Abstract
We assessed the prognostic value of five complex inflammatory and nutritional parameters, namely neutrophil-to-lymphocyte ratio (NLR), prognostic nutritional index (PNI), C-reactive protein-to-NLR ratio (C/NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR) using data from patients with malignant pleural mesothelioma (MPM) undergoing extrapleural pneumonectomy (EPP). Moreover, the correlation between these five parameters and programmed cell death protein 1 ligand-1 (PD-L1) expression in the tumor microenvironment was evaluated. This study included consecutive MPM patients who underwent EPP. The histological subtype of the eligible patients (n = 61) correlated with all five parameters. Moreover, the PD-L1 expression scores for immune cells correlated with NLR and PLR, and the PD-L1 expression scores for both tumor cells and immune cells were inversely correlated with both PNI and LMR. Univariate analysis elucidated that NLR, PNI, and C/NLR were predictors of 5-year overall survival (OS), and multivariate analysis revealed that NLR was an independent predictor of 5-year OS, suggesting that NLR is a preoperative, prognostic factor for patients with MPM who are scheduled for EPP. To the best of our knowledge, this is the first study to evaluate the prognostic potentials of NLR, PNI, C/NLR, PLR, and LMR simultaneously in patients with MPM who underwent EPP.
Similar content being viewed by others
Introduction
Malignant pleural mesothelioma (MPM) is one of the most aggressive solid tumors, although multimodal therapies1, as well as immune checkpoint inhibitors targeting the programmed cell death protein-1 (PD1)/programmed cell death protein-1 ligand 1 (PD-L1) axis2,3 have improved the clinical outcome of patients with MPM. Recently, several preoperative inflammatory and nutritional parameters such as neutrophil-to-lymphocyte ratio (NLR), prognostic nutritional index (PNI), and C-reactive protein (CRP)-to-albumin ratio have been reported as prognostic factors for surgical treatment of several types of malignant tumor4,5,6. Inflammatory parameters are useful for the prognosis of MPM, as asbestos-induced chronic inflammation is associated with the development of MPM7. We previously showed that the CRP-to-albumin ratio and CRP multiplied by the neutrophil ratio predicted the prognosis of patients with MPM undergoing extrapleural pneumonectomy (EPP)8, while most studies that have investigated NLR and PNI in MPM included inoperable cases7,9,10,11. Thus, the relationship between inflammatory and nutritional parameters and surgical outcomes in patients with MPM remains unknown.
PD-L1 is a well-known immune checkpoint molecule that suppresses T cell-induced anti-tumor immunity12. Previous studies have reported that in patients with MPM, PD-L1 overexpression in the tumor microenvironment (TME) is a poor prognostic factor13,14,15, but other studies, including our previous study, revealed that PD-L1 status has no influence on the prognosis of patients with MPM16,17,18. Although the prognostic impact of PD-L1 status in patients with MPM is controversial, the expression status of PD-L1 can be used as a preoperative prognostic marker because it can be determined via pleural biopsy. Furthermore, the relationship between PD-L1 expression and inflammatory parameters is interesting, since inflammatory cytokines directly induce PD-L1 expression in tumor cells19,20. Moreover, the relationship between PD-L1 and nutritional parameters is another important criteria because changes in nutritional status impact immune cell metabolism and function21; undernutrition is associated with immunosuppression, which manifests via a decrease in the immune cell number, particularly that of T cells22.
In this study, preoperative laboratory biomarkers for systemic inflammation and nutrition, namely NLR, PNI, CRP to NLR ratio (C/NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR), were evaluated, and we determined the relationship between each inflammatory or nutritional parameter and PD-L1 expression in the TME. Moreover, the prognostic impact of the five inflammatory/nutritional parameters was evaluated in patients with MPM who underwent EPP.
Materials and methods
Patients and specimens
This retrospective, single-center, observational study was approved by the Ethics Committee of the National Hospital Organization Yamaguchi Ube Medical Center (No. 30-5) with following consent style; written informed consent for the use of medical records and tissue samples was obtained from all living patients or patients treated at our hospital after October 2012, while written informed consent was waived; an opt-out method on the website of the National Hospital Organization Yamaguchi Ube Medical Center was applied to obtain patient consent if the patients who treated before October 2012 were dead before April 30, 2021. The study adheres to the principles of the Declaration of Helsinki. Consecutive patients who underwent EPP for MPM and achieved macroscopic complete resection (MCR) between June 2006 and March 2020 were enrolled in this study. Our routine treatment strategy for operable MPM, the method for patient follow-up, and data regarding this patient population have been described in our previous study8. Pleurodesis was not routinely performed after pleural biopsy. The interval between biopsy and blood test results before EPP is usually 3–4 weeks because it takes 3 weeks for a pathological diagnosis by immunohistochemistry (IHC) of pleural biopsy specimens. If patients had undergone pleurodesis at another hospital, EPP was performed after inflammation returned to normal or the level before pleurodesis, to minimize the disadvantages of pleurodesis-induced inflammation in the perioperative management of EPP. Patients with empyema were excluded from surgical indication, because EPP is a highly invasive procedure that requires the reconstruction of the diaphragm and pericardium with polytetrafluoroethylene patches. Five-year overall survival (OS) was estimated from the date of EPP to the date of death from any cause or the last follow-up, which was set to a maximum of 5 years, and the last follow-up date was April 30, 2021. Only OS (with a limited observation period of ≤ 5 years) was evaluated because the majority of patients were followed up at other hospitals, making it difficult to collect accurate information on cause of death and long-term prognosis. Histological diagnosis was according to the WHO 2015 criteria23. TNM classification and pathological stage (pStage) were assessed as per the criteria defined by the International Association for the Study of Lung Cancer24,25,26.
Clinical variables
The following clinicopathological data of the patients were obtained for the analysis: age, sex, laterality, histological subtype, and pathological stage. Moreover, laboratory blood test results, including white blood cell count (WBC), platelet count, neutrophil count, lymphocyte count, monocyte count, serum CRP level, and serum albumin level, were obtained; these tests were performed within 7 days before EPP. Preoperative inflammatory and nutritional parameters were calculated as follows: NLR, neutrophil count divided by lymphocyte count; PNI, 10 × serum albumin + 0.005 × lymphocyte count27; C/NLR, serum CRP level divided by NLR; PLR, platelet count divided by lymphocyte count; and LMR, lymphocyte count divided by monocyte count.
IHC
Formalin-fixed paraffin-embedded blocks from 61 MPM patients were obtained from the archives of the National Hospital Organization Yamaguchi Ube Medical Center. IHC was performed on surgically resected tissues using a mouse monoclonal anti-PD-L1 antibody (1:100, clone SP142, Spring Bioscience, Pleasanton, CA, USA), according to a previously described protocol18. The scoring criteria for PD-L1 expression by tumor cells (PD-L1 TC) and immune cells (PD-L1 IC) were used to assess the percentage of total tumor and immune cells, respectively, as per a previously described method18,28. Immunoreactivity for PD-L1 was scored by two investigators (R.O. and N.K. or N.Y.), who had no prior knowledge of the corresponding clinicopathological data. To evaluate the status of PD-L1 TC and PD-L1 IC, average scores were calculated from the two investigators.
Statistical analysis
The cutoff values of each parameter were determined according to the receiver operating characteristic (ROC) curves predicting 5-year OS, using the SPSS statistical package (version 17.0; SPSS, Chicago, IL). Chi-square test was performed to evaluate the relationships between patient characteristics and each parameter, and Spearman’s rank correlation test was performed for correlation analysis using GraphPad Prism (version 6.01; GraphPad Software, La Jolla, CA). The 5-year OS rate until death or last follow-up after EPP was evaluated by Kaplan–Meier survival analysis, and the significance of the differences in 5-year OS between groups was assessed by the log-rank Mantel–Cox test using GraphPad Prism. Univariate and multivariate analyses were performed using the Cox proportional hazards model to identify independent prognostic factors, using SPSS statistical package 17.0. The statistical significance was set at p < 0.05.
Ethics approval
This research was approved by the Yamaguchi Ube Medical Center ethics committee (No. 30-5); the study followed the principles of the Declaration of Helsinki and was in compliance with the relevant guidelines and regulations.
Consent to participate
Written informed consent for the use of tissue samples and medical records was waived, and an opt-out method on the website was applied to obtain patient consent; however, written informed consent was obtained from all living patients or patients treated in our hospital after October 2012.
Results
Patient cohort
We previously assessed PD-L1 TC and IC based on representative IHC results in 58 EPP cases of MPM to evaluate their prognostic impact18. In the present study, the number of patients was higher and the relationship between inflammatory and nutritional parameters and the expression status of PD-L1 in the TME of MPM was determined. This study included 61 consecutive patients with MPM who underwent EPP and achieved MCR. The patient characteristics are described previously, and the median length of follow-up for censored cases was 39.7 months (range, 20.2–59.3 months)8.
Preoperative inflammatory and nutritional parameters of patients with resected MPM
The WBC, platelet count, serum CRP level, serum albumin level, NLR, PNI, C/NLR, PLR, and LMR of the patients were 3180–10,700 cells/μL (mean: 6233), 11.2–53.2 × 104 cells/μL (mean 28.1), 0.01–10.48 mg/dL (mean: 1.24), 2.4–4.8 g/dL (mean: 3.7), 1.1–5.7 (mean: 2.5), 29.9–64.5 (mean: 45.1), 0.01–32.58 (mean: 4.20), 62.2–436.3 (mean: 184.9), and 1.2–10.3 (mean: 4.5), respectively. PD-L1 TC score was 0–3 (mean: 1.30) and PD-L1 IC score was 0–3 (mean: 1.45). Optimal cutoff values for each parameter determined by ROC analyses were as follows: 5965 cells/μL for WBC, 28.35 × 104 cells/μL for platelet count, 1.75 for PD-L1 TC score, 1.75 for PD-L1 IC score, 2.850 for NLR, 45.150 for PNI, 0.955 for C/NLR, 167.1 for PLR, and 5.15 for LMR (Supplementary Fig. 1, 2). Optimal cutoff values for CRP and albumin levels were 0.515 mg/dL and 3.75 g/dL, respectively, as proposed previously8. Next, the patients were divided into high and low groups based on the optimal cut-off value of each parameter for further analysis. The inflammatory and nutritional parameters of the patients are presented in Table 1.
Relationship between clinicopathological characteristics and the inflammatory and nutritional parameters
The clinicopathological characteristics of the patients were compared according to the status of each inflammatory and nutritional parameter. Among the combination parameters, NLR was associated with histology, WBC, CRP, albumin, PNI, C/NLR, PLR, and LMR; PNI was associated with histology, CRP, albumin, PD-L1 IC, NLR, C/NLR, PLR, and LMR; C/NLR was associated with histology, WBC, CRP, albumin, PD-L1 IC, NLR, PNI, PLR, and LMR; PLR was associated with histology, platelet count, CRP, albumin, PD-L1 TC, NLR, PNI, C/NLR, and LMR; and LMR was associated with histology, WBC, CRP, NLR, PNI, C/NLR, and PLR (Table 2). The correlations between each parameter were evaluated to validate these results. Linear regression with Spearman’s rank correlation analysis revealed that the PD-L1 TC score was inversely correlated with PNI (Spearman’s rank correlation coefficient rs = 0.067, p = 0.044) and LMR (rs = 0.048, p = 0.009), although no correlation with other parameters was observed (Fig. 1). The PD-L1 IC score was positively correlated with platelet count (rs = 0.068, p = 0.043), NLR (rs = 0.110, p = 0.009), and PLR (rs = 0.119, p = 0.006), and inversely correlated with albumin level (rs = 0.081, p = 0.026), PNI (rs = 0.122, p = 0.006), and LMR (rs = 0.090, p = 0.019) (Fig. 2). PNI was inversely correlated with platelet count (rs = 0.128, p = 0.005), CRP (rs = 0.211, p = 0.0002), NLR (rs = 0.428, p < 0.0001), C/NLR (rs = 0.235, p < 0.0001), and PLR (rs = 0.396, p < 0.0001) and positively correlated with LMR (rs = 0.252, p < 0.0001) (Fig. 3).
PD-L1 TC inversely correlates with PNI and LMR. Correlations between PD-L1 TC and (a) WBC, (b) platelet count, (c) CRP level, (d) Alb level, (e) NLR, (f) PNI, (g) C/NLR, (h) PLR, or (i) LMR. Data were analyzed using the Spearman’s rank correlation test. PD-L1 TC, programmed cell death 1 ligand-1 by tumor cells; WBC, white blood cell count; Plt, platelet count; CRP, C-reactive protein; Alb, albumin; NLR, neutrophil-to-lymphocyte ratio; PNI, prognostic nutritional index; C/NLR, C-reactive protein-to-NLR ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; Rs, Spearman’s rank correlation coefficient. *p < 0.05.
PD-L1 IC positively correlates with platelet count, NLR, and PLR, whereas inversely correlates with albumin, PNI and LMR. Correlation between PD-L1 IC and (a) WBC, (b) platelet count, (c) CRP level, (d) Alb level, (e) NLR, (f) PNI, (g) C/NLR, (h) PLR, and (i) LMR. Data were analyzed using the Spearman’s rank correlation test. PD-L1 IC: programmed cell death 1 ligand-1 by immune cells; WBC: white blood cell count; Plt: platelet count; CRP: C-reactive protein; Alb: albumin; NLR: neutrophil-to-lymphocyte ratio; PNI: prognostic nutritional index; C/NLR: C-reactive protein-to-NLR ratio; PLR: platelet-to-lymphocyte ratio; LMR: lymphocyte-to-monocyte ratio; Rs: Spearman’s rank correlation coefficient. *p < 0.05.
PNI positively correlates with LMR and inversely correlates with platelet count, CRP, NLR, C/NLR and PLR. Correlation between PNI and (a) WBC, (b) platelet count, (c) CRP level, (d) NLR, (e) C/NLR, (f) PLR, or (g) LMR. Data were analyzed using the Spearman’s rank correlation test. PNI, prognostic nutritional index; WBC, white blood cell count; Plt, platelet count; CRP, C-reactive protein; NLR, neutrophil-to-lymphocyte ratio; C/NLR, C-reactive protein-to-NLR ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; Rs, Spearman’s rank correlation coefficient. *p < 0.05.
Five-year OS stratified by the inflammatory and nutritional parameters
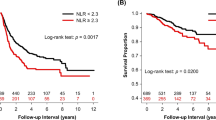
Kaplan–Meier survival analysis with log-rank test for 5-year OS demonstrated that the patients with high NLR (> 2.850) [hazard ratio (HR): 2.628, confidence interval (CI): 1.762–7.544, p = 0.0007] and high C/NLR (> 0.955) (HR: 2.122, CI: 1.266–4.368, p = 0.008) were significantly associated with poor prognosis, whereas those with high PNI (> 45.150) (HR: 0.524, CI: 0.277–0.908, p = 0.025) were significantly associated with good prognosis, and neither PLR status nor LMR status had an impact on the 5-year OS (Fig. 4a–e). For further analysis, the cases were divided into four groups according to high and low NLR (cutoff: 2.850), and high and low PNI (cutoff: 45.150), although no case of high NLR/high PNI was seen. We determined that the prognosis was similar between cases with low NLR with low PNI and low NLR with high PNI, whereas patients with high NLR with low PNI, which is the same as high NLR with any PNI, exhibited shorter survival compared to that of others (p = 0.003) (Fig. 4f).
Association of a combination of inflammatory and nutritional parameters with OS in MPM patients who underwent EPP. Kaplan–Meier plots showing 5-year OS in patients with lower or higher scores for (a) NLR, (b) PNI, (c) C/NLR, (d) PLR, (e) LMR, and (f) combination of NLR and PNI. NLR, neutrophil-to-lymphocyte ratio; PNI, prognostic nutritional index; C/NLR, C-reactive protein-to-NLR ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; HR, hazard ratio; CI, confidence interval; MST, median survival time (months). *p < 0.05.
Univariate and multivariate analysis of the inflammatory and nutritional parameters for 5-year OS
Cox regression analysis was performed to determine the predictive value of the clinical variables for 5-year OS. For the analysis, NLR, PNI, C/NLR, PLR, and LMR were assessed independently with three other parameters, including sex, histological subtype, and laterality, as these parameters could be evaluated preoperatively. Both sex29 and histology29,30 are prognostic factors, and laterality is an important predictive factor for major morbidity31. Univariate analysis showed that sex (HR:3.231, p = 0.015), histological subtype (HR: 0.400, p = 0.004), NLR (HR: 2.790, p = 0.001), PNI (HR: 0.515, p = 0.027), and C/NLR (HR: 2.183, p = 0.009) were significantly associated with 5-year OS. Multivariate analyses revealed that NLR was an independent predictor for 5-year OS in the NLR-including cohort (HR 2.062, CI: 1.054–4.031, p = 0.034), whereas PNI, C/NLR, PLR and LMR exhibited no significant impact on 5-year OS in each cohort, although histology was an independent prognostic factor in these four cohorts and sex was an independent prognostic factor in the cohorts with PLR or LMR (Table 3).
Discussion
In this study, we elucidated that NLR is an independent prognostic factor for the outcome of EPP in patients with MPM. Moreover, we found that PD-L1 TC score was inversely correlated with PNI and LMR; PD-L1 IC score was positively correlated with platelet count, NLR, and PLR and inversely correlated with albumin level, PNI, and LMR.
Elevated neutrophil and platelet counts are associated with systemic inflammation. Neutrophils promote transformation, progression, and metastasis of cancer cells while suppressing antitumor immunoreaction by proinflammatory cytokines32. Platelet-tumor interaction is important because thrombocytosis is associated with shorter survival in patients with unresectable MPM33. During thrombopoiesis, the mobilization of hematopoietic stem cells is controlled by endothelial-active cytokines, such as vascular endothelial growth factor34, which are upregulated during pleural effusion in patients with MPM35; they are secreted directly by tumor cells and indirectly by tumor-infiltrating immune cells due to tumor-related inflammation36.
The relationship between the five parameters investigated in this study and PD-L1 expression is relevant because both inflammation and nutrition affect immunological functions. Regarding LMR, monocytes release several immunosuppressive cytokines and create oxidative stress, which promotes tumor progression37. One important immunosuppressive phenotype of monocytes is myeloid-derived suppressor cells (MDSC), and their count increases in the TME of patients with MPM38. In an experimental mouse model, the tumor inflammatory microenvironment supported MDSC recruitment, and tumor-derived MDSC inactivated T cell proliferation39. Moreover, in a tumor-bearing mouse model, the inhibition of PD-L1 decreased MDSC count and increased effector-memory T cell count40. In the present study, LMR had no impact on clinical outcomes, although tumors expressing PD-L1 might have an increased number of circulating monocytes, including MDSC, whereas a decreased number of circulating effector-memory lymphocytes, which play a pivotal role in eliminating tumor cells, as LMR is inversely correlated with PD-L1 TC and IC. One reason behind why PD-L1 TC score was inversely correlated with PNI could be that chronic inflammation leads to both PD-L1 expression and malnutrition by transforming growth factor β, which is an immunosuppressive cytokine and abundant in the TME of MPM41 and implicates the promotion of cancer cachexia42. In the present study, the PD-L1 IC score was inversely correlated with PNI, but the PD-L1 IC score exhibited no relationship with non-epithelioid histology, which could be due to malnutrition-induced immune dysfunction43, and the cause of malnutrition could be chronic inflammation. In contrast, a positive correlation between PD-L1 IC and NLR or PLR might result in either PD-L1-induced inactivation of circulating lymphocytes or chronic inflammation-induced increase in the number of both neutrophils and platelets. Moreover, PNI was inversely correlated with platelet count, CRP, NLR, C/NLR and PLR, whereas it was positively correlated with LMR, suggesting that tumor-induced chronic inflammation and malnutrition coexist in patients with MPM.
Survival impact of these five parameters was determined using the log-rank test and univariate analysis, which demonstrated that NLR, PNI, and C/NLR were significant prognostic factors for patients with MPM who underwent EPP, and multivariate analysis indicated that NLR was an independent prognostic factor for 5-year OS in patients with MPM who underwent EPP. Our findings suggest that NLR test, one of the least invasive and cheapest blood tests, could predict the clinical outcome of EPP; moreover, PD-L1 status in TME does independently exhibit any impact on the outcome despite the cost, time, and effort required for its evaluation. A previous study on patients with MPM reported a high NLR (NLR > 3.0) at baseline as a poor prognostic factor in MPM treated with or without chemotherapy9. Another study reported that NLR > 3.0 was an independent prognostic marker associated with shorter survival for 85 patients who underwent EPP, which is in accordance with our results10. In contrast, Tagawa et al. reported that PLR < 215 was associated with improved survival in patients with MPM who underwent EPP, whereas NLR was not identified as a prognostic marker in their study44. Our results did not demonstrate a significant impact of PLR on the prognosis of MPM patients who underwent EPP, although a low PLR (< 167.1) predicted good prognosis. Yamagishi et al. reported that among NLR, PLR, and LMR, only LMR was an independent predictor of OS in patients with MPM, although patients with both operative and inoperative MPM were included in the study45. Therefore, it is unclear whether NLR is a definitive prognostic marker for OS, and further studies are required to establish whether inflammatory and nutritional markers such as NLR and PLR are reliable predictive markers for EPP. The combination of NLR and PNI may also be a promising parameter because in a previous study, low NLR with high PNI was reported to be a good prognostic factor for resectable hepatocellular carcinoma46, but our results demonstrate only low NLR as a good prognosis factor.
A limitation of this study is the small sample size; however, it is difficult to obtain data for a large number of patients with MPM who underwent EPP because of the rarity of this condition. The strength of this study is that to the best of our knowledge, this is the first study to evaluate five inflammatory/nutritional parameters simultaneously and to assess their relationship with PD-L1 expression in the TME of patients with MPM who underwent EPP.
Thus, we demonstrated that NLR is an independent prognostic factor for patients with MPM who underwent EPP. Preoperative assessment of NLR could predict whether the prognosis of patients with MPM can be improved by EPP, which is an invasive surgery.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MPM:
-
Malignant pleural mesothelioma
- PD1:
-
Programmed cell death protein 1
- PD-L1:
-
Programmed cell death protein 1 ligand-1
- NLR:
-
Neutrophil to lymphocyte ratio
- PNI:
-
Prognostic nutritional index
- CRP:
-
C-reactive protein
- EPP:
-
Extrapleural pneumonectomy
- TME:
-
Tumor microenvironment
- C/NLR:
-
C-reactive protein/NLR ratio
- PLR:
-
Platelet/lymphocyte ratio
- LMR:
-
Lymphocyte/monocyte ratio
- MCR:
-
Macroscopic complete resection
- IHC:
-
Immunohistochemistry
- OS:
-
Overall survival
- pStage:
-
Pathological stage
- WBC:
-
White blood cell count
- PD-L1 TC:
-
PD-L1 by tumor cells
- PD-L1 IC:
-
PD-L1 by immune cells
- ROC:
-
Receiver operating characteristics
- Rs :
-
Spearman’s rank correlation coefficient.
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- MDSC:
-
Myeloid-derived suppressor cells
- AUC:
-
Area under the curve
- MST:
-
Median survival time
References
de Perrot, M. et al. Trimodality therapy with induction chemotherapy followed by extrapleural pneumonectomy and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J. Clin. Oncol. 27, 1413–1418 (2009).
Okada, M. et al. Clinical efficacy and safety of nivolumab: Results of a multicenter, open-label, single-arm, Japanese phase II study in malignant pleural mesothelioma (MERIT). Clin. Cancer Res. 25, 5485–5492 (2019).
Baas, P. et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 397, 375–386 (2021).
Shimizu, K. et al. Preoperative neutrophil/lymphocyte ratio and prognostic nutritional index predict survival in patients with non-small cell lung cancer. World J. Surg. Oncol. 13, 291 (2015).
Chan, A. W. et al. Prognostic nutritional index (PNI) predicts tumor recurrence of very early/early stage hepatocellular carcinoma after surgical resection. Ann. Surg. Oncol. 22, 4138–4148 (2015).
Ishizuka, M. et al. Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ann. Surg. Oncol. 23, 900–907 (2016).
Pinato, D. J. et al. Inflammation-based prognostic indices in malignant pleural mesothelioma. J. Thorac. Oncol. 7, 587–594 (2012).
Okita, R., Okada, M., Inokawa, H., Murakami, T. & Ikeda, E. Prognostic values of preoperative C-reactive protein, albumin, and neutrophil ratios in patients with malignant pleural mesothelioma who underwent extrapleural pneumonectomy. Surg. Oncol. 43, 101813 (2022).
Kao, S. C. et al. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin. Cancer Res. 16, 5805–5813 (2010).
Kao, S. C. et al. Low calretinin expression and high neutrophil-to-lymphocyte ratio are poor prognostic factors in patients with malignant mesothelioma undergoing extrapleural pneumonectomy. J. Thorac. Onco. 6, 1923–1939 (2011).
Yao, Z. H. et al. Prognostic nutritional index predicts outcomes of malignant pleural mesothelioma. J. Cancer Res. Clin. Oncol. 139, 2117–2123 (2013).
Freeman, G. J. et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192, 1027–1034 (2012).
Cedres, S. et al. Analysis of expression of programmed cell death 1 ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM). PLoS One 10, e0121071 (2015).
Kao, S. C. et al. Tumor suppressor microRNAs contribute to the regulation of PD-L1 expression in malignant pleural mesothelioma. J. Thorac. Oncol. 12, 1421–1433 (2017).
Thapa, B. et al. The immune microenvironment, genome-wide copy number aberrations, and survival in mesothelioma. J. Thorac. Oncol. 12, 850–859 (2017).
Watanabe, T. et al. Four immunohistochemical assays to measure the PD-L1 expression in malignant pleural mesothelioma. Oncotarget 9, 20769–20780 (2018).
de Perrot, M. et al. Prognostic influence of tumor microenvironment after hypofractionated radiation and surgery for mesothelioma. J. Thorac. Cardiovasc. Surg. 159, 2082-2091.e1 (2020).
Okita, R. et al. Effects of tumor-infiltrating CD8+ T cells, PD1/PD-L1 axis, and expression patterns of HLA class I on the prognosis of patients with malignant pleural mesothelioma who underwent extrapleural pneumonectomy. Cancer Immunol. Immunother. https://doi.org/10.1007/s00262-022-03292-4 (2022).
Dong, H. et al. Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8, 793–800 (2002).
Okita, R., Shimizu, K., Nojima, Y., Saisho, S. & Nakata, M. Tofacitinib overcomes an IFNγ-induced decrease in NK cell-mediated cytotoxicity via the regulation of immune-related molecules in LC-2/ad. Thorac. Cancer 12, 775–782 (2021).
Alwarawrah, Y., Kiernan, K. & MacIver, N. J. Changes in nutritional status impact immune cell metabolism and function. Front. Immunol. 9, 1055 (2018).
Najera, O., Gonzalez, C., Toledo, G., Lopez, L. & Ortiz, R. Flow cytometry study of lymphocyte subsets in malnourished and well-nourished children with bacterial infections. Clin. Diagn. Lab. Immunol. 11, 577–580 (2004).
Travis, W.D. International Agency for Research on C. WHO classification of tumours of the lung, pleura, thymus and heart (International Agency for Research on cancer, 2015).
Nowak, A.K. et al. The IASLC Mesothelioma Staging Project: Proposals for revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for pleural mesothelioma. J. Thorac. Oncol. 11, 2089–2099 (2016).
Rice, D. et al. The IASLC Mesothelioma Staging Project: Proposals for revisions of the N descriptors in the forthcoming eighth Edition of the TNM Classification for pleural mesothelioma. J. Thorac. Oncol. 11, 2100–2111 (2016).
Rusch, V. W. et al. The IASLC Mesothelioma Staging Project: Proposals for the M Descriptors and for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for mesothelioma. J. Thorac. Oncol. 11, 2112–2119 (2016).
Onodera, T., Goseki, N. & Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 85, 1001–1005 (1984).
Vennapusa, B. et al. Development of a PD-L1 complementary diagnostic immunohistochemistry assay (SP142) for atezolizumab. Appl. Immunohistochem. Mol. Morphol. 27, 92–100 (2019).
Rusch, V. W. & Venkatraman, E. S. Important prognostic factors in patients with malignant pleural mesothelioma, managed surgically. Ann. Thorac. Surg. 68, 1799–1804 (1999).
Okada, M. et al. Radical surgery for malignant pleural mesothelioma: results and prognosis. Interact. Cardiovasc. Thorac. Surg. 7, 102–106 (2008).
Lauk, O. et al. Extrapleural pneumonectomy after induction chemotherapy: perioperative outcome in 251 mesothelioma patients from three high-volume institutions. Ann. Thorac. Surg. 98, 1748–1754 (2014).
Ishikawa, M., Koga, Y., Hosokawa, M. & Kobayashi, H. Augmentation of B16 melanoma lung colony formation in C57BL/6 mice having marked granulocytosis. Int. J. Cancer 37, 919–924 (1986).
Bille, A., Krug, L. M., Woo, K. M., Rusch, V. W. & Zauderer, M. G. Contemporary analysis of prognostic factors in patients with unresectable malignant pleural mesothelioma. J. Thorac. Oncol. 11, 249–255 (2016).
Hattori, K. et al. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J. Exp. Med. 193, 1005–1014 (2001).
Strizzi, L. et al. Vascular endothelial growth factor is an autocrine growth factor in human malignant mesothelioma. J. Pathol. 193, 468–475 (2001).
Freeman, M. R. et al. Peripheral blood T lymphocytes and lymphocytes infiltrating human cancers express vascular endothelial growth factor: a potential role for T cells in angiogenesis. Cancer Res. 55, 4140–4145 (1995).
Mao, Y., Poschke, I. & Kiessling, R. Tumour-induced immune suppression: role of inflammatory mediators released by myelomonocytic cells. J. Intern. Med. 276, 154–170 (2014).
Salaroglio, I. C. et al. Potential diagnostic and prognostic role of microenvironment in malignant pleural mesothelioma. J. Thorac. Oncol. 14, 1458–1471 (2019).
Meyer, C. et al. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc. Natl. Acad. Sci. U S A 108, 17111–17116 (2011).
Bazhin, A.V. et al. Pivotal antitumor role of the immune checkpoint molecule B7-H1 in pancreatic cancer. Oncoimmunology 11, 2043037; https://doi.org/10.1080/2162402X.2022.2043037 (2022).
DeLong, P. et al. Regulatory T cells and cytokines in malignant pleural effusions secondary to mesothelioma and carcinoma. Cancer Biol. Ther. 4, 342–346 (2005).
Zugmaier, G. et al. Transforming growth factor beta 1 induces cachexia and systemic fibrosis without an antitumor effect in nude mice. Cancer Res. 51, 3590–3594 (1991).
Bourke, C. D., Berkley, J. A. & Prendergast, A. J. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. 37, 386–398 (2016).
Tagawa, T. et al. Clinical role of a new prognostic score using platelet-to-lymphocyte ratio in patients with malignant pleural mesothelioma undergoing extrapleural pneumonectomy. J. Thorac Dis. 7, 1898–1906 (2015).
Yamagishi, T. et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with malignant pleural mesothelioma. Lung Cancer 90, 111–117 (2015).
Qu, Z. et al. Preoperative prognostic nutritional index and neutrophil-to-lymphocyte ratio predict survival outcomes of patients with hepatocellular carcinoma after curative resection. Front. Oncol. 11, 823054; https://doi.org/10.3389/fonc.2021.823054 (2021).
Acknowledgements
The authors thank all pathologists, physicians, and medical staff who diagnosed and treated the patients with malignant pleural mesothelioma. The authors also thank Yuka Mimura-Kimura for providing technical assistance. We would like to thank Editage (www.editage.com) for English language editing.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS) Kakenhi Grant (19K09298 and 22K08991) awarded to R. Okita.
Author information
Authors and Affiliations
Contributions
R.O.: Conceptualization, data curation, methodology, validation, formal analysis, investigation, resources, writing the original draft, visualization, supervision, project administration, and funding acquisition. N.K.: Data curation, investigation, and resources. M.O.: Data curation and resources. H.I.: Data curation and resources. N.Y.: Investigation and validation; T.M.: Data curation and resources; E.I.: Data curation and resources. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Okita, R., Kawamoto, N., Okada, M. et al. Preoperative neutrophil-to-lymphocyte ratio correlates with PD-L1 expression in immune cells of patients with malignant pleural mesothelioma and predicts prognosis. Sci Rep 13, 5263 (2023). https://doi.org/10.1038/s41598-023-31448-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-31448-4
- Springer Nature Limited