Abstract
This study aimed to investigate the metabolite profile and inflammatory state of follicular fluid (FF) in women with stage III–IV ovarian endometriosis (OE) who underwent in vitro fertilization (IVF). A cohort of 20 consecutive patients with OE were recruited and received progestin-primed ovary stimulation (PPOS) protocol (study group), while another 20 OE patients received one-month ultra-long term protocol (control group) for IVF in this prospective, nonrandomized study. FF samples were obtained from dominant follicles during oocyte retrieval, and liquid chromatography-mass spectrometry (LC–MS) was used to investigate the metabolites profile of FF. Results showed that significant increases in the levels of proline, arginine, threonine, and glycine in patients who received PPOS protocol compared to the control group (P < 0.05). A panel of three metabolites (proline, arginine, and threonine) was identified as specific biomarkers of OE patients using PPOS protocol. Additionally, levels of interleukin-1β, regulated on activation, normal T cell expressed and secreted, and tumor necrosis factor-α markedly decreased in women who received PPOS protocol compared to the control group (P < 0.05). In conclusion, PPOS protocol regulates the metabolism of several amino acids in the FF, which may play critical roles in the oocyte development and blastocyst formation, and their specific mechanism should be further elucidated.
Similar content being viewed by others
Introduction
Endometriosis is an identifiable cause of infertility and also known as a chronic inflammatory status dependent on estrogen1,2. In vitro fertilization (IVF) has been a choice for pregnancy in the infertility women. Even though mild ovarian endometriosis (OE) might have an adverse effect on the fertility by affecting the oocyte and embryo development along with embryo implantation3,4. Nevertheless, the precise pathophysiological mechanisms underlying the effects of endometriosis on the infertility remain unclear5,6,7. To date, reproductive specialists have devoted to exploring the best IVF regimen for endometriosis patients. Progestins have been applied in the therapy of endometriosis for many years. The therapeutic efficacy of progestins in endometriosis-associated pelvic pain may be owed to its suppression on the regulated on activation, normal T cell expressed and secreted (RANTES) production and inflammatory responses in the pelvis8. Fechner et al. proposed that progestins can modulate the local estrodiol (E2) biosynthesis and suppress the growth of ectopic endometrium in the endometriosis women9. Some researchers have covered the usage of medroxyprogesterone acetate (MPA) for controlled ovarian hyperstimulation (COH) during IVF in patients, and it is an alternative for the treatment of stage III-IV endometriosis10,11. Studies have been conducted to investigate the follicular fluid (FF), aiming to determinate the biomarkers as well as metabolic pathways related to the oocyte and embryo outcomes12. The compositions of FF represent the interchanges between oocyte and its associated microenvironment. Oocyte metabolomics profiling may be helpful to investigate the influence of latest assisted reproduction technologies (ART) on the oocyte outcomes12. Functional analysis of oocyte quality at the distinctive oocyte level will provide worthy standpoints into the oocyte developmental ability. There is evidence showing that uterine endometriosis has mitochondrial dysfunction; ovarian endometrioma (OMA) has the imbalance between anaerobic glycolysis and β-oxidation. Both of them may affect the fertility of endometriosis women. Since the composition of follicular fluid has been proved to be related to the oocyte development and post fertilization implantation, these findings may help explain the high infertility rate in these patients13. In addition, some studies show that endometriosis is related to abnormal lipid metabolism, which can provide the necessary energy to adapt to the inflammatory response by mobilizing lipid metabolism14. On the contrary, non-invasive determination of oocyte metabolism have suggested a relationship between metabolism and embryo developmental ability. Metabolomics profile is influenced by the oocyte meiotic progression, age of a patient, regimen of controlled ovarian stimulation (COS) and disease etiology12,15. Currently, little is known about the change of cytokines in the local microenvironment of endometriosis, leading to the less acceptance and awareness of the significance of anti-inflammatory factors in the pathogenesis of endometriosis. The microenvironment of FF is complicated in patients with ovarian endometriosis diagnosed by laparoscopy13,15. Recent studies have indicated that the metabolomics profiling of FF in endometriosis patients can be employed to assess the possibility of integrating data acquired by different approaches13,15. Metabolomics profile has been recommended as a tool which can discriminate between oocytes and embryos with the developmental potential to the blastocysts as well as implantation ability. The utilization rate of amino acid in bovine oocytes has shown to be a good predictive factor of oocyte developmental competence to experience fertilization and early cleavage12,13. A variety of studies have indicated that the ultra-long term protocol can improve the quality of oocytes and embryos in patients with moderate to severe endometriosis16. The ultra-long term protocol is a common protocol used for the ovulation induction in patients with severe endometriosis. The PPOS protocol is an original protocol developed in our center for ovulation induction, and the goal of this protocol is to inhibit the premature ovulation in the ovulation induction cycle by using progesterone. Our previous study indicated the PPOS protocol in severe endometriosis patients achieves similar oocyte and embryo outcomes to the ultra-long protocol17. Studies have confirmed that progesterone can improve the inflammatory status of patients with endometriosis8. Thus, we speculate that progesterone in the PPOS protocol may improve the inflammatory status during follicular development in severe endometriosis patients, which is helpful for the improvement of oocyte and embryo quality, finally achieving similar clinical outcomes to ultra-long term protocol. In clinical studies of our group, the pregnancy outcomes of in vitro fertilization-embryo transfer (IVF-ET) after the application of PPOS protocol were explored in the endometriosis patients18,19. For these patients, the improvement of inflammatory environment in the follicular development of endometriosis patients is helpful for the improvement of oocyte quality and embryo outcomes. Metabonomic studies have indicated that the energy metabolism and inflammatory metabolism change in the FF of endometriosis patients15. However, no metabolomic studies have been undertaken in OE women who underwent the progestin-primed ovarian stimulation (PPOS) protocol. In the present prospective study, metabonomics was employed to compare the metabolites in the FF of endometriosis patients who received IVF-ET with two different ovulation induction protocols (PPOS protocol vs ultra-long term protocol). Our findings may provide theoretic evidence on the application of metabonomics in the evaluation of ovulation induction protocol for IVF-ET in the endometriosis patients, which may be helpful for the development of treatment to improve the outcomes of endometriosis patients after IVF-ET.
Materials and methods
This was a prospective, non-randomized study. Forty infertile women undergoing the first in vitro fertilization /intracytoplasmic sperm injection (IVF/ICSI) in Shanghai Ninth People’s Hospital were consecutively recruited between March 2017 to September 2017. These women were divided into 2 groups depending on the protocols used: 20 women received treatment with the PPOS protocol and 20 women with the ultra-long term protocol (control group). The inclusion criteria were as follows: (1) ovarian endometriomas (> 3 cm) were diagnosed as staged III-IV according to the revised American Fertility Society (AFS) classification20; (2) patients were younger than 39 years, but older than 20 years; (3) the baseline follicle-stimulating hormone (FSH) was lower than 10 IU/ml and the antral follicles count (AFC) was greater than or equal to 5; (4) the body mass index (BMI) was in normal range; (5) the menstrual cycle (MC) was regular (27–35 days). Women with male factor infertility, uterine or tubal infertility, or unexplained infertility, polycystic ovary syndrome, premature ovarian failure, hydrosalpinx or adenomyosis were excluded from this study. Of these patients, FF was collected from 20 patients in each group and processed for metabolitics analysis. The study was approved by the Ethics Review Committee of the Ninth People’s Hospital of Shanghai, China ([2014]109). This study has been performed in accordance with the Declaration of Helsinki. Informed consent was obtained from all patients before study.
Protocols
The MPA + hMG protocol used during IVF has been reported in detail elsewhere10,11. HCG (2000 IU) (Lizhu Pharmaceutical Trading Co, Zhuhai, China) and Decapeptyl (0.1 mg) (Ferring International Center SA, Germany) were used at the last stage of oocyte maturation10. Viable embryos were cryopreserved. The one-month ultra-long GnRHa scheme was used in the control group. The ultra-long triptorelin at 3.75 mg were administered in patients beginning on MC day 2, and day 5 or 6 weeks later, and hMG was administered at 225 IU once daily. In the GnRHa protocol, hCG (5000 IU) was used at the final stage of oocyte maturation. In the ultra-long term protocol group, fresh embryo transfer was the first choice except for the patients who had to undergo frozen embryo transfer due to some reasons. If the fresh embryo transfer (ET) was not prepared due to high risk of ovarian hyperstimulation syndrome (OHSS), elevated progesterone (P) on the triggering day, unqualified endometrium, or other personal reasons, all good-quality embryos were frozen, and frozen-thawed embryo transfer (FET) was done later.
Sample preparation and mass spectrometry and liquid chromatography
Under the guidance of vaginal ultrasonography, 2–3 follicles with a diameter of about 18 mm were selected for puncturing, and the FF without obvious blood was used in the following analysis.
The FF was thawed at 4 °C. Then, 200 µL of FF was transferred into a 1.5 mL EP tube, following by addition of 800 µL of methanol. After vertexing for 60 s, the fluid was centrifuged at 12,000 rpm for 10 min at 4 °C. The supernatant was collected and transferred into a 1.5 ml EP tube, followed by drying in the vacuum. The sediment was dissolved with 300 µL of 80% methanol, and the solution was filtered through a filter with the pore size of 0.22 µm. The resultant filtrate was harvested. Then, 20 µL of the filtrate from each sample was mixed into the quality control (QC) sample which was used to adjust the deviation in the assay of mixed sample and the system error. The remaining samples were subjected to LC–MS.
The sample preparation for the metabolomic analysis was based on sample-preparation mean by LC–MS21. The specific courses were previously reported22. The pooled QC and blank (pure acetonitrile) sample were injected to run for assessing the stability and repeatability. On the basis of QC, quality assurance (QA) was carried out to delete the features with poor repeatability in the QC samples, and higher quality data set was obtained to facilitate the detection of biomarkers. In the QC samples, the proportion of characteristic peaks with RSD < 30% reached 70%, which indicated that the data quality was repeatability. Interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and RANTES concentrations in FF were detected by enzyme linked immunosorbent assay (ELISA; BOSTER, Biological technology Ltd, USA) according to the manufacturer’s instructions. The standard curve was made according to the concentration and optical density (OD) of the standard substance, and then the sample concentration was calculated according to the standard curve. Chromatographic separation was completed in an Acquity LC system. The specific operation process has been described in detail in previous articles23.
Statistical analysis and processing of data
The data were collated by the pheat map package in R version 3.3.2 (Auckland, New Zealand), and the samples and metabolites were bi-directionally clustered. The metabolites were characterized by comparisons with reference standards or MS/MS fragment information obtained from, Human Metabolome Database (HMDB) (http://www.hmdb.ca), Metlin (http://metlin.scripps.edu), LipidMaps (http://www.lipidmaps.org) and mzclound (https://www.mzcloud.org) database. The confirmed metabolites were related to biochemical pathways in KEGG (http://www.kegg.jp/pathway). Metabolic pathway topological analysis and enrichment were implemented using the MetPA database (www.metabo analyst.ca) to evaluate metabolic pathways related to different metabolites24. Pearson correlation coefficient was used to estimate the correlation between metabolites25. P ≤ 0.05 + variable importance in projection (VIP) ≥ 1 was considered statistically significant indifferences metabolites26. Orthogonal Projections to Latent Structures Discriminant Analysis (OPLS-DA) can effectively reduce the complexity of the model and enhance the interpretation ability of the model without reducing the prediction ability of the model, which maximizes the intergroup differences. OPLS-DA uses orthogonal signal correction technology to decompose the X matrix information into Y-relevant and Y-irrelevant information, and then the information that is irrelevant to classification is filtered out. The relevant information is mainly concentrated in the first prediction component. Generally, the Variable Importance for the Projection (VIP) is used to explain the importance of X dataset and relevant Y dataset. The sum of squares of all VIP values are equal to the total number of variables in the model. Thus, its mean is 1. The VIP of larger than 1 is indicative of important role of this variable. Thus, this is usually used for the screening of potential biomarkers.
Results
Clinical characteristics of patients
The clinical features of 40 women are displayed in Table 1. No obvious differences were found in the age, infertility duration, baseline levels of hormones, AFC and BMI between 2 groups. Furthermore, the MPA + HMG protocol had a lower stimulation dose of hMG, and the duration of stimulation was significantly different between two groups (P < 0.05). There were no marked differences in the numbers of oocytes retrieved, mature embryos and high quality embryo (P > 0.05).
Metabolomics analysis
OPLS-DA predicted metabolic profiles
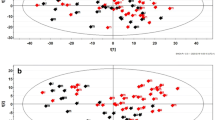
When redundant peaks were eliminated, LC–MS was applied to test 1158 differential metabolites from each FF sample. In view of the profiled metabolites, OPLS-DA was performed to distinguish PPOS protocol from controls in OE patients. The model exhibited gratifying results in distinction of two groups with one predictive component and one orthogonal component (R2Xcum = 0.306, R2Ycum = 0.998, and Q2cum = 0.886) (Fig. 1). These results revealed that the variation of FF metabolomic profile could be applied to separate two different protocols in OE patients.
Biomarker identification and diagnosis evaluation
Variables that were helpful for the distinction of two different protocols in OE patients were considered as potential biomarkers. In Table 2, the analytes that were displayed differentially (nonparametric Wilcoxon–Mann–Whitney, P < 0.005) between two groups were incorporated into the biomarker set. A total of 1158 differential metabolites were established and MS/MS fragments were further confirmed with commercial standards. As described in Table 2, the levels of proline, arginine, and threonine, p-Salicylic acid, glycine, Testosterone, 11b-Hydroxyandrost-4-ene-3,17-dione and 17-Hydroxyprogesteronelevels were significantly different between two groups. Agglomerate hierarchical clustering was used to show the levels of different metabolites in two groups (Fig. 2). The data set was scaled through the heat map package in R (v3.3.2). The differential metabolite correlation analysis was used to study the consistency of the change trend between 19 metabolites. Metabolite correlations often display the synergy of changes among metabolites (Fig. 3).
The receiver operating characteristic (ROC) curve was further used to evaluate the identified biomarkers. As displayed in Table 3, 17 metabolites related to PPOS protocol, the area under the ROC curve (AUC) of 17 biomarkers ranged from 0.708 to 0.887, demonstrating that they were moderately predictive markers. Table 3 shows the metabolites with AUC > 0.7.
Efficacy of PPOS on the metabolic pathway of OE
Multivariate analysis was performed on the differential metabolites to test the efficacy of PPOS on relevant metabolite pathways. The more discrete the point, the greater the disturbance was. The influences of PPOS on 17 metabolic pathways of FF were analyzed, and results showed the most affected pathways were phenylalanine metabolism pathway, which involves glycine, salicylic acid and cinnamic acid, and the aminoacyl-tRNA biosynthesis pathway, arginine and proline metabolism pathway, which involves proline (AUC: 0.709), arginine (AUC: 0.803) and threonine (AUC: 0.689) (Fig. 4; Table 4).
Inflammatory factors in two groups
The levels of IL-1β, RANTES and TNF-α in the FF of PPOS protocol group were considerably lower than in the ultra-long protocol group (IL-1β: 32.2523,37 ng/mL vs 35.0430,49 ng/mL; RANTES: 231.81 ± 21.41 ng/mL vs 258.42 ± 16.26 ng/mL; TNF-α: 48.88 ± 5.26 ng/mL vs 58.01 ± 7.23 ng/mL; P < 0.05).
Discussion
In the present study, LC–MS was performed to explore the metabolomics profile of FF in patients undergoing IVF/ICSI with PPOS or ultra-long term protocol. Three differentially expressed amino acids (proline, arginine, and threonine) were identified to be associated with the PPOS protocol that achieved high-quality oocytes and satisfactory pregnancy outcomes17. The levels of IL-1β, RANTES and TNF-α in the FF of PPOS protocol group were remarkably lower than in the ultra-long protocol group. Metabolomics analysis of the FF has allowed the identification of biomarkers and metabolic pathways related to several pathologies which affect oocyte quality. In the present study, the metabolic pathways related to the oocyte quality included phenylalanine metabolic pathway, synthesis of aminoacyl proline and arginine metabolism.
The administration of GnRH agonists before IVF/ICSI may advance the pregnancy rate. The 2006 Cochrane guideline proposes the use of GnRH analogs for more than 2 months before IVF cycle to “inhibit the inflammatory state”, thereby improving the pregnancy rate16. It has been confirmed that endometriosis is detrimental to the ovaries. Toxic factors (such as reactive oxygen species [ROS], free iron and inflammatory molecules) from an endometrioma may cause adverse events such as increased oxidative stress, reduced follicular maturation and impaired fertilization27,28. A 3-month ultra-long GnRHa creates a more favorable environment for oocyte maturation, with better oocyte and embryo quality as well as fertilization rate. The effect of ultra-long term protocol on the oocyte and embryo quality is still controversial. Some studies indicate that the ultra-long term protocol is able to improve the oocyte and embryo quality, but others fail to reveal the improvement of oocyte and embryo quality after the ultra-long term protocol29. Whether this vicious cycle of damage can be ameliorated by MPA treatment during IVF/ICSI is still unclear. MPA is clinically useful for the treatment of pain and may improve the overall comforts in most women with endometriosis30,31. There is still controversy on the therapeutic efficacy of MPA, such as danazol, stems from the pituitary inhibition and atrophy of endometriotic lesions32. Another possible attribute is that MPA alleviates inflammation at the site of endometriotic implants. Prolonged treatment (8 days) with MPA decreases the luciferase activity by 36% and reduces the RANTES protein expression by 50%. Nevertheless, short treatment (2 or 4 d) with MPA has no significant effect. Our previous study also showed that 8-d MPA increased progesterone receptor (PR) expression8. General metabolite profiling has been supposed to be a tool which can distinguish between pronucleate zygotes and embryos with the developmental potential into the blastocysts and implantation12. In the present study, no significant differences were observed in the number of mature oocytes, number of high-quality embryo and fertilization, and pregnancy rate between two groups. Although the PPOS protocol did not significantly elevate the pregnancy rate, it at least achieved the pregnancy outcome similar to those after ultra-long term protocol17, and the inflammatory cytokines reduced significantly, suggesting the improvement of microenvironment of oocyte development. These findings support that oocyte metabolism reflects oocyte quality. Thus, more studies are needed to investigate the association of functional metabolites with the quality of human oocytes having embryo developmental competence. Our study was to explore the differential metabolites during the COH between endometriosis patients with PPOS protocol and those with ultra-long term protocol. Some studies shown that individual bovine MII oocytes with the capacity to support zygote cleavage and blastocyst development in vitro following IVF display a different preference for amino acids compared with oocytes which failed to fertilize or undergo embryo cleavage33. The study also demonstrated, that amino acid depletion/appearance by human oocytes is a function of patient age, and gonadotrophin preparation and dose used for COS12. These findings indicate that metabolite profiling is useful to identify the ‘nutritional fingerprint’ related to the oocyte quality in endometriosis patients, which may be used to enhance the efficacy of ART33.
The signalling pathways that promote the resumption and progression of meiosis of bovine oocytes after the luteinizing hormone (LH) peak are not fully understood34. One of the factors involved in the oocyte maturation that have been investigated is nitric oxide (NO). NO is produced by the nitric oxide synthase (NOS), which catalyzes the production of l-citrulline and NO from l-arginine (l-Arg). The utilization rate of L-Arg for NO synthesis in the medium is one of the keys in the in vitro synthesis of NO35.
It has been confirmed that some key enzymes and metabolites in the arginine metabolism pathway play important roles in the development of follicles36. They can directly affect the follicles, or indirectly improve the development of follicles by promoting the proliferation of granulosa cells. Therefore, the arginine level after the use of PPOS regimen in patients with endometriosis significantly increased, which may be a key factor to improve the developmental microenvironment of follicles and regulate the function of granulosa cells.
At the same time, FF has its own antioxidant system, such as superoxide dismutase (SOD)37,38,39. The antioxidative activity of FF has an adverse effect on the oocyte and embryo developmental potential as well as pregnancy outcome40. The increased ROS are the principal consideration which influences mammalian embryo development in vitro41,42. The roles of dietary supplementation with arginine in the regulation of inflammation, antioxidation, and mRNA expression of antioxidation-related molecules in the spleen have been confirmed in rats under oxidative stress43. Studies have shown that Arg facilitates the protein synthesis, improves the reproductive performance, and facilitates the intestinal cell migration44,45. These indicate that Arg may influence the antioxidative defense in the spleen. The antioxidative capacity has a correlation with the inflammatory response46, which is regulated by some inflammatory cytokines. In pigs, Arg is able to decrease the transcription of TNF-αand IL-6 in the jejunum challenged by lipopolysaccharide47. Moreover, Arg intake may increase the expression of transforming growth factor-β (TGF-β) and IL-10 in carps, which is partially ascribed to the upregulated mTOR expression48. Further investigations indicate that this may be affected by the transcription factor nuclear factor κB (NF-κB), which may induce the resistance to programmed cell death by up-regulating the expression of anti-apoptotic genes49. These findings indicate that Arg may affect the pro-inflammatory and anti-inflammatory cytokines in animals50. In our study, the levels of IL-1β, RANTES and TNF-α in the FF of PPOS protocol group remarkably reduced as compared to the ultra-long term protocol group. LC–MS has been used to compare the metabolites of FF between non-endometriosis and endometriosis samples15. Results showed β-hydroxybutyric acid and glutamine significantly increased, whereas tryptophan markedly decreased in the endometriosis patients. Karaer et al.51 conducted metabonomic analysis of FF, and results showed that lactic acid, β-glucose, pyruvate and valine significantly increased in the FF of endometriosis women when compared with the controls, which was related to the inhibition of follicular development in these women. In patients with repeated fertilization failure, valine in the FF increased, which may be related to infertility in these patients. Marianna et al.52 found the levels of alanine, proline, aspartic acid, glucose, valine, leucine, lysine, choline and phosphocholine in the FF decreased, while those of lactic acid, phospholipid and other lipids increased in the FF of endometriosis women. Excessively high or low levels of these metabolites may exert adverse effects on the follicular development. Increased metabolites may induce oxidative stress in the FF, affecting the cytoplasm and nucleus of oocytes. Valine, leucine and lysine are essential amino acids, leucine and lysine are ketogenic amino acids, and lysine is a branched chain amino acid. They are involved in the synthesis of ATP and can be metabolized into ketone bodies. The reduction of these amino acids is closely related to the abnormal energy metabolism in the FF. Proline, arginine, threonine, and glycine significantly increased in women with PPOS protocol as compared to controls. These amino acids are also related to the energy metabolism and inflammation, and may be involved in the ovulation induction with PPOS protocol.
No study has been conducted to explore the antioxidative effect of L-Arg in the FF of patients with endometriosis. The progression of endometriosis is estrogen dependent, while progesterone can suppress the effect of estrogen. Although the estrogen level in the PPOS protocol group was significantly higher than in the ultra-long protocol group, the levels of inflammatory factors significantly reduced. The relationship between l-Arg level and expression of antioxidant enzymes is still unclear. However, above studies showed that the arginine content significantly increased in patients with endometriosis receiving PPOS protocol, and arginine might promote the development of oocytes and embryos by improving the inflammation and immune status of patients with endometriosis through important signaling pathways.
The phenylalanine metabolism is interesting. In the plasma, phenylalanine may be metabolized into tyrosine, mainly in the liver. Tyrosine is closely related to some hormones and neurotransmitters. Thus, it would be a kind of “environmental estrogen” effect where the action of estrogens is modulated by other phenolic compounds.
There were still limitations in the present study. In our study, ovarian endometriosis patients were included, and they might have deep endometriosis. Endometriosis is a heterogeneous disease, and its influence on the FF may be dependent on the severity of endometriosis. In the ultra-long term protocol, the conventional pre-treatment often lasts for 3 months or more, but the time of downregulation is longer, side effects are serious, medical cost is high, the treatment is long, the therapeutic response is poor, and the patient’s compliance is also unacceptable, which may cause early ending of clinical studies. Thus, in our study, the time of downregulation was one cycle. In addition, there was no ideal control group to study the metabolic characteristics of FF in endometriosis patients. The endometriosis patients receiving IVF for tubal infertility will be the suitable controls. In addition, the sample size was small in the present study, which might bias the results of pregnancy outcomes. Thus, the pregnancy outcomes were not displayed in this study. Further studies with large sample size and elegant design are needed to confirm our findings and reveal the mechanism(s) for the alteration of metabolite profiles in the FF.
Data availability
All data generated or analysed during this study are included in this published article.
References
Johnson, N. P. et al. World endometriosis society consensus on the classification of endometriosis. Hum. Reprod. 32, 315–324 (2017).
Johnson, N. P., Hummelshoj, L., World Endometriosis Society Montpellier C. Consensus on current management of endometriosis. Hum. Reprod. 28, 1552–1568 (2013).
Signorile, P. G. & Baldi, A. Endometriosis: New concepts in the pathogenesis. Int. J. Biochem. Cell Biol. 42, 778–780 (2010).
Harb, H. M., Gallos, I. D., Chu, J., Harb, M. & Coomarasamy, A. The effect of endometriosis on in vitro fertilisation outcome: A systematic review and meta-analysis. BJOG 120, 1308–1320 (2013).
Gupta, S. et al. Pathogenic mechanisms in endometriosis-associated infertility. Fertil. Steril. 90, 247–257 (2008).
Vercellini, P., Vigano, P., Somigliana, E. & Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 10, 261–275 (2014).
Carvalho, L. F. et al. From conception to birth: How endometriosis affects the development of each stage of reproductive life. Minerva Ginecol. 65, 181–198 (2013).
Zhao, D., Lebovic, D. I. & Taylor, R. N. Long-term progestin treatment inhibits RANTES (regulated on activation, normal T cell expressed and secreted) gene expression in human endometrial stromal cells. J. Clin. Endocrinol. Metab. 87, 2514–2519 (2002).
Vercellini, P. et al. Progestogens for endometriosis: Forward to the past. Hum. Reprod. Update. 9, 387–396 (2003).
Kuang, Y. et al. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil. Steril. 104, 62–70 (2015).
Wang, Y. et al. Controlled ovarian stimulation using medroxyprogesterone acetate and hMG in patients with polycystic ovary syndrome treated for IVF: A double-blind randomized crossover clinical trial. Medicine 95, e2939 (2016).
Hemmings, K. E. et al. Amino acid turnover by human oocytes is influenced by gamete developmental competence, patient characteristics and gonadotrophin treatment. Hum. Reprod. 28, 1031–1044 (2013).
Pocate-Cheriet, K. et al. The follicular fluid metabolome differs according to the endometriosis phenotype. Reprod. Biomed. Online. 41, 1023–1037 (2020).
Ni, Z. et al. Correlation of fecal metabolomics and gut microbiota in mice with endometriosis. Am. J. Reprod. Immunol. 84, e13307 (2020).
Murgia, F. et al. Metabolic profile of patients with severe endometriosis: A prospective experimental study. Reprod. Sci. 28, 728–735 (2021).
Sallam, H. N., Garcia-Velasco, J. A., Dias, S. & Arici, A. Long-term pituitary down-regulation before in vitro fertilization (IVF) for women with endometriosis. Cochrane Database Syst. Rev. 2006, 004635 (2006).
Guo, H. et al. The comparison of two different protocols ultra-long versus medroxyprogesterone acetate in women with ovarian endometriosis: A prospective randomized controlled trial. Reprod. Health. 19, 198 (2022).
Guo, H. et al. Use of medroxyprogesterone acetate in women with ovarian endometriosis undergoing controlled ovarian hyperstimulation for in vitro fertilization. Sci. Rep. 7, 11927 (2017).
Guo, H. et al. Efficacy of different progestins in women with advanced endometriosis undergoing controlled ovarian hyperstimulation for in vitro fertilization: A single-center non-inferiority randomized controlled trial. Front. Endocrinol. 11, 129 (2020).
Canis, M. et al. Revised American Society for reproductive medicine classification of endometriosis: 1996. Fertil. Steril. 67, 817–821 (1997).
Vuckovic, D. Current trends and challenges in sample preparation for global metabolomics using liquid chromatography-mass spectrometry. Anal. Bioanal. Chem. 403, 1523–1548 (2012).
Blasco, H. et al. Metabolomics in cerebrospinal fluid of patients with amyotrophic lateral sclerosis: An untargeted approach via high-resolution mass spectrometry. J. Proteome Res. 12, 3746–3754 (2013).
Want, E. J. et al. Global metabolic profiling procedures for urine using UPLC-MS. Nat. Protoc. 5, 1005–1018 (2010).
Wang, G. et al. ROS mediated EGFR/MEK/ERK/HIF-1α loop regulates glucose metabolism in pancreatic cancer. Biochem. Biophys. Res. Commun. 500, 873–878 (2018).
Rao, G., Sui, J. & Zhang, J. Metabolomics reveals significant variations in metabolites and correlations regarding the maturation of walnuts (Juglans regia L.). Biol. Open. 5, 829–836 (2016).
Heischmann, S. et al. Exploratory metabolomics profiling in the kainic acid rat model reveals depletion of 25-hydroxyvitamin D3 during epileptogenesis. Sci. Rep. 6, 31424 (2016).
Sanchez, A. M. et al. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: From pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum. Reprod. Update. 20, 217–230 (2014).
Gupta, S., Ghulmiyyah, J., Sharma, R., Halabi, J. & Agarwal, A. Power of proteomics in linking oxidative stress and female infertility. Biomed. Res. Int. 2014, 916212 (2014).
Georgiou, E. X. et al. Long-term GnRH agonist therapy before in vitro fertilisation (IVF) for improving fertility outcomes in women with endometriosis. Cochrane Database Syst. Rev. 2019, 1–10 (2019).
Harrison, R. F. & Barry-Kinsella, C. Efficacy of medroxyprogesterone treatment in infertile women with endometriosis: A prospective, randomized, placebo-controlled study. Fertil. Steril. 74, 24–30 (2000).
Vercellini, P., Cortesi, I. & Crosignani, P. G. Progestins for symptomatic endometriosis: A critical analysis of the evidence. Fertil. Steril. 68, 393–401 (1997).
Henzl, M. R. et al. Administration of nasal nafarelin as compared with oral danazol for endometriosis: A multicenter double-blind comparative clinical trial. N. Engl. J. Med. 318, 485–489 (1988).
Hemmings, K. E., Leese, H. J. & Picton, H. M. Amino acid turnover by bovine oocytes provides an index of oocyte developmental competence in vitro. Biol. Reprod. 86(165), 1–12 (2012).
Bilodeau-Goeseels, S. Cows are not mice: The role of cyclic AMP, phosphodiesterases, and adenosine monophosphate-activated protein kinase in the maintenance of meiotic arrest in bovine oocytes. Mol. Reprod. Dev. 78, 734–743 (2011).
Hobbs, A. J. Nitric oxide biology & pathobiology (3rd edition) editors: Louis Ignarro & Bruce Freeman. Nitric Oxide 90, 66 (2019).
Dubeibe, D. F. et al. L-Arginine affects the IVM of cattle cumulus-oocyte complexes. Theriogenology 88, 134–144 (2017).
Carbone, M. C. et al. Antioxidant enzymatic defences in human follicular fluid: Characterization and age-dependent changes. Mol. Hum. Reprod. 9, 639–643 (2003).
Lambrinoudaki, I. V. et al. Measurable serum markers of oxidative stress response in women with endometriosis. Fertil. Steril. 91, 46–50 (2009).
Ozkaya, M. O. & Naziroglu, M. Multivitamin and mineral supplementation modulates oxidative stress and antioxidant vitamin levels in serum and follicular fluid of women undergoing in vitro fertilization. Fertil. Steril. 94, 2465–2466 (2010).
Nishihara, T., Matsumoto, K., Hosoi, Y. & Morimoto, Y. Evaluation of antioxidant status and oxidative stress markers in follicular fluid for human in vitro fertilization outcome. Reprod. Med. Biol. 17, 481–486 (2018).
Johnson, M. H. & Nasr-Esfahani, M. H. Radical solutions and cultural problems: could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro?. BioEssays 16, 31–38 (1994).
Kitagawa, Y., Suzuki, K., Yoneda, A. & Watanabe, T. Effects of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology 62, 1186–1197 (2004).
Huang, B. et al. Antioxidant capacity of follicular fluid from patients undergoing in vitro fertilization. Int. J. Clin. Exp. Pathol. 7, 2273–2282 (2014).
Gao, K. et al. Dietary l-arginine supplementation enhances placental growth and reproductive performance in sows. Amino Acids 42, 2207–2214 (2012).
Kong, X. et al. L-Arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. J. Nutr. Biochem. 23, 1178–1183 (2012).
Bub, A. et al. Fruit juice consumption modulates antioxidative status, immune status and DNA damage. J. Nutr. Biochem. 14, 90–98 (2003).
Liu, Y. et al. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br. J. Nutr. 100, 552–560 (2008).
Chen, G. et al. Effect of dietary arginine on the immune response and gene expression in head kidney and spleen following infection of Jian carp with Aeromonas hydrophila. Fish Shellfish Immunol. 44, 195–202 (2015).
Manu, K. A. & Kuttan, G. Ursolic acid induces apoptosis by activating p53 and caspase-3 gene expressions and suppressing NF-kappaB mediated activation of bcl-2 in B16F-10 melanoma cells. Int. Immunopharmacol. 8, 974–981 (2008).
Mo, W. et al. Roles of dietary supplementation with arginine or N-carbamylglutamate in modulating the inflammation, antioxidant property, and mRNA expression of antioxidant-relative signaling molecules in the spleen of rats under oxidative stress. Anim. Nutr. 4, 322–328 (2018).
Karaer, A., Tuncay, G., Mumcu, A. & Dogan, B. Metabolomics analysis of follicular fluid in women with ovarian endometriosis undergoing in vitro fertilization. Syst. Biol. Reprod. Med. 65, 39–47 (2019).
Marianna, S. et al. Metabolomic profiling and biochemical evaluation of the follicular fluid of endometriosis patients. Mol. Biosyst. 13, 1213–1222 (2017).
Kanehisa, M., Furumichi, M., Sato, Y., Ishiguro-Watanabe, M. & Tanabe, M. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 49, D545–D551 (2021).
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951 (2019).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Acknowledgements
This study was supported partially by the National Natural Science Foundation of China (No. 81971448, 81903324, 82273634), Cross-disciplinary Research Fund of Shanghai Ninth People's Hospital, Shanghai JiaoTong university School of Medicine (JYJC202213).
Author information
Authors and Affiliations
Contributions
H.G. and B.L. designed this study. H.G., Q.Z., H.G., Q.L., W.C., L.W., and B.L. collected the data. H.G. analysed the data. H.G. drafted the manuscript. B.L. reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, H., Zhu, Q., Gao, H. et al. Metabolomics analysis of follicular fluid in ovarian endometriosis women receiving progestin-primed ovary stimulation protocol for in vitro fertilization. Sci Rep 13, 5747 (2023). https://doi.org/10.1038/s41598-023-32797-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-32797-w
- Springer Nature Limited