Abstract
This study aimed to investigate the prognostic impact of lymphovascular and perineural invasions in patients with squamous cell carcinoma of the tongue who received surgery-based treatment at our institution between January 2013 and December 2020. Patients were divided into four groups based on the presence of perineural (P−/P +) and lymphovascular invasions (V−/V +): P–V−, P–V + , P + V−, and P + V + . Log-rank and Cox proportional hazard models were used to evaluate the association between perineural /lymphovascular invasion and overall survival (OS). Altogether, 127 patients were included, and 95 (74.8%), 8 (6.3%), 18 (14.2%), and 6 (4.7%) cases were classified as P–V−, P–V + , P + V−, and P + V + , respectively. Pathologic N stage (pN stage), tumor stage, histological grade, lymphovascular invasion, perineural invasion, and postoperative radiotherapy were significantly associated with OS (p < 0.05). OS was significantly different among the four groups (p < 0.05). Significant between-group differences in OS were detected for node-positive (p < 0.05) and stage III–IV (p < 0.05) cases. OS was the worst in the P + V + group. Lymphovascular and perineural invasions are independent negative prognostic factors for squamous cell carcinoma of the tongue. Patients with lymphovascular and/or perineural invasion may have significantly poorer overall survival than those without neurovascular involvement.
Similar content being viewed by others
Introduction
Oral cavity cancer is the 16th most common malignant neoplasm worldwide, with approximately 355,000 new cases and approximately 177,000 cancer-related deaths annually1,2,3. Cancer of the anterior two-thirds of the tongue accounts for approximately 37% of newly diagnosed cases of malignancy involving the oral cavity4, and approximately 84–97% of tongue cancers are squamous cell carcinomas5,6. Over the past decade, the mortality rate of squamous cell carcinoma has been high7. Approximately 60% of the five-year survival rate of patients with oral squamous cell carcinoma (OSCC) is unoptimistic despite advances in diagnosing and treating oral cavity cancer8,9. Oral cavity cancer is typically diagnosed when it is relatively advanced with a poor prognosis owing to the lack of public awareness; however, this disease has gradually attracted the public’s attention in recent years10. The concept of perineural invasion (PNI) and lymphovascular invasion (LVI) has often been discussed in research on OSCC11,12,13,14. The pathways of tumor cells invading the nerves and vessels are shown in Fig. 1.
Pathways of tumor cells invading the nerves and vessels. Tumor cells are transmitted indirectly via lymphatic or lymphatic vein exchange channels rather than directly via blood vessels. LVI refers to the localization of tumor cells in the vascular lumen or local adhesion to the vascular wall. PNI refers to cells being close to the nerves and involves 33% of tumor cells or the invasion of three layers of the nerve sheath. A black and thickened arrow in the figure indicates the direction of tumor cell invasion. PNI perineural invasion, LVI lymphovascular invasion.
Cruveiheir first described PNI in head and neck cancer in 18356. OSCC is a neurotropic malignancy in which neurotropism is a significant feature representing tumor cells and stroma affinity to the surrounding nerve tissue15. The incidence rates of PNI in tongue cancer and buccal mucosa carcinoma were approximately 28.3% and 16%, respectively, and this may be attributed to the rich nerve distribution in the tongue16. LVI is defined as tumor embolism within the space in which tumor cells invade the vascular wall and/or that in which endothelial cells are arranged17. Tumor cells are transmitted indirectly via lymphatic or lymphatic vein exchange channels rather than directly via blood vessels18. Although LVI and PNI are widely used as indicators of aggressive tumor cells, there is no definite report of LVI and PNI in squamous cell carcinoma of the tongue (SCCT)19,20. The staging of SCCT affects risk stratification, which is the first step toward personalized treatment21. The American Joint Committee on Cancer (AJCC) tumor node metastasis is widely available, but whether LVI and PNI can be used as additional staging criteria remains uncertain2,22,23. Therefore, this study aimed to investigate the prognostic impact of LVI and PNI in patients with SCCT to establish a complete risk classification.
Methods
Patient characteristics
This retrospective study included patients with SCCT who underwent surgery-based treatment at our institution between January 2013 and December 2020. Patients without clinical evidence of lymph node metastasis (N−) usually underwent selective neck dissection, whereas those with lymph node metastasis (N +) underwent modified radical neck dissection24,25. Clinicopathological data, including histopathology and surgical records, were retrieved from the patient’s medical records, and the follow-up time was set from the date of surgery to death, loss of visit, or May 2022. Given that the etiology and prognosis of squamous cell carcinoma in the posterior part of the tongue are different from those of the anterior part, only the anterior two-thirds of the tongue was studied in the present research. The inclusion criteria were (1) diagnosis of SCCT based on preoperative imaging and postoperative pathology; (2) no preoperative chemotherapy, radiotherapy, immunotherapy, or endocrine therapy; (3) first onset and treatment occurring during the study period; (4) no distant metastasis detected before surgery; and (5) complete clinical and pathological data. Patients were excluded from the study if they had incomplete records, were lost to follow-up, or had concomitant malignant tumors or a malignant tumor history.
This study was carried out following the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans that were further updated, and the protocol was reviewed and approved by the Medical ethics committee of the Second Affiliated Hospital of Fujian Medical University(no. 274, 2022). All procedures conducted in studies involving human participants met the ethical standards of the Institutional Research Committee. Informed consent was obtained from all patients. All methods were performed in accordance with the relevant guidelines and regulations.
Clinicopathological features
The eighth edition of the American Joint Committee on Cancer (AJCC) tumor node metastasis (AJCC-TNM 8th) was used to stage all the patients with SCCT. The pathologists at our institution examined the surgical specimens by using the AJCC criteria in a blinded manner. The pathological sections were stained with hematoxylin and eosin to analyze histopathological parameters, including histological grade, staging, pathologic T stage (pT stage), pathologic N stage (pN stage), LVI, and PNI. Patients with LVI, PNI, or lymph node metastasis should undergo postoperative radiotherapy (PORT); hence, PORT was also analyzed as a prognostic factor in our study. The patients were divided into four groups based on the presence of LVI (V−/V +) and PNI (P−/P +): P–V−, P–V + , P + V− and P + V + .
Statistical methods
Fisher’s exact and the chi-square tests were used to determine differences in clinical features among the four groups. Pearson’s chi-square test was used to identify the correlation between clinicopathological features and overall survival (OS). OS was defined as the time from surgery to death, loss of visit, or May 2022. The Kaplan–Meier method was used to plot univariate survival curves, and a log-rank test was used to determine the statistical differences. Cox proportional hazard modeling was used to estimate the independent prognostic impact of patient- and tumor-related factors on survival, and hazard ratios (HRs) and 95% confidence intervals (CIs) were used to report the magnitude of the differences and the strength of association26. All statistical analyses were performed using SPSS software version 26 (SPSS Inc., Chicago, Illinois, USA). Statistical significance was set at p < 0.05.
Results
Clinical parameters
The study had 219 eligible patients with SCCT who received treatment at our institution from January 2013 to December 2020. However, those who did not have medical records (n = 4), had other malignant tumors (n = 13), were receiving preoperative radiotherapy and chemotherapy (n = 13), refused surgical treatment (n = 46), had postoperative pathology suggestive of other pathological types (n = 4), and were lost to follow-up (n = 12) were excluded. A total of 127 eligible patients were included in the analysis (Fig. S1). Among the 127 patients evaluated in this study, the median age was 57 years (range, 28–87 years), and 68 (53.5%) were male. Table 1 shows the patient demographic information and histological evaluation of the lesional tissue. Significant differences were observed in pT stage, pN stage, and tumor stage. No significant differences among the four groups were detected in sex, age, histological grade, or postoperative radiotherapy. The relationship between these factors and OS is summarized in Fig. 2.
One-, three-, and five-year survival rates
Among the 127 patients who were included, 95 patients (74.8%) were classified as P–V−, 8 (6.3%) were P–V + , 18 (14.2%) were P + V−, and 6 (4.7%) were P + V + (Fig. 3A–D). The mean follow-up time was 41 months (range, 3–111 months), and 33 patients died during the follow-up period. The 1-year (χ2 = 11.139, p = 0.011), 3-year (χ2 = 29.237, p < 0.001), and 5-year survival rates (χ2 = 7.664, p = 0.022) were significantly different among the four groups (Table S1).
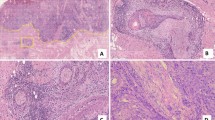
Pathological features of the four groups. Pathological sections of SCCT were stained with hematoxylin and eosin. (A) P–V−, PNI negative with LVI negative (× 20); (B) P–V + , PNI negative with LVI positive (× 40); (C) P + V−, PNI positive with LVI negative (× 40); (D) P + V + , PNI positive with LVI positive (× 40). The black arrow indicates tumor cells invading the blood and lymphatic vessels. The white arrow indicates tumor cells invading the nerves. SCCT squamous cell carcinoma of the tongue, PNI perineural invasion, LVI lymphovascular invasion.
Cox proportional hazard modeling for LVI and PNI
Univariate analysis was used to evaluate the prognostic significance of PNI, LVI, and other pathological factors. In univariate analysis, the OS rate was negatively influenced by six tumor-related factors: pN stage, tumor stage, histological grade, postoperative radiotherapy, PNI, and LVI. Sex, age, and pT stage were not risk factors for OS (Fig. S2). The results of multivariate analysis showed that PNI (HR = 3.309; 95% CI 1.466–7.472; p = 0.004) and LVI (HR = 4.537, 95% CI 1.871–11.001; p = 0.001) were significantly correlated with OS and were independent risk factors for the prognosis of SCCT. The univariate and multivariate analyses of LVI, PNI, and various factors are shown in Table 2.
Hierarchical analysis of LVI and PNI
The pT stage (T1–T2 vs. T3–T4), pN stage (N0 vs. N +), tumor stage (I–II vs. III–IV), and histological grade were analyzed. Significant differences were detected in the OS rates of the four groups at T1–T2 and T3–T4, and the OS in the P–V− group was significantly higher than that in the other three groups. No statistical difference was noted in the OS rates among the four groups in stages I–II, whereas statistical differences were detected in stages III–IV, with the OS in the P–V− group being the highest and that in the P + V + group being the lowest. Significant differences were found between the four groups in moderate and good differentiation. A significant difference was observed in the OS of the four groups with positive lymph nodes. In cases with positive lymph nodes, the OS rates in the four groups in T1–T2 and T3–T4 were compared, and the results showed statistical differences in T3–T4 between the four groups, and the OS rates in the P–V + , P + V−, and P + V + groups were significantly poorer than that in the P–V− group. Similarly, the OS rates in the four groups was analyzed, in which lymph node metastasis occurred in patients with stages III–IV. The results showed significant differences in the OS rates among the four groups in stages III–IV combined with positive lymph nodes. The OS in the P + V + group was the lowest, whereas no significant difference was detected in cases with negative lymph nodes (p = 0.979). Figures 4 and S3 show the relationships of LVI and PNI with OS in different situations.
Survival time for the four groups
The median survival time was 47 ± 30.1 months (95% CI 41.1–53.3) in the P–V− group, 25 ± 25.3 months (95% CI 3.9–46.1) in the P–V + group, 29 ± 16.6 months (95% CI 20.5–37.1) in the P + V− group, and 10 ± 4. 4 months (95% CI 5.5–14.8) in the P + V + group. The results showed that the P + V + group had a shorter survival time (only 10 months on average). The OS for P + V− patients was longer than that for P–V + patients but shorter than that for P–V− patients. The 3-year survival rate in the P + V + group was significantly lower than that in the other three groups, whereas the 5-year survival rate in the P–V− group was significantly higher.
Discussion
Sex and age
The high prevalence of SCCT worldwide may be related to poor habits, such as chewing betel nuts, smoking, and alcohol consumption, which are risk factors for SCCT27,28. It has been reported that males are twice as likely to develop SCCT3. In most countries, the average age of patients with SCCT is between 51 and 55 years27, with an incidence of approximately 17% in patients < 40 years old29,30. This was consistent with our study that included 35 people < 50 years old, with a minimum age of 28 years31. A previous study indicated no age or sex differences in the survival rate27. Our study also showed that age and sex were not prognostic indicators and that there were no significant differences in LVI/PNI.
Univariate and multivariate analysis
PNI is a common pathological finding in head and neck cancers, including squamous cell and adenoid cystic carcinomas32. PNI is a pathological process in which tumor cells invade the nerves and spread along the nerve sheaths, not in the form of multiple lymph or blood but as a direct extension of the primary tumor33,34. PNI is a histological manifestation of tumor cell infiltration34, which is an important cause of morbidity and mortality, and its prognosis is poor35,36,37. Cancer cell extension along nerves has been reported to increase the recurrence rate and decrease the survival rate38. The presence of LVI indicates that a significant number of tumor cells enter the blood vessels, which is the first step in potential metastasis39. In our study, pN stage, tumor stage, histological grade, PORT, PNI, and LVI were risk factors for poor prognosis. The results of the multivariate analysis showed that PNI/LVI was significantly correlated with OS and was an independent risk factor for SCCT prognosis.
LVI, PNI, and their correlation with clinicopathologic parameters
Recent studies have shown that PNI is more common in advanced tumors, thus suggesting a poor prognosis6. LVI and PNI were significantly different in pT, pN, and tumor stages and were more common in T3–T4, N + , and stage III–IV patients. Many studies have consistently shown that LVI and PNI increase the risk of lymph node metastasis and that lymphatic vessel invasion is the first step in metastasis development37,40,41. Survival rates decreased significantly among patients with early tongue cancer when LVI was associated with other adverse prognostic factors42.
Correlations of LVI and PNI with OS
PNI is considered an important prognostic factor for head and neck cancers, can be observed in the absence of lymphatic or vascular invasion, and often occurs in small and unnamed nerve fibers with an incidence of 42–52%34,35,43. LVI is an early indication of metastasis to lymph nodes and other organs (indicating a tendency for tumor cell invasion17) and is a risk factor for lymph node metastasis44. It has been reported that the presence of LVI and PNI suggests poor survival and can be used as prognostic biomarkers for patients with OSCC18,45. A strong correlation was found between LVI and survival outcomes in recurrent tumors40. Thus, we divided all participants into four groups according to LVI and PNI. OS in the four groups was statistically different; it was highest for P–V− and lowest for P + V + . The results showed that the P + V + group had a shorter survival time (only 10 months on average). Patients with P + V- had a longer OS than those with P–V + but had a shorter OS than those with P–V−. The 3-year survival rate in the P + V + group was significantly lower, whereas the 5-year survival rate in the P–V− group was significantly higher than that in the other three groups. This indicates that the prognosis of simultaneous neurovascular invasion is the poorest and that the prognosis of vascular invasion alone is poorer than that of neurotransplantation.
Hierarchical analysis of the four groups
For patients with positive lymph nodes, a significant difference in OS was found among the four groups. Therefore, when patients with SCCT develop lymph node metastases, the prognosis of P + V + is the poorest among groups. OS in the P–V− group was higher than that in the other three groups at T1–T2 and T3–T4. OS was significantly different among the four groups for stage III–IV cases, and OS in the P + V + group was poorer than the OS rates in the P–V−, P–V + , and P + V− groups. Significant between-group differences were observed in terms of moderate and good differentiation, and the P + V + group showed the poorest prognosis for patients with moderately differentiated tumors. Patients in T3–T4 with lymph node metastases in the P–V + , P + V− and P + V + groups have poorer prognoses than those in the P–V− group. Patients with SCCT with both PNI and LVI have the poorest prognosis if lymph node metastases occur in stages III–IV.
Postoperative radiotherapy and prognosis
Radiation therapy is known to be effective for oral cancer because it provides a high dose of radiation locally while preserving the surrounding healthy tissue46. The 2016 National Comprehensive Cancer Network guidelines recommend adjuvant radiation therapy when PNI and/or LVI are diagnosed after surgery47. Some studies have shown that appropriate postoperative radiotherapy can improve local control for patients with LVI and who were PNI positive16,18,34. Postoperative radiotherapy is recommended for patients with adverse features (stage IV), positive margins, LVI, and PNI48,49,50. Previous research has shown that PORT does not provide significant survival benefits in patients with stage I–II OSCC with PNI and/or LVI as the sole risk factor and may not be recommended51. In the current study, patients receiving postoperative radiotherapy generally had advanced lymph node metastases, which also predicted a poor prognosis. According to a relevant report, adding chemotherapy to radiotherapy for high-risk locally advanced head and neck cancer improved local control rates and OS52. For patients with stage III–IV disease not receiving PORT, there was a significant difference in survival among the four groups, with OS being the highest in the P + V− group. Therefore, a precise conclusion on whether patients with SCCT with LVI/PNI should receive radiation therapy after surgery could not be reached. Further studies are warranted to address this issue.
Limitations
First, this study was limited by its retrospective design, including selection bias and the lack of medical record data. Patients deemed suitable for surgery were included in the analysis, which may have also yielded selection bias. Second, further analyses of LVI and PNI may be particularly undermotivating to achieve significant results because of the limited number of patients in this subgroup. Third, patients receiving postoperative radiotherapy are mainly concentrated in advanced or lymph node metastases, thus limiting further studies on radiation therapy. Finally, due to the absence of available data, further analysis of disease-specific survival cannot be achieved in the present research. Future studies should be focused on this issue.
Conclusion
LVI and PNI are independent negative prognostic factors for patients with SCCT. Patients with LVI and/or PNI had a significantly lower OS than those without neurovascular involvement. The classification of patients with SCCT based on the TNM staging system combined with LVI and PNI may be more comprehensive and accurate. Future studies with larger sample sizes are warranted to confirm our findings, preferably with prospective designs.
Data availability
The raw data supporting the conclusions of this article will be made available by corresponding authors(Chaohui Zheng), without undue reservation.
Abbreviations
- AJCC:
-
American joint commission on cancer
- LVI:
-
Lymphovascular invasion
- OSCC:
-
Oral squamous cell carcinoma
- OS:
-
Overall survival
- PORT:
-
Postoperative radiotherapy
- PNI:
-
Perineural invasion
- SCCT:
-
Squamous cell carcinoma of the tongue
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68(6), 394–424 (2018).
Rodrigues, R. M. et al. How pathological criteria can impact prognosis of tongue and floor of the mouth squamous cell carcinoma. J. Appl. Oral Sci. 28, e20190198 (2019).
Warnakulasuriya, S. & Kerr, A. R. Oral cancer screening: past, present, and future. J. Dent. Res. 100(12), 1313–1320 (2021).
Gad, Z. S., El-Malt, O. A., El-Sakkary, M. A. T. & Abdal Aziz, M. M. Elective neck dissection for management of early- stage oral tongue cancer. Asian Pac. J. Cancer Prev. 19(7), 1797–1803 (2018).
Kruaysawat, W., Aekplakorn, W. & Chapman, R. S. Survival time and prognostic factors of oral cancer in Ubon Ratchathani Cancer Center. J. Med. Assoc. Thai. 93(3), 278–284 (2010).
Quintana, D. M. V. O., Dedivitis, R. A. & Kowalski, L. P. Prognostic impact of perineural invasion in oral cancer: A systematic review. Acta Otorhinolaryngol. Ital. 42(1), 17–25 (2022).
Santarelli, A. et al. Survivin-based treatment strategies for squamous cell carcinoma. Int. J. Mol. Sci. 19(4), 971 (2018).
Sarode, G., Maniyar, N., Sarode, S. C., Jafer, M. & Patil, S. Epidemiologic aspects of oral cancer. Dis. Mon. 66(12), 100988 (2020).
Mascitti, M. et al. American Joint Committee on Cancer staging system 7th edition versus 8th edition: Any improvement for patients with squamous cell carcinoma of the tongue?. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 126(5), 415–423 (2018).
Adeoye, J., Thomson, P. & Choi, S. W. Prognostic significance of multi-positive invasive histopathology in oral cancer. J. Oral Pathol. Med. 49(10), 1004–1010 (2020).
Mohammed Nur, M., Al Saadi, M., O’Regan, E. M., Van Harten, M. & Toner, M. Small and thin oral squamous cell carcinomas may exhibit adverse pathologic prognostic features. Head Neck Pathol. 15(2), 461–468 (2021).
Ting, K. et al. Perineural invasion/lymphovascular invasion double positive predicts distant metastasis and poor survival in T3–4 oral squamous cell carcinoma. Sci. Rep. 11(1), 19770 (2021).
Colonia-García, A., Salazar-Peláez, L. M., Serna-Ortiz, C. A., Álvarez-Sánchez, L. G. & de Andrade, C. R. Prognostic value of lymphovascular and perineural invasion in squamous cell carcinoma of the tongue. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 133(2), 207–215 (2022).
Sowmya, S. V., Rao, R. S. & Prasad, K. Development of clinico-histopathological predictive model for the assessment of metastatic risk of oral squamous cell carcinoma. J. Carcinog. 19, 2 (2020).
Manjula, M., Angadi, P. V., Priya, N. K., Hallikerimath, S. & Kale, A. D. Assessment of morphological parameters associated with neural invasion in oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 23(1), 157 (2019).
Nair, D. et al. Perineural invasion: Independent prognostic factor in oral cancer that warrants adjuvant treatment. Head Neck 40(8), 1780–1787 (2018).
Fujikawa, H. et al. The clinical significance of lymphovascular invasion in gastric cancer. In Vivo 34(3), 1533–1539 (2020).
Huang, S., Zhu, Y., Cai, H., Zhang, Y. & Hou, J. Impact of lymphovascular invasion in oral squamous cell carcinoma: A meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 131(3), 319-328.e1 (2021).
Jardim, J. F. et al. Prognostic impact of perineural invasion and lymphovascular invasion in advanced stage oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 44(1), 23–28 (2015).
Singh, M. et al. Incorporation of adverse features in advanced oral cancer improves precision in staging and patient prognostication. Head Neck 44(4), 964–974 (2022).
Almangush, A. et al. Staging and grading of oral squamous cell carcinoma: An update. Oral Oncol. 107, 104799 (2020).
Hortobagyi, G. N., Edge, S. B. & Giuliano, A. New and important changes in the TNM staging system for breast cancer. Am. Soc. Clin. Oncol. Educ. Book 38, 457–467 (2018).
Mascitti, M. et al. Histological features and survival in young patients with HPV-negative oral squamous cell carcinoma. Oral Dis. 26(8), 1640–1648 (2020).
Farrokhian, N. et al. Development and validation of machine learning models for predicting occult nodal metastasis in early-stage oral cavity squamous cell carcinoma. JAMA Netw. Open 5(4), e227226 (2022).
Hanai, N., Asakage, T., Kiyota, N., Homma, A. & Hayashi, R. Controversies in relation to neck management in N0 early oral tongue cancer. Jpn. J. Clin. Oncol. 49(4), 297–305 (2019).
Abbas, S. A., Saeed, J., Tariq, M. U., Baksh, A. R. & Hashmi, S. Clinicopathological prognostic factors of oral squamous cell carcinoma: An experience of a tertiary care hospital. J. Pak. Med. Assoc. 68(7), 1115–1119 (2018).
Krishna Rao, S. V., Mejia, G., Roberts-Thomson, K. & Logan, R. Epidemiology of oral cancer in Asia in the past decade—An update (2000–2012). Asian Pac. J. Cancer Prev. 14(10), 5567–5577 (2013).
Bhurgri, Y. et al. Cancer of the oral cavity and pharynx in Karachi–identification of potential risk factors. Asian Pac. J. Cancer Prev. 4(2), 125–130 (2003).
Halboub, E. S., Al-Anazi, Y. M. & Al-Mohaya, M. A. Characterization of Yemeni patients treated for oral and pharyngeal cancers in Saudi Arabia. Saudi Med. J. 32(11), 1177–1182 (2011).
Mukdad, L. et al. Oral tongue squamous cell carcinoma survival as stratified by age and sex: A surveillance, epidemiology, and end results analysis. Laryngoscope 129(9), 2076–2081 (2019).
Tagliabue, M. et al. A systematic review and meta-analysis of the prognostic role of age in oral tongue cancer. Cancer Med. 10(8), 2566–2578 (2021).
Wei, P. Y., Li, W. Y. & Tai, S. K. Discrete perineural invasion focus number in quantification for T1–T2 oral squamous cell carcinoma. Otolaryngol. Head Neck Surg. 160(4), 635–641 (2019).
Chablani, P. et al. Perineural invasion predicts for distant metastasis in locally advanced rectal cancer treated with neoadjuvant chemoradiation and surgery. Am. J. Clin. Oncol. 40(6), 561–568 (2017).
Bakst, R. L. et al. Perineural invasion and perineural tumor spread in head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 103(5), 1109–1124 (2019).
Chinn, S. B. et al. Impact of perineural invasion in the pathologically N0 neck in oral cavity squamous cell carcinoma. Otolaryngol. Head Neck Surg. 149(6), 893–899 (2013).
Saidak, Z., Lailler, C., Clatot, F. & Galmiche, A. Perineural invasion in head and neck squamous cell carcinoma: Background, mechanisms, and prognostic implications. Curr. Opin. Otolaryngol. Head Neck Surg. 28(2), 90–95 (2020).
Schmitd, L. B., Scanlon, C. S. & D’Silva, N. J. Perineural invasion in head and neck cancer. J. Dent. Res. 97(7), 742–750 (2018).
Binmadi, N. O. & Basile, J. R. Perineural invasion in oral squamous cell carcinoma: A discussion of significance and review of the literature. Oral Oncol. 47(11), 1005–1010 (2011).
Adel, M. et al. Evaluation of lymphatic and vascular invasion in relation to clinicopathological factors and treatment outcome in oral cavity squamous cell carcinoma. Medicine (Baltimore) 94(43), e1510 (2015).
Subramaniam, N. et al. Factors affecting survival in surgically salvaged locoregional recurrences of squamous cell carcinoma of the tongue. J. Oral Maxillofac. Surg. 76(5), 1133.e1-1133.e6 (2018).
Chatterjee, D. et al. Tumor budding and worse pattern of invasion can predict nodal metastasis in oral cancers and associated with poor survival in early-stage tumors. Ear Nose Throat J. 98(7), E112–E119 (2019).
Mascitti, M. et al. Lymphovascular invasion as a prognostic tool for oral squamous cell carcinoma: A comprehensive review. Int. J. Oral Maxillofac. Surg. 51(1), 1–9 (2022).
Liebig, C., Ayala, G., Wilks, J. A., Berger, D. H. & Albo, D. Perineural invasion in cancer: A review of the literature. Cancer 115(15), 3379–3391 (2009).
Choi, S. et al. Lymphovascular invasion: Traditional but vital and sensible prognostic factor in early gastric cancer. Ann. Surg. Oncol. 28(13), 8928–8935 (2021).
Saidak, Z., Lailler, C., Clatot, F. & Galmiche, A. Perineural invasion in head and neck squamous cell carcinoma: Background, mechanisms, and prognostic implications. Curr. Opin. Otolaryngol. Head Neck Surg. 28(2), 90–95 (2020).
Yoshimura, R. I. et al. Efficacy and safety of induction chemotherapy and/or external beam radiotherapy followed by brachytherapy in patients with tongue cancer. Anticancer Res. 41(12), 6259–6266 (2021).
Adelstein, D. et al. NCCN guidelines insights: Head and neck cancers, version 2.2017. J. Natl. Compr. Cancer Netw. 15(6), 761–770 (2017).
Quinlan-Davidson, S. R. et al. Outcomes of oral cavity cancer patients treated with surgery followed by postoperative intensity modulated radiation therapy. Oral Oncol. 72, 90–97 (2017).
Cassidy, R. J. et al. Association of lymphovascular space invasion with locoregional failure and survival in patients with node-negative oral tongue cancers. JAMA Otolaryngol. Head Neck Surg. 143(4), 382–388 (2017).
Harris, B. N. et al. Association of adjuvant radiation therapy with survival in patients with advanced cutaneous squamous cell carcinoma of the head and neck. JAMA Otolaryngol. Head Neck Surg. 145(2), 153–158 (2019).
Chen, T. C. et al. The impact of perineural invasion and/or lymphovascular invasion on the survival of early-stage oral squamous cell carcinoma patients. Ann. Surg. Oncol. 20(7), 2388–2395 (2013).
Lau, L., Eu, D., Loh, T., Ahmed, Q. & Lim, C. M. Histopathologic prognostic indices in tongue squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 278(7), 2461–2471 (2021).
Acknowledgements
We thank Prof. Chaohui Zheng and Prof. Feng Zheng for critically reading the manuscript and his useful comments.
Funding
This work was financially supported by Fujian health education joint project (WKJ2016-2-36), Quanzhou City Science & Technology Program of China (Grant Number 2022NS084) and DoctoralStartup Fund of the Second Affiliated Hospital of Fujian Medical University (Grant Number BS202205).
Author information
Authors and Affiliations
Contributions
Study conception and design, methodology, monitoring, supervision, C.Z.; statistical analysis, study monitoring, and data interpretation, W.H. and F.Z.; study conception, methodology, and data acquisition and interpretation, Q.H.; histopathology reporting, analysis, and data interpretation, D.F. and Y.Y.; study monitoring and data interpretation, Y.H.; data acquisition and interpretation, C.C.; study conception, study design, methodology, and data interpretation, Y.Z. and J.Z.; data acquisition, analysis, and interpretation, C.X., M.L. and Y.X.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, Q., Huang, Y., Chen, C. et al. Prognostic impact of lymphovascular and perineural invasion in squamous cell carcinoma of the tongue. Sci Rep 13, 3828 (2023). https://doi.org/10.1038/s41598-023-30939-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30939-8
- Springer Nature Limited
This article is cited by
-
Insights into metastatic roadmap of head and neck cancer squamous cell carcinoma based on clinical, histopathological and molecular profiles
Molecular Biology Reports (2024)
-
Triple Positive Oral Squamous Cell Carcinoma Patients Predict Poor Survival Outcomes: Multiple Factor Positivity Warrants the Need for Modified Treatment Approaches
Journal of Maxillofacial and Oral Surgery (2024)
-
Novel Scoring Formula to Predict Survival in Patients of Primary Tongue Cancer Belonging to Tobacco Chewing Population
Indian Journal of Surgical Oncology (2023)