 4). The results of the “leave-one-out” analysis showed that there was no abnormal IV in this analysis affecting the overall results (Supplementary File 3, Fig. S14). The forest plots and funnel plots in the analysis are shown in Supplementary File 3, Figs. S4 and S9.
The causal relationship between TGs and DVT
Based on the results of IVW (random effects), weighted median and weighted mode, methods we found a suggested negative causal relationship between TGs and DVT (IVW (random effects): Beta = − 0.004, Pbeta = 0.038; weighted median: Beta = − 0.004, Pbeta = 0.017; weighted mode: Beta = − 0.003, Pbeta = 0.038) (Table 1). However, based on the results of simple mode and MR‒Egger methods, there was no causal relationship between TGs and DVT (simple mode: Beta = − 0.003, Pbeta = 0.270; MR‒Egger: Beta = − 0.003, Pbeta = 0.335). The multivariable MR analysis also showed that there was no causal relationship between TGs and DVT (Beta = − 0.006, P = 0.039) (Table 3).
The scatter plot shows the estimated impact of SNPs on the exposure (TG) and outcome (DVT) (Fig. 5). The heterogeneity test showed some heterogeneity (IVW and MR–Egger Q value < 0.05). The MR–Egger intercept in the analysis showed that there was no horizontal multiplicity (MR–Egger intercept p value > 0.05) (Table 2).
The results of the “leave-one-out” method test in the analysis showed that no abnormal IV in the analyses affected the overall results (Supplementary File 3, Fig. S15). The forest plots and funnel plots in the analysis are shown in Supplementary File 3, Figs. S5 and S10.
Discussion
In this study, we used two-sample Mendelian randomization to analyse summary GWAS data and found that our two-sample MR analysis failed to detect a statistically significant causal relationship between five common circulating lipids and DVT. This result differs from those of previous clinical studies. For example, Morelli et al. found that lower levels of APOA1 and APOB were associated with an increased risk of DVT4. In addition, Sabine et al. found that patients with high levels of APOA1 and HDL had a lower risk of recurrent DVT24. Petter et al. found no association between the risk of DVT and total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, glucose or smoking25. In our previous study26, we found that there was no significant causal relationship between three traditional lipids (LDL, HDL and TGs) and VTE (DVT and PE) from a genetic point of view. The GWAS data used in our current Mendelian randomization study are very different from those used in previous studies, and we used new GWAS data to further prove that there is no causal relationship between LDL, HDL, TGs and DVT. In addition, this study found that there was no causal relationship between APOA1 and APOB and DVT, which adds new evidence to the study of the causal relationship between blood lipids and DVT.
Compared with major lipids, there are few reports on the relationship between DVT and APOA1 and APOB levels. Apolipoprotein was not associated with the risk of DVT in cohort studies7,27. In other clinical studies, these apolipoprotein-DVT associations have been limited to certain subgroups; for example, in a case–control study that included only men, low levels of APOA1 were associated with an increased risk of DVT28. High levels of APOA1 in the Women's Health Study were associated with an increased risk of blood clots in hormone users29. In another small case–control study, high APOB levels appeared to increase the risk of DVT in men30. These contradictory results may be related to the adjustment for potential confounding factors, as the MR method can reduce confounding factors in observational studies. Our study showed that APOA1 and APOB had no significant causal relationship with DVT from a genetic perspective.
Statins and fibrates are commonly used to reduce blood lipids. A meta-analysis also found that statins may reduce the risk of DVT, whereas fibrates may increase this risk31. The authors did not provide an effective explanation for this result, but our results showed that TGs had no protective effect on DVT. Therefore, well-designed in vitro and in vivo studies are strongly encouraged, and DVT events should be included as a major end point in new clinical studies of fibrates. In recent years, an increasing number of studies have found that statins can effectively reduce the incidence of DVT32,33; for example, rosuvastatin significantly reduces the incidence of symptomatic venous thromboembolism34, and in vitro and observational studies have found that statins may have beneficial effects on blood vessel walls and antithrombotic formation35,36. A meta-analysis found that the use of statins and antiplatelet therapy was associated with a significant reduction in the incidence of venous thromboembolism37. It is well known that statins not only have lipid-lowering effects but also have a variety of vascular protective effects that are independent of changes in cholesterol levels. These effects are attributed to the anti-inflammatory and antithrombotic properties of statins, which can alter endothelial dysfunction and regulate angiogenesis38. Therefore, the documentation that statins can effectively reduce the incidence of DVT does not conflict with our results. However, there are also contrary findings about the efficacy of statins in preventing DVT39. Therefore, more clinical and basic studies are needed to explore whether statins can prevent DVT at this stage.
In this study, we selected SNPs with a genome-wide association and independent inheritance without any LD as IVs to detect a causal relationship between circulating lipids (APOA1, APOB, LDL, HDL and TGs) and DVT. To make our conclusions more robust and reliable, we used several analysis methods for comparison and verification. The F value in each analysis was greater than 10, which effectively avoided the weak tool variable bias. Of course, our analysis was somewhat restrictive. First, because our analysis was based on publicly available aggregate data, the data provided did not allow us to conduct other subgroup analyses to address associations with specific factors such as age, sex and other DVT risk factors. Second, this study was limited to participants of European origin. Therefore, our results may not apply to people of other races. Finally, regarding the results that were not statistically significant in this study, we could not completely rule out an association between these circulating lipids and DVT because this may reflect the small sample size and the lack of statistical power of the MR analysis.
Conclusions
Based on our results, our two-sample MR analysis failed to detect a statistically significant causal relationship between five common circulating lipids and DVT. Subsequent studies or MR analysis based on more genetic instruments are needed to validate these findings and clarify the potential mechanism of the effect of circulating lipids on the development of DVT.
Data availability
The data used in the present study are all publicly available at https://gwas.mrcieu.ac.uk/.
Abbreviations
- DVT:
-
Deep venous thrombosis
- VT:
-
Venous thrombosis
- SNPs:
-
Single-nucleotide polymorphisms
- GWAS:
-
Genome-wide association study
- IVW:
-
Inverse variance weighting
- LDL:
-
Low-density lipoprotein
- HDL:
-
High-density lipoprotein
- TGs:
-
Triglycerides
- APOB:
-
Apolipoprotein B
- APOA1:
-
Apolipoprotein A1
- IV:
-
Instrumental variable
- ORs:
-
Odds ratios
- LD:
-
Linkage disequilibrium
- CIs:
-
Confidence intervals
References
Ageno, W. et al. Rivaroxaban treatment for six weeks versus three months in patients with symptomatic isolated distal deep vein thrombosis: Randomised controlled trial. BMJ 379, e072623. https://doi.org/10.1136/bmj-2022-072623 (2022).
Brill, A. Multiple facets of venous thrombosis. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22083853 (2021).
Zhang, H., Weng, H., Yu, K. & Qiu, G. Clinical risk factors and perioperative hematological characteristics of early postoperative symptomatic deep vein thrombosis in posterior lumbar spinal surgery. Spine 46, E1042-e1048. https://doi.org/10.1097/brs.0000000000003963 (2021).
Morelli, V. M., Lijfering, W. M., Bos, M. H. A., Rosendaal, F. R. & Cannegieter, S. C. Lipid levels and risk of venous thrombosis: Results from the MEGA-study. Eur. J. Epidemiol. 32, 669–681. https://doi.org/10.1007/s10654-017-0251-1 (2017).
Spasić, I., Ubavić, M., Šumarac, Z., Todorović, M. & Vučković, B. Influence of lipid metabolism disorders on venous thrombosis risk. J. Med. Biochem. 40, 245–251. https://doi.org/10.5937/jomb0-27106 (2021).
Li, Y. & Shan, J. Study on the correlation between high density lipoprotein and lower extremities deep venous thrombosis in patients undergoing hip arthroplasty. Phlebology 37, 516–521. https://doi.org/10.1177/02683555221090309 (2022).
van Schouwenburg, I. M. et al. Lipid levels do not influence the risk of venous thromboembolism: Results of a population-based cohort study. Thromb. Haemost. 108, 923–929. https://doi.org/10.1160/th12-06-0426 (2012).
Weghuber, D. et al. Once-weekly semaglutide in adolescents with obesity. N. Engl. J. Med. 387, 2245–2257. https://doi.org/10.1056/NEJMoa2208601 (2022).
Caiano, L. M. et al. Role of statins in the prevention of post-thrombotic syndrome after a deep vein thrombosis event: A systematic review and meta-analysis. J. Thromb. Haemost. 21, 944–952. https://doi.org/10.1016/j.jtha.2022.12.008 (2023).
Holmes, M. V., Ala-Korpela, M. & Smith, G. D. Mendelian randomization in cardiometabolic disease: Challenges in evaluating causality. Nat. Rev. Cardiol. 14, 577–590. https://doi.org/10.1038/nrcardio.2017.78 (2017).
Sekula, P., Del Greco, M. F., Pattaro, C. & Köttgen, A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol 27, 3253–3265. https://doi.org/10.1681/asn.2016010098 (2016).
Emdin, C. A., Khera, A. V. & Kathiresan, S. Mendelian randomization. Jama 318, 1925–1926. https://doi.org/10.1001/jama.2017.17219 (2017).
Hartwig, F. P., Borges, M. C., Horta, B. L., Bowden, J. & Davey Smith, G. Inflammatory biomarkers and risk of schizophrenia: A 2-sample mendelian randomization study. JAMA Psychiatry 74, 1226–1233. https://doi.org/10.1001/jamapsychiatry.2017.3191 (2017).
Xu, J. et al. No genetic causal association between iron status and osteoporosis: A two-sample Mendelian randomization. Front. Endocrinol. 13, 996244. https://doi.org/10.3389/fendo.2022.996244 (2022).
Sudlow, C. et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779. https://doi.org/10.1371/journal.pmed.1001779 (2015).
Kettunen, J. et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat. Commun. 7, 11122. https://doi.org/10.1038/ncomms11122 (2016).
Willer, C. J. et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 45, 1274–1283. https://doi.org/10.1038/ng.2797 (2013).
Burgess, S. & Thompson, S. G. Multivariable Mendelian randomization: The use of pleiotropic genetic variants to estimate causal effects. Am. J. Epidemiol. 181, 251–260. https://doi.org/10.1093/aje/kwu283 (2015).
Sanderson, E., Davey Smith, G., Windmeijer, F. & Bowden, J. An examination of multivariable mendelian randomization in the single-sample and two-sample summary data settings. Int. J. Epidemiol. 48, 713–727. https://doi.org/10.1093/ije/dyy262 (2019).
Yuan, S. et al. Lifestyle and metabolic factors for nonalcoholic fatty liver disease: Mendelian randomization study. Eur. J. Epidemiol. 37, 723–733. https://doi.org/10.1007/s10654-022-00868-3 (2022).
Bowden, J. et al. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat. Med. 36, 1783–1802. https://doi.org/10.1002/sim.7221 (2017).
Bowden, J., Davey Smith, G. & Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through egger regression. Int. J. Epidemiol. 44, 512–525. https://doi.org/10.1093/ije/dyv080 (2015).
Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314. https://doi.org/10.1002/gepi.21965 (2016).
Eichinger, S. et al. High-density lipoprotein and the risk of recurrent venous thromboembolism. Circulation 115, 1609–1614. https://doi.org/10.1161/circulationaha.106.649954 (2007).
Quist-Paulsen, P. et al. Arterial cardiovascular risk factors and venous thrombosis: Results from a population-based, prospective study (the HUNT 2). Haematologica 95, 119–125. https://doi.org/10.3324/haematol.2009.011866 (2010).
Lin, L. et al. A bidirectional Mendelian randomized study of classical blood lipids and venous thrombosis. Sci. Rep. 13, 3904. https://doi.org/10.1038/s41598-023-31067-z (2023).
Chamberlain, A. M., Folsom, A. R., Heckbert, S. R., Rosamond, W. D. & Cushman, M. High-density lipoprotein cholesterol and venous thromboembolism in the longitudinal investigation of thromboembolism etiology (LITE). Blood 112, 2675–2680. https://doi.org/10.1182/blood-2008-05-157412 (2008).
Deguchi, H., Pecheniuk, N. M., Elias, D. J., Averell, P. M. & Griffin, J. H. High-density lipoprotein deficiency and dyslipoproteinemia associated with venous thrombosis in men. Circulation 112, 893–899. https://doi.org/10.1161/circulationaha.104.521344 (2005).
Everett, B. M., Glynn, R. J., Buring, J. E. & Ridker, P. M. Lipid biomarkers, hormone therapy and the risk of venous thromboembolism in women. J. Thromb. Haemost. 7, 588–596. https://doi.org/10.1111/j.1538-7836.2009.03302.x (2009).
Delluc, A. et al. Lipid parameters, lipid lowering drugs and the risk of venous thromboembolism. Atherosclerosis 220, 184–188. https://doi.org/10.1016/j.atherosclerosis.2011.10.007 (2012).
Squizzato, A. et al. Statins, fibrates, and venous thromboembolism: A meta-analysis. Eur. Heart J. 31, 1248–1256. https://doi.org/10.1093/eurheartj/ehp556 (2010).
Brækkan, S. K. et al. Statin use and risk of recurrent venous thrombosis: Results from the MEGA follow-up study. Res. Pract. Thromb. Haemost. 1, 112–119. https://doi.org/10.1002/rth2.12003 (2017).
Biere-Rafi, S. et al. Statin treatment and the risk of recurrent pulmonary embolism. Eur. Heart J. 34, 1800–1806. https://doi.org/10.1093/eurheartj/eht046 (2013).
Glynn, R. J. et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N. Engl. J. Med. 360, 1851–1861. https://doi.org/10.1056/NEJMoa0900241 (2009).
Pignatelli, P. et al. Immediate antioxidant and antiplatelet effect of atorvastatin via inhibition of Nox2. Circulation 126, 92–103. https://doi.org/10.1161/circulationaha.112.095554 (2012).
Chaffey, P. et al. Usefulness of statins for prevention of venous thromboembolism. Am. J. Cardiol. 121, 1436–1440. https://doi.org/10.1016/j.amjcard.2018.02.024 (2018).
Khemasuwan, D. et al. Dose-related effect of statins in venous thrombosis risk reduction. Am. J. Med. 124, 852–859. https://doi.org/10.1016/j.amjmed.2011.04.019 (2011).
Undas, A., Celińska-Löwenhoff, M., Kaczor, M. & Musial, J. New nonlipid effects of statins and their clinical relevance in cardiovascular disease. Thromb. Haemost. 91, 1065–1077. https://doi.org/10.1160/th04-02-0064 (2004).
Poredos, P. & Jezovnik, M. K. Dyslipidemia, statins, and venous thromboembolism. Semin. Thromb. Hemost. 37, 897–902. https://doi.org/10.1055/s-0031-1297368 (2011).
Funding
This work was supported by the National Natural Science Foundation of China (82072432).
Author information
Authors and Affiliations
Contributions
P.L.: Writing—original draft. Q.L.Y. and X.J.W.: Conceptualization, project administration, and writing—review and editing. M.Y.Y. and Q.L.Y.: Data curation and methodology. P.X. and P.L.: Formal analysis, validation, visualization and software. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luo, P., Yuan, Q., Wan, X. et al. A two-sample Mendelian randomization study of circulating lipids and deep venous thrombosis. Sci Rep 13, 7432 (2023). https://doi.org/10.1038/s41598-023-34726-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34726-3
- Springer Nature Limited