Abstract
The co-existence of diabetic ketoacidosis (DKA) with acute pancreatitis (AP) is associated with unfavorable clinical outcomes. However, diagnosing AP in DKA patients is challenging and often missed due to overlapping symptoms. The aim of this retrospective observational study was to compare the clinical characteristics and outcomes of patients with concomitant DKA and AP or DKA alone. Data of patients with DKA admitted between January 2015 to August 2021 to four hospitals in Qatar was extracted from the electronic health record (Cerner). American Diabetes Association criteria and Atlanta criteria were used for DKA and AP diagnosis, respectively. Independent T-test or Mann–Whitney U test was used to analyze continuous variables, whereas categorical variables were analyzed via Chi-square or Fischer exact tests as appropriate. Univariate and multivariate logistic regression models were generated to assess the correlations. A p-value of < 0.05 was considered statistically significant. Of 936 patients with DKA, 84 (9.0%) had coexisting AP. AP was most common in the Asian race (66%, p < 0.001). Patients with DKA and AP were older, had higher admission anion-gap, white cell count, hemoglobin (hb), neutrophil/lymphocyte ratio, urea, creatinine, maximum blood glucose during the episode, total cholesterol and triglyceride level (TGL) (p < 0.05). They had a lower admission venous pH and bicarbonate at 6 h. Patients in the DKA with AP group also had a longer length of stay (LOS), DKA duration and a higher rate of ICU admission (p-values ≤ 0.001). In-hospital mortality, 3-month all-cause readmission, 6-month and 12-month DKA recurrence did not differ between the two groups. Univariate logistic regression analysis showed age, Asian ethnicity, male gender, T2D, admission WBC count, hb, urea, creatinine, potassium, venous pH, bicarbonate, anion gap, total cholesterol, TGL and LDL level were significantly associated with the development of DKA with AP (p < 0.05). In multivariate logistic regression analysis, age and total cholesterol level were associated with concomitant DKA and AP (p < 0.05). Patients with concomitant DKA and AP have more severe derangement in markers of DKA severity, inflammation, kidney injury and metabolic profile, along with a longer DKA duration, LOS and requirement for ICU support compared to DKA patients without AP. This highlights the clinical significance of diagnosing the co-existence of DKA with AP, as the combination results in significantly worse clinical outcomes and greater healthcare utilization than in patients with only DKA.
Similar content being viewed by others
Introduction
Diabetic ketoacidosis (DKA) is a serious metabolic complication of diabetes mellitus (DM) characterized by the development of ketosis, hyperglycemia and a high anion gap metabolic acidosis1,2. It is more common in type 1 diabetes (T1D) patients than in type 2 diabetes (T2D). The incidence of DKA in T1D patients is reported to be 178.6/10,000 patient-years, while in T2D, it is 20/10,000 patient-years3. The disease is associated with a very high risk of short-term mortality, with a study reporting a mortality rate of 5.2% over a 4-year follow-up after DKA admission4. Acute pancreatitis (AP) is another life-threatening condition with an estimated incidence of 33.74 cases per 100,000 patient years and 1.6 deaths per 100,000 patient-years5. The development of concomitant DKA and AP has been reported in the literature. Recognition of the co-existence of these two entities is associated with unfavorable clinical outcomes but is challenging to recognize because of the overlapping symptoms. Abdominal pain, the most common symptom of AP, is present in up to 46% of cases of DKA and is strongly associated with more severe metabolic acidosis6. Interestingly, some case reports reported concomitant AP and DKA as even the initial manifestation of both T1D and T2D7,8.
Several studies have compared patients with coexisting DKA with AP to patients with AP alone. Yuan et al. reported a higher rate of acute kidney injury (AKI), length of stay (LOS), and severity of AP in patients of the DKA and AP group as compared to the AP group9. Wang et al. also reported a higher incidence of AKI, an increase in intensive care unit (ICU) admission and a higher APACHE II score in DKA with AP patients compared to AP only patients10. Similar results were described by Fu et al., who found statistically significant differences in ICU admission and LOS between the two groups11. However, data comparing patients with concomitant DKA and AP to patients with DKA alone is limited. Ma et al. compared the characteristics of DKA patients with and without pancreatitis. Their study showed a longer LOS and a higher ICU admission rate in patients with a combination of DKA and AP compared to DKA alone. However, the study only included 25 patients with coexisting AP and excluded patients with T1D12. Our study aimed to assess the differences in clinical characteristics and outcomes of patients with these two coexisting conditions compared to those with DKA alone. Early identification of this combination of illnesses can alert physicians to patients at high risk of developing worse clinical outcomes.
Materials and methods
This is a retrospective, cross-sectional study and included consecutive patients with DKA diagnosis presenting to the emergency department (ED) of four hospitals of Hamad Medical Corporation (HMC), Doha, Qatar, between January 2015 and August 2021. HMC is Qatar's main tertiary healthcare provider and one of the leading healthcare systems in the Middle East.
Inclusion criteria
All patients fulfilling the criteria of DKA were included in the study. We used American Diabetes Association (ADA) criteria for establishing the diagnosis of DKA, i.e., blood glucose greater than 250 mg/dL, high anion gap metabolic acidosis (pH < 7.3, bicarbonate < 18 mmol/L, anion gap > 10 mmol/L) and ketonemia/ketonuria13. Patients fulfilling the DKA diagnostic criteria were further assessed for the presence of concomitant pancreatitis using the Atlanta criteria, which includes the presence of two of the following three features: (1) Persistent upper abdominal pain, (2) Serum amylase or lipase level at least three times the upper limit of normal (3) Imaging evidence of AP14.
Exclusion criteria
Patients aged less than 14 years, pregnant patients and patients with starvation or alcoholic ketosis were excluded from the analysis.
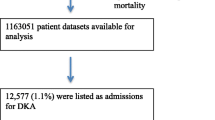
A total of 936 patients fulfilled the criteria of DKA and were included in the study (Fig. 1).
Data were manually extracted from electronic medical records (EMR) by the research study's members. Demographic data included age, gender, ethnicity, weight, height and co-morbid conditions. All laboratory results at admission and sequential DKA related laboratory results during hospital stay (venous pH, bicarbonate, anion gap, lactate, electrolytes) were also recorded. Data related to outcome included length of stay (LOS), duration of DKA, a requirement for ICU admission, in-hospital mortality, 30-day all-cause readmission, 6-month DKA recurrence and 12-month DKA recurrence.
To ensure adequate data quality, it was collected manually from EMR and subsequently reviewed again for quality by the primary investigator. There was no missing data in laboratory variables used to establish DKA diagnosis and in variables to establish clinical outcomes of DKA. No formal method to deal with the missing data was used for other variables that had missing data.
Statistical analysis
Continuous variables were described as either means (± standard deviation) or median (interquartile range), and an independent t-test or Mann–Whitney U test was used for comparison as appropriate. Categorical variables were described as percentages, and comparisons were performed using the chi-square and Fisher's tests. Factors associated with the development of coexisting DKA and AP were assessed using univariate and multivariate logistic regression analysis and reported as odds ratio (OR) and 95% confidence intervals (CI). A p-value of < 0.05 was considered significant. Stata version 17 was used for analysis.
Ethical declaration
The study is original and is not under consideration for publication in another journal. The study has been approved by the Medical Research Centre (MRC) at Hamad Medical Corporation, Qatar. All methods were performed in accordance with the relevant guidelines and regulations and according to the principles laid down in the Declaration of Helsinki. All the authors reviewed and approved the final manuscript.
Informed consent
Due to the nature of this retrospective study and the preserved anonymity of patients, a waiver of informed consent was obtained from the Medical Research Centre (MRC) at Hamad Medical Corporation, Qatar.
Results
Baseline characteristics of the study population
A total of 936 patients fulfilling the DKA criteria were included in the analysis, of which 84 (9.0%) patients had concomitant AP. DKA patients with coexisting AP were older (Mean (SD) age of 35.3 ± 14.5 vs. 42.7 ± 12.8 years, p < 0.001) as compared to those without AP. Both groups had a predominance of males (male to female 61.2% vs. 38.8% in the DKA group; 79.8% vs. 20.2% in the DKA and AP group; p < 0.001). Notably, most DKA patients belonged to the Arab ethnicity (54%). However, coexisting AP was most common in the Asian race (65.47%, p < 0.001). More patients in the DKA with AP group had T2D (75%, p < 0.001). No statistically significant difference between mean BMI, duration of DM and co-morbid conditions was observed between the two groups (Table 1).
Laboratory investigation results
In comparison to patients with DKA alone, DKA patients with AP had a statistically significant higher mean anion gap (22.9 ± 6.8 vs. 24.4 ± 7.7 mEq/L, p 0.044), median white cell count (WBC) (11.6 (IQR 8.2–17) vs. 15.1 (IQR 10.6–20.6) × 103/µL; p < 0.001) median neutrophil to lymphocyte ratio (NLR) 6 (IQR 2.5–11.2) vs. 7.6 (IQR 4.2–12.7); p 0.01), mean hemoglobin (14.2 ± 2.1 vs. 15 ± 2.4 g/dL; p 0.001), median serum urea level (5.7 (IQR 4–8.1) vs. 7.8 (IQR 4.9–12.4) mmol/L; p < 0.001), median serum creatinine 85 (IQR 64–120) vs. 109 (IQR 78–152) µmol/L; p < 0.001), median total cholesterol 4.3 (IQR 3.3–5.5) vs. 4.7 (3.5–7) mmol/L; p 0.01) and median serum triglyceride (TG) level 1.8 (IQR 1.2–2.5) vs. 2.2 (1.4–5.3) mmol/L; p 0.006) at admission as compared to the patients without AP. DKA patients with AP had lower mean potassium level (3.4 ± 0.5 vs. 3.2 ± 0.6 mmol/L; p 0.001) and lower mean sodium (131.5 ± 4.6 vs. 130 ± 6.6 mmol/L; p 0.048) during the DKA episode. These patients also had a lower mean venous pH at admission (7.15 ± 0.13 vs. 7.09 ± 0.15; p 0.001), median venous pH at 6-h 7.26 (IQR 7.2–7.32) vs. 7.24 (IQR 7.15–7.29); p 0.03) and mean bicarbonate at 6-h (15 ± 5 vs. 13.7 ± 5 mmol/L; p 0.007) in comparison to the DKA only group. There was no statistically significant difference between hbA1c, beta-hydroxybutyrate (BHB) and lactate levels at presentation between the two groups (Table 2).
Clinical outcomes
DKA patients with AP had a longer median length of hospital stay (2.4 (IQR 1.07–4.47) vs. 4.8 (IQR 2.6–7.2) days; p < 0.001), longer median DKA duration (17 (IQR 10–27) vs. 27 (IQR 13.3–41) hours; p < 0.001) and a higher rate of ICU admission (23.1 vs. 39.3%; p 0.001) in comparison to the patients without concomitant AP. In-hospital mortality, 3-month all-cause readmission, 6-month and 12-month DKA recurrence were not statistically significant between the two groups (Table 3).
Univariate logistic regression analysis (Table 4) showed age (OR 1.03 (1.01–1.04), p < 0.001) Asian ethnicity (OR 6.52 (1.95–20.98), p 0.002), male gender (OR 2.5 (1.44–4.33), p 0.001), T2D (OR 3.61(2.16–6.02), p < 0.001), admission WBC count(OR 1.04 (1.02–1.07), p 0.001), hb (OR 1.2 (1.08–1.35), p 0.001), urea (OR 1.04 (1.02–1.07), p < 0.001), creatinine (OR 1.003 (1.001–1.004), p < 0.001), potassium (OR 1.4 (1.09–1.8), p 0.009), venous pH at admission (OR 0.05 (0.01–0.23), p < 0.001), bicarbonate at admission (OR 0.94 (0.89–0.98), p 0.01), anion gap at admission (OR 1.03 (1.0006–1.064), p 0.045), total cholesterol (OR 1.15 (1.06–1.25), p 0.001), LDL (OR 1.19 (1.008–1.40), p 0.03) and TG (OR 1.04 (1.005–1.09), p 0.02) to be the statistically significant factors (p < 0.05) associated with concomitant AP in DKA patients. Multivariate logistic regression analysis (Table 5) revealed only age and total cholesterol level to be associated with the co-existence of DKA and AP.
Discussion
This retrospective study compared the differences in demographics, biochemical and clinical outcomes of patients with concomitant DKA and AP to those with DKA alone. Patients with DKA and AP were older, predominantly Asians and had T2D. These patients had higher anion gap, WBC count, hb, NLR, urea, creatinine, total cholesterol and TG while a lower venous pH at admission than those with DKA alone (p < 0.05). LOS, DKA duration and ICU admission rate was also higher in patients with DKA and AP than in DKA alone. Age, Asian ethnicity, male gender, T2D, admission WBC count, hb, urea, creatinine, potassium, venous pH, bicarbonate, anion gap, total cholesterol, TGL and LDL level were significant factors associated with the development of coexisting DKA and AP in univariate logistic regression analysis. In multivariate logistic regression analysis, age and total cholesterol level were associated with concomitant DKA and AP.
Studies assessing the prevalence of acute pancreatitis in DKA patients are minimal. A prospective study of 100 DKA patients reported an 11% prevalence of AP in DKA patients15. Ma et al. found the prevalence of AP in DKA patients to be 15.53% in a cohort of patients with T2D only12. In our cohort consisting of both T1D and T2D patients, 9% of patients with DKA had evidence of concurrent AP. In particular, a statistically significant difference between T1D and T2D groups was observed as 4.1% (21/482) T1D patients, and 13.5% (63/486) T2D patients with DKA had coexisting AP. This highlights the need for particular attention to the presence of this combination in T2D patients.
Early recognition of coexisting DKA and AP is of particular significance as it carries a higher risk of unfavorable outcomes. Madsen et al. described a case of a 27-year-old patient who had a delay in the diagnosis of coexisting DKA with AP and died within 36 h of initial presentation16. Ma et al. reported worse biochemical markers in patients with DKA and AP than in DKA alone. Patients with DKA and AP had a lower pH, higher anion gap and evidence of haemoconcentration (high hemoglobin and hematocrit)12. Nair et al. also reported a lower pH, higher anion gap and higher blood glucose levels in DKA patients with AP compared to patients without AP15. In addition to the similar results concerning the parameters mentioned above, our study also found a higher WBC count, urea and creatinine in patients with DKA and AP. NLR is used widely as a marker of acute stress and increases rapidly following any pathological condition (within 6 h). Normal NLR is between 1–2, and higher values are associated with pathological states, including inflammation and infections17,18. King et al. reported an NLR cut-off value of 7.45 or above as predictive of an increased risk of the requirement of intensive care support and death19. On the other hand, Liu et al. reported a cut-off of 3.13 or above as predictive of ICU support in patients with COVID-19 infection aged 50 years or above20. To our knowledge, NLR in patients with concomitant DKA with AP has not been studied earlier. Our study found a higher NLR (p-value 0.01) in DKA with AP patients compared to the DKA only group indicating increased severity of illness in these patients. A careful assessment of this simple and readily available marker can alert physicians to the possibility of underlying coexisting AP in DKA patients.
An important finding in this study is the greater percentage of T2D (75%) patients in the DKA and AP groups. Noel et al. reported 2.83 fold increased risk of AP in patients with T2D than those without T2D21. A meta-analysis conducted by Alexandra et al. also concluded an increased risk of local and systemic complications of AP in patients with DM22. This is most likely because patients with T2D have multiple risk factors, including obesity and deranged lipid profile (especially TG levels), that increase the risk of AP. Further studies are needed to understand the relationship between T2D and the risk of AP development.
An interesting aspect of the study was finding a higher percentage of concomitant DKA and AP in patients of Asian ethnicity. Patients of Asian ethnicity comprised 33% of the study population, but 65.5% of patients in DKA with AP group were Asians. The univariate analysis also revealed a statistically significant association between Asian ethnicity and the development of AP with DKA with an odds ratio of 6.52, revealing a higher risk of developing this coexisting condition in this cohort of patients. This could be related to a higher prevalence of metabolic risk factors in the Asian population. Ooi et al. reported lower bicarbonate and higher lactate levels in Asian T2D patients with DKA compared to the White ethnicity23. Patients belonging to the Asian race develop metabolic complications at a lower BMI than other racial groups due to a higher percentage of fat at a lower BMI24,25. Obesity has also been identified as a risk factor for increased severity and local and systemic complications in AP26. An interplay of increased metabolic risk due to high body fat and DM might explain the increased risk of AP in Asians. However, the exact mechanism for developing an increased risk of AP in patients of Asian ethnic group needs to be further assessed in more extensive studies.
Clinical relevance
Recognition of patients with DKA with AP is clinically relevant due to its impact on patient outcomes and the utilization of healthcare resources. Each DKA admission can cost between $10,000 to $284,00027. A longer LOS further considerably increases healthcare costs28. Two previous studies comparing DKA patients with and without AP revealed a higher ICU admission rate and a longer LOS in patients with concomitant DKA and AP12,15. Our study also confirmed these findings with a longer duration of DKA, a longer LOS and a higher risk of ICU admission in DKA with AP cohort compared to DKA only patients. Knowledge regarding worse clinical outcomes in these patients can help physicians identify and effectively manage these high-risk patients.
Strengths and limitations
Our study has several strengths. To our knowledge, of the studies comparing DKA patients with coexisting acute pancreatitis to DKA alone, our study has the largest number of patients with concomitant DKA and acute pancreatitis. Furthermore, the inclusion of patients from multiple ethnic backgrounds adds further to the study's strengths. In addition, our study included both T1D and T2D patients with DKA as opposed to a previous study in which patients with T1D were excluded12. We strictly followed ADA criteria for DKA diagnosis and Atlanta criteria for AP diagnosis during manual data collection to include patients in the study, thus excluding patients who were wrongly coded as either DKA or AP in the EMR. This robust method of data gathering adds to the authenticity of the study.
Our study has some limitations as well. An important limitation is its retrospective design, due to which adjustment for confounders cannot be performed. Another limitation is the lack of comparison of symptoms between DKA patients with and without pancreatitis. However, DKA and AP have a significant overlap in symptoms, so a comparison of symptoms between DKA versus DKA and AP groups is less meaningful. Severity scores for AP were not calculated and compared as the study involved comparing DKA patients with and without AP. Whether there is any difference in treatment modalities between these two groups of DKA patients was not studied. Larger prospective studies are required to understand the differences between DKA patients with and without AP to provide a higher level of evidence.
Conclusion
This study showed that patients with concomitant DKA and AP have more severe derangement in the laboratory markers of DKA severity, inflammation, kidney injury and metabolic profile, highlighting much higher severity of illness than patients with DKA alone. Furthermore, this combination also significantly impacts healthcare resources utilization as DKA patients with AP have a longer DKA duration, LOS and requirement for ICU support than DKA patients without AP. More awareness among physicians regarding this combination of life-threatening conditions can lead to early recognition and improved clinical outcomes in patients with DKA.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Fazeli Farsani, S. et al. Incidence and prevalence of diabetic ketoacidosis (DKA) among adults with type 1 diabetes mellitus (T1D): a systematic literature review. BMJ Open 7(7), e016587 (2017).
Ooi, E. et al. Clinical and biochemical profile of 786 sequential episodes of diabetic ketoacidosis in adults with type 1 and type 2 diabetes mellitus. BMJ Open Diabetes Res Care. 9(2), e002451 (2021).
Davis, T. M. E. & Davis, W. Incidence and associates of diabetic ketoacidosis in a community-based cohort: the Fremantle Diabetes Study Phase II. BMJ Open Diabetes Res. Care 8(1), e000983 (2020).
Gibb, F. W., Teoh, W. L., Graham, J. & Lockman, K. A. Risk of death following admission to a UK hospital with diabetic ketoacidosis. Diabetologia 59(10), 2082–2087 (2016).
Xiao, A. Y. et al. Global incidence and mortality of pancreatic diseases: A systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol. Hepatol. 1(1), 45–55 (2016).
Umpierrez, G. & Freire, A. X. Abdominal pain in patients with hyperglycemic crises. J. Crit. Care. 17(1), 63–67 (2002).
Kumar, P., Sakwariya, A., Sultania, A. R. & Dabas, R. Hypertriglyceridemia-induced acute pancreatitis with diabetic ketoacidosis: A rare presentation of type 1 diabetes mellitus. J. Lab. Phys. 9(4), 329–331 (2017).
Kong, M. T., Nunes, M. P. & Leong, K. F. Diabetic ketoacidosis with acute severe hypertriglyceridaemia-induced pancreatitis as first presentation of type 2 diabetes. BMJ Case Rep. 14(4), e239727 (2021).
Yuan, S. et al. Acute pancreatitis concomitant with diabetic ketoacidosis: A cohort from South China. J. Int. Med. Res. 48(3), 300060520912128 (2020).
Wang, Y. et al. Concurrent diabetic ketoacidosis in hypertriglyceridemia-induced pancreatitis: How does it affect the clinical course and severity scores?. Pancreas 46(10), 1336–1340 (2017).
Fu, Y. et al. Clinical characteristics of concomitant diabetic ketoacidosis in type 2 diabetes patients with acute pancreatitis. Diabetes Metab. Syndr. Obes. 15, 111–119 (2022).
Ma, L. P. et al. Diabetic ketoacidosis with acute pancreatitis in patients with type 2 diabetes in the emergency department: A retrospective study. Front. Med. (Lausanne). 9, 813083 (2022).
Kitabchi, A. E., Umpierrez, G. E., Miles, J. M. & Fisher, J. N. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 32(7), 1335–1343 (2009).
Oppenlander, K. M., Chadwick, C., Do, M. R. & Carman, K. D. Acute pancreatitis: Rapid evidence review. Am. Fam. Phys. 106(1), 44–50 (2022).
Nair, S., Yadav, D. & Pitchumoni, C. S. Association of diabetic ketoacidosis and acute pancreatitis: Observations in 100 consecutive episodes of Dka. ACG. 95(10), 2795–2800 (2000).
Madsen, K. R. Fatal hypertriglyceridemia, acute pancreatitis and diabetic ketoacidosis possibly induced by quetiapine. BMJ Case Rep. 2014, bcr2013202039 (2014).
Zahorec, R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl Lek Listy. 122(7), 474–488 (2021).
Abaza, N. M., El-Latif, E. M. A. & Gheita, T. A. Clinical significance of neutrophil/lymphocyte ratio in patients with granulomatosis with polyangiitis. Reumatol. Clín. (Engl. Ed.). 15(6), 363–367 (2019).
King, A. H. et al. A high neutrophil-lymphocyte ratio is associated with increased morbidity and mortality in patients with coronavirus disease 2019. Crit. Care Explor. 3(5), e0444 (2021).
Liu, J. et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J. Transl. Med. 18(1), 206 (2020).
Noel, R. A., Braun, D. K., Patterson, R. E. & Bloomgren, G. L. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: A retrospective cohort study. Diabetes Care 32(5), 834–838 (2009).
Mikó, A. et al. Preexisting diabetes elevates risk of local and systemic complications in acute pancreatitis: Systematic review and meta-analysis. Pancreas 47(8), 917–923 (2018).
Ooi, E. et al. Clinical and biochemical profile of 786 sequential episodes of diabetic ketoacidosis in adults with type 1 and type 2 diabetes mellitus. BMJ Open Diabetes Res. Care 9(2), e002451 (2021).
Yi, S. S., Kwon, S. C., Wyatt, L., Islam, N. & Trinh-Shevrin, C. Weighing in on the hidden Asian American obesity epidemic. Prev. Med. 73, 6–9 (2015).
Mui, P., Hill, S. E. & Thorpe, R. J. Jr. Overweight and obesity differences across ethnically diverse subgroups of Asian American men. Am. J. Mens Health. 12(6), 1958–1965 (2018).
Martínez, J. et al. Is obesity a risk factor in acute pancreatitis? A meta-analysis. Pancreatology 4(1), 42–48 (2004).
Lyerla, R. et al. Recurrent DKA results in high societal costs—A retrospective study identifying social predictors of recurrence for potential future intervention. Clin. Diabetes Endocrinol. 7(1), 13 (2021).
Cyganska, M. The impact factors on the hospital high length of stay outliers. Proc. Econ. Finance. 39, 251–255 (2016).
Acknowledgements
Open Access funding was provided by the Qatar National Library. The authors would like to appreciate the efforts of Mr. Kris Choda from the Business Intelligence Unit (BIU) at Hamad Medical Corporation for his assistance in retrieving data from electronic medical records.
Funding
Open Access funding provided by the Qatar National Library. No funding was acquired to conduct this study.
Author information
Authors and Affiliations
Contributions
A.A.K.: Principal investigator, conceptualization, methodology, literature review, statistical analysis, manuscript writing. F.A.: Literature review, manuscript writing, data interpretation. Z.Y.: Statistical analysis, data interpretation, prepared tables. M.S.A., M.N.S., A.M., B.D., M.H., B.M. and A.M.M.: Data collection, prepared tables, literature review. A.K.: Supervision, manuscript writing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khan, A.A., Ata, F., Yousaf, Z. et al. A retrospective study on comparison of clinical characteristics and outcomes of diabetic ketoacidosis patients with and without acute pancreatitis. Sci Rep 13, 4347 (2023). https://doi.org/10.1038/s41598-023-31465-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-31465-3
- Springer Nature Limited