Abstract
Poly(acrylic acid-co-acrylamide-co-maleic acid) (p(AA-co-AM-co-MA)) superabsorbent polymer was synthesized from acrylic acid (AA), acrylamide (AM), and maleic acid (MA) via free radical copolymerization. Results showed the presence of maleic acid in structure of superabsorbent has the key and superior role in creating a smart superabsorbent. The structure, morphology, and strength of the superabsorbent were characterized using FT-IR, TGA, SEM, and rheology analysis. The effect of different factors was investigated to determine the ability of water absorbency of the superabsorbent. According to optimized conditions, the water absorbency capacity of the superabsorbent in distilled water (DW) was 1348 g/g and in a solution containing 1.0 wt.% NaCl (SCS) was 106 g/g. The water retention ability of the superabsorbent was also investigated. The kinetic swelling of superabsorbent was identified by Fickian diffusion and Schott's pseudo-second-order model. Furthermore, the reusability of superabsorbent was studied in distilled water and saline solution. The ability of superabsorbent was investigated in simulated urea and glucose solutions, and very good results were obtained. The response ability of the superabsorbent was confirmed by swelling and shrinking behavior against changes of temperature, pH, and ionic strength.
Similar content being viewed by others
Introduction
One of the most important industrial materials in the world is superabsorbent polymers (SAPs). Global demand for manufacturing of SAPs is very abundant because they are used in hospital bed pads, baby and adult diapers1 as well as agriculture2, food processing3, water/sewage treatment3, tissue engineering4, sensors5, and drug delivery6. SAPs are a type of hydrophilic hydrogels that can absorb and hold massive amounts of water or other aqueous solutions up to hundreds of times their dry weight. These three-dimensional polymeric networks are not dissolved in water and physiological solutions owing to chemical or physical crosslinks7. Although a great deal of attention has been paid to the production of superabsorbents using natural materials, it has not yet been industrialized due to the high price of these materials. Still, AA and AM are the best monomers in the industry to synthesize superabsorbents due to easy production, low-cost, availability and rapid polymerization8,9. AA and AM have high hydrophilicity, excellent flexibility, chelating properties, and biodegradablity10. Moreover, it was found that copolymerization of AA and AM in a polymeric network can improve the strength of the gel11. On the other hand, maleic acid (MA) is the other industrially synthetic compound that has lately gained popularity in the production of polymers due to its lower cost, good compatibility, and nontoxicity12,13. Thus, preparation of a novel superabsorbent with these cheap molecules and worthwhile properties is very vital.
Sensitive hydrogels have attracted much attention due to absorbing/releasing materials and swelling/deswelling behavior in response to physical, chemical and biological stimuli14,15,16. Among them, temperature, pH, and ionic strength are very important owing to simplicity and no requirement for complex devices and expensive materials17. In regard to the preparation of temperature-sensitive hydrogels, it has been proven that the presence of hydrophilic and hydrophobic functional groups plays a decisive role18. When hydrophilic monomers are copolymerized with N-isopropylacrylamide (having isopropyl hydrophobic part) create a temperature-sensitive hydrogel19. Also, chitosan, cellulose, gelatin, and poloxamers and their derivatives are suitable starting materials for the synthesis of thermo-responsive hydrogels20. On the other hand, it has been specified the presence of anionic and cationic functional groups has a vital role in the synthesis of pH-sensitive hydrogels21,22,23. When acrylic acid derivatives react with natural polymers such as carboxy methyl dextran, albumin, gelatin, alginate, and chitosan are synthesized pH-responsive hydrogels21,22,23. Thus, to find a new monomer structure in production thermo- and pH-sensitive property in preparation of smart hydrogels can be very important. There is no smart superabsorbent from AA, AM and MA monomers.
Thus in this article, not only the synthesis of a worthy superabsorbent using cheap and available starting materials is described (Scheme 1), but also MA is introduced as a vital monomer in the preparation of intelligent superabsorbent which can respond to various stimuli such as temperature, pH and ionic strength media.
Materials and methods
Materials
N,N'-methylenebisacrylamide (98%) (MBA), acrylamide (99.9%), maleic acid (99%), acrylic acid (99%), sodium hydroxide, and ammonium persulfate (98%) (APS) were provided in analytical grade from Merck.
Synthesis of p(AA-co-AM-co-MA) Superabsorbent
To prepare the smart superabsorbent (p(AA-co-AM-co-MA)), AA (1.80 g, 25 mmol), AM (0.73 g, 10 mmol), and MA (1.47 g, 12 mmol) were completely dissolved in 13 mL DW in the beaker using a magnetic stirrer. Subsequently, NaOH (2.00 g, 50 mmol) and MBA (0.0045 g, 0.029 mmol) were added to the reaction mixture. The solution was then allowed to heat above 60 °C. In the following, (0.018 g, 0.079 mmol) of APS was poured into the previous solution, and the polymerization reaction was done for 2 h. Then, the prepared superabsorbent was dried at room temperature. Then, the obtained superabsorbent was immersed in DW to remove homopolymers and unreacted materials. Finally, the obtained superabsorbent was dried in oven at 60 °C.
All the reactions of the optimization stages were performed in the same way but with different amounts of AM, AM, MA, APS, NaOH, and MBA. In addition, the p(AA-co-AM) superabsorbent was prepared with this method without using the MA monomer.
Characterization techniques
FT-IR spectra were obtained at room temperature using the KBr disk method with a JASCO FT/IR-6300 spectrometer to identify functional groups in polymeric chains. Rheological characterization was done to determine the strength superabsorbent by Anton-Paar Physica MCR 301 machinery. Thermal stability was evaluated by the thermogravimetric analysis (TGA-Perkin Elmer) from 25 to 800 °C with a heating rate of 10 °C/min under N2 flow. A DME Dualscop C-26 microscope was used to study the morphology of the superabsorbent. A Metrohm pH meter (827 pH lab) was used to control pH of solutions. To perform these analyses (FT-IR, Rheology, TGA, and FE-SEM), an FD-5005-BT freeze dryer was used to lyophilize the superabsorbents, p(AA-co-AM-co-MA) and p(AA-co-AM), at − 75 °C for 48 h.
Measurement methods
Three equal samples were used for calculations, and the results were expressed as an average.
The capacity of water absorbency
A certain amount of the superabsorbent was immersed for 24 h in various solutions (including different pH solutions and various kinds of salt solutions) at 25 °C. The swollen superabsorbent was then taken out from the solution, and the capacity of the water absorbency was calculated according to Eq. (1).24
In this equation, Q (g/g), W1 and W0 are the capacity of water absorbency, the swollen and dry weights of the superabsorbent, respectively.
Swelling and shrinking
Swelling/shrinking behavior of the superabsorbent was measured utilizing the typical gravimetric method. At first, the dried sample was swelled in aqueous media with clear features and special conditions (in terms of ionic concentration, pH, and temperature). Next, the swollen superabsorbent was separated from the medium and weighted at certain times. Subsequently, to observation of the amount of shrinking, the swollen superabsorbent was put in another medium with different features and conditions. Then, its weight was measured as long as it was swollen. These swelling and shrinking investigations were performed for three cycles.
Water absorbency kinetics
To determine the kinetic of water absorbency, one gram of the superabsorbent was soaked in excess DW or SCS and calculated the amount of Q. These kinetic data were recorded at the equal time distances. In the subsequent studies, a Fickian diffusion model (Eq. 2) and a pseudo-second-order kinetic model (Eq. 3) were applied to fitting the Q amounts at different times (t)9,24.
In the above equation, the water absorbency of the superabsorbent at time t and after it becomes equilibrium are Qt (g/g) and Qe (g/g), respectively. In Eq. (2), K and n correspond to the characteristic constant and the diffusional exponent of the superabsorbent, respectively. K2 is the rate constant of swelling in Eq. (3).
Water retention studies
To find the amount of the superabsorbent water retention, the samples were weighed upon equilibration (Qe) in DW or SCS. In the following step, the samples were placed in oven at various temperatures of 25 and 50 °C. After that, each sample was weighed once every hour (Qt) for nine hours. The water retention (Wr) was calculated by Eq. (4)25.
Results and discussions
To optimize condition for the synthesis of the superabsorbent
To find a superior condition in the synthesis of a p(AA-co-AM-co-MA) superabsorbent with maximum water absorbency various parameters were considered in DS or SCS. To find the desired amount of starting materials, the amounts of AA, AM, MA, APS, NaOH, and NMBA were changed. When obtaining the optimal amount of one substance, the amounts of the other materials were kept constant. The results of various parameters are as follows:
Varying the amount of AA monomer on the ability of the water absorbency was investigated (Fig. 1a). It was found that the maximum water absorbency capacity in DW was 816 ± 79 g/g, and in a SCS was 78 ± 4 g/g with amount 1.80 g of AA monomer. At first, the amount of water absorbency is increased with the addition of AA content. Acrylic acid is a monomer that causes superabsorbent flexibility. With the increase in flexibility, the amount of water absorption by superabsorbent increases. The water absorbency capacity decreases after reaching maximum, because by increasing the concentrations of AA homopolymerization was acquired with a greater rate and the rest of the molecules do not participate in the reaction26. Thus, synergic effects of other functional groups has been reduced. After that by addition AA the portion of MA immediately declines and in 3.6 g from AA, the role of MA is completely omitted. Subsequently, polymerization is happened between AA and AM, and by increasing the number of AA again by addition of flexibility along the chains the amount of water absorption is improved up to 556 g/g water absorbency with 5.67 g AA (the second maximum water absorbency). After this, water absorbency reduces again owing to AA homopolymerization and creation of cross-linkers between acid groups.
The water absorbency capacity of the p(AA-co-AM-co-MA) superabsorbent under the effect of the following variables: (a) Amount of AA. (b) Amount of AM. (c) Amount of APS initiator. (d) Amount of MBA cross-linker. (e) Neutralization amount. (f) Reaction time. Data for DW (blue color), Data for SCS (red color).
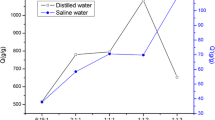
AM as a non-ionic monomer was used and optimized because it has excellent salt resistance feature27. Fig. 1b shows the effect of AM amounts on water absorbency capacity. The results showed that the maximum absorbency capacity reached 1348 ± 153 g/g in DS and in SCS gained 106 ± 10 g/g with 0.73 g AM. The initial increase up to the first maximum is due to the hydrophilic nature of AM monomer. After maximum, similar to the increasing AA, the portion of MA (that is the main material in absorption factor) reduces by addition of AM. When the amount of AM arrived to 1.35 g, MA completely remove. Then, the amount of water absorbency increases again by accumulative the amount of AM, which is due to the hydrophilicity of amide groups. But the increase did not continue. Dropping water absorbency after 1.59 is owing to increasing medium viscosity which prevents the movement of free radicals and active species of reactants in the medium28.
Changes in the concentration of MA showed that this compound with 1.47 g has the maximum amount of water absorption. But in lower than this amount the water absorbency was decreased and higher than this the value of water absorbency is constant.
Changing the amounts of APS on the amount of water absorbency has been shown in Fig. 1c. Results directed the maximum water absorbency capacity in DS is 1348 ± 153 g/g, and in SCS is 106 ± 10 g/g with amount 0.018 g of APS. According to Allcock and Frederick studies, the increased amount of the initiator decreases the molecular weight of the polymer in free radical polymerization. This decrease in the molecular weight causes an increase in polymer chain ends25. It seems that with increasing initiator content, the cross-linked density enhances, which affects the capacity of water absorbency. The capacity of water absorbency by the superabsorbent was decreased with the initiator content lower than 0.45 wt.%. Possibly this is due to the reduced content of free radicals generated by the initiator, so the network cannot be effectively formed29.
According to the Flory's network theory, cross-linker is a significant factor that affects network density and the water absorbency capacity of the superabsorbent. In this theory, the increasing cross-linkers raise the network nodes and density30. Consequently, the more amount of cross-linker increases the rigidity of the structure and limits the amount of water absorbency30. According to Fig. 1d, the maximum absorbency in DS is 1744 ± 87 g/g with the cross-linker amount in the range of 0.0030–0.0040 g. However, this amount was not selected as the optimum amount because the dimensional structure of the superabsorbent was unstable, and the mechanical property was negligible. With amounts, lower than 0.0030 g is only created viscous gel. According to the mechanical strength obtained from the rheology analysis, the fabricated superabsorbent with 0.0045 g of cross-linker was selected as the optimum amount. The water absorbency capacity by this amount in DW was 1348 ± 153 g/g, and in SCS was 106 ± 10 g/g.
The degree of neutralization is an important parameter of the polymerization rate and three-dimensional network charge density. As a result, it considerably affects the amount of water that can be absorbed by the superabsorbent. The neutralization degree was defined as the molar percentage of carboxyl groups in AA and MA neutralized by NaOH29. The maximum capacity of water absorbency is shown in Fig. 1e with 2 g neutralization which was 1348 ± 153 g/g and 106 ± 10 g/g in DW and SCS, respectively. The capacity to absorb water enhances with an increasing amount of neutralization until 2 g, due to the more active carboxylate groups of the AA and MA. It can be attributed to the electrostatic forces repulsion between carboxylate groups that are created by the neutralization of acrylic acid and the number of osmotically active ionic carboxylate groups in the copolymer31. When the neutralization degree is more than 2 g, the water absorbency capacity is decreased. It may be due to hydrolysis of cross-linkers and the increasing solubility of the product owing to the further number of carboxylate groups29.
As shown in Fig. 1f, the effect of reaction time was studied on the water absorbency capacity of the superabsorbent. At 2 h, the maximum capacity in DW was 1348 ± 153 g/g, and SCS was 106 ± 10 g/g. In shorter times polymerization is presumably incomplete, thus causing to increase the solubility of the polymer fraction and insufficient formation of cross-linkers. Longer reaction times lead to a high number of cross-linkers in the polymer network, which inhibits the stretching of the polymer chains in the network and reduces the capacity of water absorbency29,32.
According to the obtained results, the best conditions for the synthesis of p(AA-co-AM-co-MA) superabsorbent, with maximum water absorbency capacity includes AA (1.80 g), AM (0.73 g), MA (1.47 g), and NaOH (2 g), 2 h reaction time, and 60–70 °C reaction temperature. To prove the effect of MA, the p(AA-co-AM) superabsorbent was synthesized under the optimum condition without MA, and its water absorbency capacity was also measured (866 ± 41 g/g). Thus, results confirmed the efficiency of malic acid in the structure of the p(AA-co-AM-co-MA) superabsorbent.
Characterizations
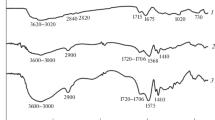
FT-IR spectrum of the p(AA-co-AM-co-MA) superabsorbent is shown in Fig. 2a. The broadband at 3411.5 cm−1 is ascribed to the stretching vibration of the NH group of the AM unit. Also, it has overlapped with the stretching vibration of the –OH group of carboxylate units33. The band at about 2946.7 cm−1 is related to the C–H absorption band of the methylene groups of p(AA-co-AM-co-MA). The absorption bands at 1723 cm−1 and 1677.7 cm−1 are assigned to the C=O stretching vibrations of carboxyl and amide groups, respectively34,35. Also, –N–H bending of the amide has overlapped with C=O of amide groups. The band at 1452.1 cm−1 is attributed to the symmetric stretching −CH=CHCOO−Na+ and asymmetric stretching vibrations of groups COO− is at 1569.7 cm−136. In addition, the bands at 1405.8 cm−1 and 1172.5 cm−1 correspond to the C–N and C–O stretching of the –CONH2 and COOH units, respectively37,38. The FT-IR results clearly proved that the p(AA-co-AM-co-MA) superabsorbent was successfully prepared. Also, the comparison of changes in the peak intensities of the two superabsorbents indicates the successful copolymerization of MA in the structure of the polymer network.
Figure 2b shows the TGA and DTG curves of the p(AA-co-AM-co-MA) and p(AA-co-AM) superabsorbents. The curves of the p(AA-co-AM-co-MA) superabsorbent show a Five-step decomposition. The first step of weight loss is at around 40–170 °C because of water loss, which is absorbed on the surface and in the network pores. The second step was observed in the range of 175 to 290 °C, which is due to the water elimination from two carboxyl groups of the MA units and cyclic anhydride formation39. In the third step, range of 260 to 384 °C, the amide groups of AM units and the cross-linkers on the polymer chains were decomposed. The fourth step, between 394 to 520 °C, is owing to remove of carboxylate and anhydride groups on the polymer chain. The final step (above 520 °C) is the breaking of the main polymer chains and the destruction of the polymer network40,41,42. While, the curves of the p(AA-co-AM) superabsorbent show a four-step decomposition. The first step weight reducing similar to the p(AA-co-AM-co-MA) superabsorbent is at range 50–218 °C because of surface and network water loss. The second step in the range of 230 to 352 °C, the amide groups of AM units and the cross-linkers are removed from the polymer chains. The third step, in the range of 360 to 537 °C, is related to the destruction of the carboxyl groups of AA units from the polymer chain. The fourth step of decomposition, above 540 °C is ascribed to the breakdown of the main chain and the organic residues40,41,42. Therefore, TGA and DTG analysis confirm that p(AA-co-AM) superabsorbent has one stage less than p(AA-co-AM-co-MA) superabsorbent, this can be attributed to the presence of maleic acid.
The appearance of p(AA-co-AM-co-MA) superabsorbent in dried and swollen states is shown in Fig. 3a,b. There was a considerable difference between dried and swollen superabsorbents. The form of swollen superabsorbents is completely spherical contrary to flat form of the dried superabsorbent.
Figure 3c,d shows the SEM micrograph of the superabsorbent. The structure of the prepared superabsorbent from AA, AM, and MA, due to the presence of the large interconnected pores, provided excellent surface and absorption functions43. Also, these interconnected pores are in the form of large hollow channels that can facilitate the rapid movement of water or other solvents. Also, SEM micrographs of the p(AA-co-AM) superabsorbent are presented in Fig. 3e,f. These indicate the structure and size of pores are different relative to the surface of the p(AA-co-AM-co-MA) superabsorbent. This can be attributed to the participation of the MA monomer.
Rheological analysis was used to study the viscoelastic properties of the prepared superabsorbents. For this aim, the linear viscoelastic range of the superabsorbent, LVE (linear viscoelastic), was first determined through stress sweep tests. In this range, the storage modulus (G′), and the loss modulus (G″) do not depend on applied stress (Fig. 4a). Also, this Figure shows that the superabsorbent with smaller absorbency (1347.72 ± 153.09 g/g in DW) has greater critical stress (σc) than the superabsorbent with higher absorbency (1743.59 ± 86.83 g/g in DW). This indicates its greater resistance to external stresses. Another significant rheological parameter in determining the strength of the gel is the damping coefficient (tan δ). Figure 4b shows the plot of the shear stress-damping coefficient. An increase in tan δ is indicative of liquid behavior (tan δ greater than 1), while a decrease in the value indicates solid-like (gel) behavior (tan δ less than 1)44. Smaller tan δ values indicate stronger interactions within the polymer system45. As shown in Fig. 4b, the tan δ value of the superabsorbent with high absorbency was rather close to 1, indicating that it had a more liquid-like behavior (less gel strength). Therefore, it showed that water absorbency capacity and gel strength are inversely related44, as well as the phase angles of two superabsorbents, were investigated. The phase angle of a perfectly elastic material is 0° (0 π rad), and that of a perfectly viscous material is 90° (π/2 rad)46. It can be seen from the results that the deflection angle of the superabsorbent with less absorbency (1347.72 ± 153.09 g/g) in a range of stresses (0.1–1) is close to that of fully elastic material (Fig. 4c). In contrast, the higher absorbency superabsorbent is close to the viscous material.
Subsequent to the stress sweep experiments, the frequency sweep tests were accomplished in the range of 0.1 to 1000 rad/s. Figure 4d shows the results of these experiments. G′ values of the superabsorbent with smaller absorbency were significantly higher than G″ values at all frequencies, indicating more solid-like (gel-like) behavior. Thus, the gel of this superabsorbent with smaller absorbency had greater strength than the gel of the superabsorbent with higher absorbency capacity44. In gels of the superabsorbent with smaller absorbency, G′ is greater than G″, showing that elastic behavior overcomes viscoelastic behavior. In addition, the samples tend to be more rigid at higher frequencies because their flexibility is reduced47.
Measurement
Kinetic studies
As shown in Fig. 5a,b, swell kinetics curves in DW and SCS for the p(AA-co-AM-co-MA) superabsorbent are similar. As depicted, in both DW and SCS, the water absorbency rises quickly in the initial steps and gradually increases to reach equilibrium. This swelling behavior can be explained by variations in osmotic pressure differences. In the structure of the p(AA-co-AM-co-MA) superabsorbent, the osmotic pressure difference is initially significant because of the presence of hydrophilic groups as well as electrostatic repulsion between carboxylate functional groups (anionic groups). Consequently, the water molecules can easily penetrate the superabsorbent polymer network, which increases the swelling rate. Nevertheless, the osmotic pressure difference is reduced by increasing water molecules in the network. Thus, the swelling rate gradually slows down and reaches equilibrium48.
The Fickian diffusion model was also applied to explain the mechanism of water molecules penetrating into the superabsorbent in DW and SCS during the initial step. For this aim, ln (f) versus ln (t) was plotted to find the amounts of n (slope) and k (intercept), based on Eq. (2) (Table 1). In Fig. 5c, the curves show good linear correlations. Based on the diffusion mechanism category7,24, the water absorbency behavior of superabsorbent in DW is governed by both Fickian diffusion and polymer chain relaxation (non-Fickian diffusion). Whereas, the water absorbency in SCS is dominant by the Fickian diffusion.
Pseudo-second-order model was applied to investigate the kinetic mechanism. Using Eq. (3), the plot of t/Qt against time was achieved in DS and SCS (Fig. 5d). Table 1 also lists the calculated kinetic parameters. Due to the high correlation coefficients (R2), in DW and SCS, the pseudo-second-order kinetic model, is suitable to describe the kinetic behavior of superabsorbent. Also, experimental results for this kinetic model were almost confirmed by using the calculated Qe, cal amounts.
Water retention time at various temperatures
In terms of the practical application of superabsorbents, measuring their water retention behavior is very important. Therefore, the water retention time of p(AA-co-AM-co-MA) in DW and SCS at various temperatures (25 and 50 °C) are shown in Fig. 6. As seen, the rate of water loss of swollen superabsorbents was different in various conditions. When the superabsorbent was placed in saline at two temperatures and in DW at 50 °C, a large amount of water was quickly lost. However, this behavior was not observed for DW at 25 °C. The results indicate that water retention of the superabsorbent is 63.54% and 9.36% in DW at 25 and 50 °C, respectively. Also, water retention in the 1wt. % NaCl solution is 11.98% and 0.96% at 25 and 50 °C, for 9 h, respectively. The water retaining can be attributed to the hydrogen bonding interaction and Van der Waals force between the water molecules and the superabsorbent49. It can be seen from these results that with an increasing temperature, the water retention capability decreases seriously. The cause of this behavior is probably related to the weakening of H-bonds and their breakdown at high temperatures50.
Reusability
The reusability and reswelling of the superabsorbent are essential factors in its efficiency. For this purpose, one gram of the superabsorbent was soaked in either DW or SCS to achieve equilibrium. The sample was then dried and used for subsequent measurements. Figure 7 shows the reusability of the p(AA-co-AM-co-MA) superabsorbent in DW and SCS. Accordingly, recycled superabsorbent was used constantly in five cycles under the same conditions. After each reusing, the water absorbency capacity of the superabsorbent a few decreases, and they can absorb 876 g/g and 48 g/g of solutions (DW and SCS) after five reswelling times, respectively. This high absorbency capacity after five cycles may be due to its stable polymeric network51. The breakdown of physical crosslinking in the superabsorbent structure may cause weak retention of water and ultimately decrease water absorbency capacity52.
Application of superabsorbent
Application of superabsorbent in simulated physiological fluids
To expand the potential application of the prepared superabsorbent, their absorbency capacity was investigated in four kinds of simulated biological fluids, including synthetic urine, physiological saline, urea solution, and D-glucose. Among simulated physiological fluids, the capacity of superabsorbent in urea solution was greater in compared with DW medium. Also, the absorbency capacity of the superabsorbent was the same in the D-glucose solution and DW. The absorbency capacity of superabsorbent in synthetic urine and physiological saline water was reduced (Fig. 8). These results demonstrated the charge screening effect of cations (Na+, K+, Mg2+, and Ca2+) in physiological saline water and synthetic urine. This event leads to the decrease of anion–anion electrostatic repulsions (carboxylate groups on the backbones superabsorbent). As a result, the difference in osmotic pressure decrease between the polymeric network and the external medium53. Based on the Donnan osmotic pressure equilibrium, the more mobile cations in a solution, the lower the difference in osmotic pressure; thus, the superabsorbent shrinks54.
The order of the absorbency capacity of the superabsorbent in different biological fluids is as follows: urea solution > D-glucose solution > physiological saline water > synthetic urine. In addition, the absorbency capacity was more in the urea solution than the value measured in DW. In addition, the absorbency capacity of the superabsorbent in synthetic urine was similar to the physiological saline water. This phenomenon is ascribed to the presence of NaCl, which is an important contribution to the absorbency capacity of the superabsorbent55.
Application of superabsorbent as a sensitive material
Salt-sensitivity and modulation behavior of superabsorbent
Figure 9a displays the ability of p(AA-co-AM-co-MA) superabsorbent to absorb water at two different concentrations of diverse salt solutions, including NaCl, MgCl2, and FeCl3. The results indicate that in salt solutions, the water absorbency capacities of superabsorbent decrease. The reason can be attributed to the protective effect of the cations in salt solutions. Salt solutions by protecting the carboxylate groups with cations prevent effective electrostatic repulsions. In this situation, the osmotic pressure difference between the polymeric network and salt solutions decreases, and as a result, the water absorbency capacity of the superabsorbent decreases. Figure 9a demonstrates the water absorbency capacity falls at upper salt concentration solutions. The phenomenon results from excess cations in solutions with higher concentrations. As well as, at an equal concentration of the salt solution, with increasing cationic charge, the capacity of the superabsorbent to absorb water decreases. The event is in accord with the theory of Flory's equation (cationic charge: univalent > divalent > trivalent)33.
In addition, Eq. (5) was used to calculate the dimensionless salt sensitivity factor (f). The f values of the superabsorbent for the three salts with different cations charges are shown in Fig. 9a26. The results show salt cations sensitivity of the superabsorbent is Na+ < Mg2+ < Fe3+, respectively56.
The modulation of the p(AA-co-AM-co-MA) and p(AA-co-AM) superabsorbents was investigated by soaking the sample in DW and SCS, respectively. The superabsorbents was swelled in DW for one hour, then soaked in SCS for one hour, and then the water absorbency capacity of the superabsorbent was determined as a function of time. Regular interval time studies show that superabsorbent swells in DW while shrinks in salt solution (Fig. 9b). This on–off switching is still present after three swelling-shrinking cycles, whereas p(AA-co-AM) superabsorbent shows a little response to ionic strength and after every time this property is decreased (Fig. 9b). By adding salt, the osmotic pressure differential between the out medium and the superabsorbent network decreases. As a result, water molecules go out of the superabsorbent and shrink57.
pH-Sensitivity behavior of superabsorbent
The sensitivity to pH was determined by analyzing the water absorbency capacities of p(AA-co-AM-co-MA) superabsorbent in different pH solutions (Fig. 10a). As the water absorbency capacity of the superabsorbent is affected by the ionic strength of the solution, buffer solutions were not utilized. Therefore, NaOH solution (alkaline pH), HCl solution (acidic pH), and DW were used to adjust the pH value of the final solution. The changes in the amount of water absorbed can be explained by the repulsion of –NH3+ groups in acidic media and –COO- groups in basic media. With increasing the pH from 2 to 7, the water absorbency capacity increases sharply, then from 7–8 decreased and at pH 8 to 9 is almost constant, and it decreases intensely from pH 9 to 14. Water absorbency is negligible in acidic conditions due to protonation of most –COO− groups and conversion to –COOH groups. Thus, anion–anion repulsive force weakens, and the network shrinks. Furthermore, hydrogen bonding interactions are enhanced due to more protonation, and the extra physical cross-linking in the polymer network is generated, leading to a decrease in water absorbency capacity56. As well as in acidic conditions, the charge-screening effect of the Cl- counter ions shielded the ammonium cations in the polymeric network and hindered an effective repulsion56. With increasing pH value, the water absorbency capacities increased since the electrostatic repulsions between carboxylate groups were enhanced. Thus, the polymeric network is expanded, and water absorbency capacities achieve maximum value at pH = 6. However, most acidic groups (–COOH) and basic groups (–CONH2) are non-ionized forms in the pH range of 7–9. Thus, hydrogen bonds between carboxylic acid and amide groups likely cause a kind of cross-linking, and as a consequence, the capacity to absorb water decreases. In highly alkaline solutions (pH > 10.0), the water absorbency capacity decreases since the excess Na+ cations prevent electrostatic repulsive interactions58.
The modulation of the pH values in which the superabsorbent presents a shrinking/swelling state can be used for controlled drug delivery systems. As shown in Fig. 10b, the p(AA-co-AM-co-MA) swollen superabsorbent in pH 6.0 solution shrinks quickly in solution with pH 3.0, and an outstanding reversible on/off switch is achieved within 15 min. This on–off switching was done even after three swelling-shrinking cycles, but the p(AA-co-AM) superabsorbent did not respond to pH media59.
Thermo-sensitivity behavior of superabsorbent
Studying modulation behavior is an essential for the potential use of superabsorbents in various applications. Figure 11a,b illustrates the swelling-shrinking behavior of the prepared superabsorbent when subjected to two temperatures of 25 and 50 °C for 24 h in either DW or SCS. From the results can be found that water absorbency in both solutions at 25 °C was higher than 50 °C, whereas the p(AA-co-AM) superabsorbent did not respond to temperature stimulus. This water absorbency reduction can be attributed to destroying hydrogen bonds between hydroxyl and carboxyl groups in a superabsorbent network structure by water molecules25. Also, it may be that by expansion of the polymer chains in the swollen state, the water molecules can remove from the polymeric network structure easily7.
Conclusion
The goal of this work was to prepare an efficient smart p(AA-co-AM-co-MA) superabsorbent with introducing maleic acid as a new structure in the synthesis of smart superabsorbents. The different analyses confirmed exactly the occurrence of the copolymerization process. Important factors in every superabsorbent are absorption aptitude, rate, strength, and retention time. In this superabsorbent, these factors are very good compared to other reported superabsorbents. The maximum water absorption capacity in DW and SCS is 1348 g/g and 106 g/g, respectively. Absorption capacity, especially in physiological solutions such as urea (1627) and D-glucose (1127) is very higher than most of the reported amount in the literature. The superabsorbent showed a good absorption rate in both DW and saline solutions. Kinetic model of water absorbency at the initial step in DW and SCS followed the mechanism of the non-Fickian type and dominant Fickian diffusion, respectively. Also, the experimental results were in good agreement with the pseudo-second-order kinetic models. The rheology analysis confirmed the preservation of the shape and strength of the superabsorbent. In regard responsibility properties of the superabsorbent, consequences showed well on–off switching reversibility behavior in salt, pH, and temperature solutions. Also, the superabsorbent was recycled and reused Five times without substantially decreasing water absorbency capacity. This superabsorbent with faster swelling, the ability to retain a large amount of water, and the ability to respond to multistimuli could be useful for a wide variety of applications, from agriculture to biomedicine fields.
Data availability
All data generated or analyzed during this study are included in this published article [its supplementary information files].
References
Chawla, P., Ranjan Srivastava, A., Pandey, P. & Chawla, V. Hydrogels: A journey from diapers to gene delivery. Mini Rev. Med. Chem. 14, 154–167 (2014).
Wang, X. et al. Highly efficient adsorption of ammonium onto palygorskite nanocomposite and evaluation of its recovery as a multifunctional slow-release fertilizer. Chem. Eng. Sci. 252, 404–414 (2014).
Paulino, A. T., Belfiore, L. A., Kubota, L. T., Muniz, E. C. & Tambourgi, E. B. Efficiency of hydrogels based on natural polysaccharides in the removal of Cd2+ ions from aqueous solutions. Chem. Eng. Sci. 168, 68–76 (2011).
Stalling, S. S., Akintoye, S. O. & Nicoll, S. B. Development of photocrosslinked methylcellulose hydrogels for soft tissue reconstruction. Acta Biomater. 5, 1911–1918 (2009).
Dalida, M. L. P. et al. Adsorptive removal of Cu(II) from aqueous solutions using non-crosslinked and crosslinked chitosan-coated bentonite beads. Desalination 275, 154–159 (2011).
Singh, B., Chauhan, N. & Kumar, S. Radiation crosslinked psyllium and polyacrylic acid based hydrogels for use in colon specific drug delivery. Carbohydr. Polym. 73, 446–455 (2008).
Huang, Y., Zhang, B., Xu, G. & Hao, W. Swelling behaviours and mechanical properties of silk fibroin–polyurethane composite hydrogels. Compos. Sci. Technol. 84, 15–22 (2013).
Anil, I., Gunday, S. T., Alagha, O. & Bozkurt, A. Synthesis, characterization, and swelling behaviors of poly (acrylic acid-co-acrylamide)/pozzolan superabsorbent polymers. J. Polym. Environ. 27, 1086–1095 (2019).
Zhang, M. et al. Synthesis, characterization, and swelling behaviors of salt-sensitive maize bran–poly (acrylic acid) superabsorbent hydrogel. J. Agric. Food Chem. 62, 8867–8874 (2014).
Joshi, S. J. & Abed, R. M. Biodegradation of polyacrylamide and its derivatives. Environ. Process. 4, 463–476 (2017).
Ilgin, P., Durak, H. & Gür, A. A novel pH-responsive p (AAm-co-METAC)/MMT composite hydrogel: Synthesis, characterization and its absorption performance on heavy metal ions. Polym. Plast. Technol. Eng. 54, 603–615 (2015).
Tuncel, A. T. et al. Maleic acid-but not structurally related methylmalonic acid-interrupts energy metabolism by impaired calcium homeostasis. PLoS ONE 10, e0128770 (2015).
Alpaslan, D., Erşen Dudu, T., Kubilay, Ş & Aktaş, N. Synthesis and characterization of biocompatible poly (maleic acid-co-citric acid) microparticles as a smart carrier for thiamine. Polym. Bull. 78, 6305–6320 (2021).
Samal, S. K., Dash, M., Dubruel, P. & Van Vlierberghe, S. (2014). Smart polymer hydrogels: properties, synthesis and applications. In Smart polymers and their applications 237–270
Peppas, N. A. & Khare, A. R. Preparation, structure and diffusional behavior of hydrogels in controlled release. Adv. Drug Deliv. Rev. 11, 1–35 (1993).
Sikdar, P. et al. Recent advances in the synthesis of smart hydrogels. Mater. Adv. 2, 4532–4573 (2021).
Li, L., He, Y., Zheng, X., Yi, L. & Nian, W. Progress on preparation of pH/temperature-sensitive intelligent hydrogels and applications in target transport and controlled release of drugs. Int. J. Polym. Sci. https://doi.org/10.1155/2021/1340538 (2021).
Katono, H., Sanui, K., Ogata, N., Okano, T. & Sakurai, Y. Drug release off behavior and deswelling kinetics of thermo-responsive IPNs composed of poly (acrylamide-co-butyl methacrylate) and poly (acrylic acid). Polym. J. 23, 1179–1189 (1991).
Lue, S. J., Chen, C.-H. & Shih, C.-M. Tuning of lower critical solution temperature (LCST) of poly (N-isopropylacrylamide-co-acrylic acid) hydrogels. J. Macromol. Sci. B. 50, 563–579 (2011).
Klouda, L. & Mikos, A. G. Thermoresponsive hydrogels in biomedical applications. J. Pharm. Biopharm. 68, 34–45 (2008).
Kocak, G., Tuncer, C. & Bütün, V. pH-Responsive polymers. Polym. Chem. 8, 144–176 (2017).
Reyes-Ortega, F. (2014). pH-responsive polymers: properties, synthesis and applications. In Smart polymers and their applications 45–92
Dai, S., Ravi, P. & Tam, K. C. pH-Responsive polymers: Synthesis, properties and applications. Soft Matter 4, 435–449 (2008).
Dai, H. & Huang, H. Enhanced swelling and responsive properties of pineapple peel carboxymethyl cellulose-g-poly (acrylic acid-co-acrylamide) superabsorbent hydrogel by the introduction of carclazyte. J. Agric. Food Chem. 65, 565–574 (2017).
Wu, Y. et al. A green route to prepare fluorescent and absorbent nano-hybrid hydrogel for water detection. Sci. Rep. 7, 1–11 (2017).
Makhado, E., Pandey, S., Nomngongo, P. N. & Ramontja, J. Fast microwave-assisted green synthesis of xanthan gum grafted acrylic acid for enhanced methylene blue dye removal from aqueous solution. Carbohydr. Polym. 176, 315–326 (2017).
He, G. et al. Preparation and properties of quaternary ammonium chitosan-g-poly (acrylic acid-co-acrylamide) superabsorbent hydrogels. React. Funct. Polym. 111, 14–21 (2017).
Sawut, A., Yimit, M., Sun, W. & Nurulla, I. Photopolymerisation and characterization of maleylatedcellulose-g-poly (acrylic acid) superabsorbent polymer. Carbohydr. Polym. 101, 231–239 (2014).
Liu, M., Liang, R., Zhan, F., Liu, Z. & Niu, A. Synthesis of a slow-release and superabsorbent nitrogen fertilizer and its properties. Polym. Adv. Technol. 17, 430–438 (2006).
Pourjavadi, A., Barzegar, S. & Mahdavinia, G. MBA-crosslinked Na-Alg/CMC as a smart full-polysaccharide superabsorbent hydrogels. Carbohydr. Polym. 66, 386–395 (2006).
Hebeish, A., Elrafie, M., Rabie, A., Aly, A. & Refaat, D. Synthesis and properties of superabsorbent carboxymethyl cellulose graft-poly (acrylic acid-co-acrylamide). Egypt J. Chem. 58, 721–739 (2015).
Liu, M., Liang, R., Zhan, F., Liu, Z. & Niu, A. Preparation of superabsorbent slow release nitrogen fertilizer by inverse suspension polymerization. Polym. Int. 56, 729–737 (2007).
Wu, F., Zhang, Y., Liu, L. & Yao, J. Synthesis and characterization of a novel cellulose-g-poly (acrylic acid-co-acrylamide) superabsorbent composite based on flax yarn waste. Carbohydr. Polym. 87, 2519–2525 (2012).
Murali Mohan, Y., Sudhakar, K., Keshava Murthy, P. & Mohan Raju, K. Swelling properties of chemically crosslinked poly (acrylamide-co-maleic acid) hydrogels. Int. J. Polym. Mater. 55, 513–536 (2006).
Cheng, D. et al. Preparation of low cost superabsorbent hydrogel by urea and acrylic acid. Mater. Lett. 204, 16–18 (2017).
Mizuguchi, M., Nara, M., Kawano, K. & Nitta, K. FT-IR study of the Ca2+-binding to bovine α-lactalbumin: Relationships between the type of coordination and characteristics of the bands due to the Asp COO− groups in the Ca2+-binding site. FEBS Lett. 417, 153–156 (1997).
Xie, L., Liu, M., Ni, B. & Wang, Y. Utilization of wheat straw for the preparation of coated controlled-release fertilizer with the function of water retention. J. Agric. Food Chem. 60, 6921–6928 (2012).
Zou, W. et al. Effects of amylose/amylopectin ratio on starch-based superabsorbent polymers. Carbohydr. Polym. 87, 1583–1588 (2012).
Świtała-Żeliazkow, M. Thermal degradation of copolymers of styrene with dicarboxylic acids–II: Copolymers obtained by radical copolymerisation of styrene with maleic acid or fumaric acid. Polym. Degrad. Stab. 91, 1233–1239 (2006).
Liu, Z., Miao, Y., Wang, Z. & Yin, G. Synthesis and characterization of a novel super-absorbent based on chemically modified pulverized wheat straw and acrylic acid. Carbohydr. Polym. 77, 131–135 (2009).
Wang, W. & Wang, A. Preparation, characterization and properties of superabsorbent nanocomposites based on natural guar gum and modified rectorite. Carbohydr. Polym. 77, 891–897 (2009).
Wen, P. et al. Rapid synthesis of a corncob-based semi-interpenetrating polymer network slow-release nitrogen fertilizer by microwave irradiation to control water and nutrient losses. Arab. J. Chem. 10, 922–934 (2017).
Guilherme, M. R. et al. Morphology and water affinity of superabsorbent hydrogels composed of methacrylated cashew gum and acrylamide with good mechanical properties. Polymer 46, 7867–7873 (2005).
Seetapan, N., Wongsawaeng, J. & Kiatkamjornwong, S. Gel strength and swelling of acrylamide-protic acid superabsorbent copolymers. Polym. Adv. Technol. 22, 1685–1695 (2011).
Pourjavadi, A. & Hosseinzadeh, H. Synthesis and properties of partially hydrolyzed acrylonitrile-co-acrylamide superabsorbent hydrogel. Bull. Korean Chem. Soc. 31, 3163–3172 (2010).
Parvathy, P. C. & Jyothi, A. N. Rheological and thermal properties of saponified cassava starch-g-poly (acrylamide) superabsorbent polymers varying in grafting parameters and absorbency. J. Appl. Polym. Sci. 131, 40368 (2014).
Ramazani-Harandi, M., Zohuriaan-Mehr, M., Yousefi, A., Ershad-Langroudi, A. & Kabiri, K. Rheological determination of the swollen gel strength of superabsorbent polymer hydrogels. Polym. Test. 25, 470–474 (2006).
Li, Q. et al. Synthesis, characterization and swelling behavior of superabsorbent wheat straw graft copolymers. Bioresour. Technol. 118, 204–209 (2012).
Zhou, Y., Fu, S., Zhang, L. & Zhan, H. Superabsorbent nanocomposite hydrogels made of carboxylated cellulose nanofibrils and CMC-gp (AA-co-AM). Carbohydr. Polym. 97, 429–435 (2013).
Etminani-Isfahani, N., Mohammadbagheri, Z. & Rahmati, A. 4-(6-Aminohexyl) amino-4-oxo-2-butenoic acid as a novel hydrophilic monomer for synthesis of cellulose-based superabsorbents with high water absorption capacity. Carbohydr. Polym. 250, 116959 (2020).
Xu, X., Bai, B., Ding, C., Wang, H. & Suo, Y. Synthesis and properties of an ecofriendly superabsorbent composite by grafting the poly (acrylic acid) onto the surface of dopamine-coated sea buckthorn branches. Ind. Eng. Chem. Res. 54, 3268–3278 (2015).
Tanan, W., Panichpakdee, J. & Saengsuwan, S. Novel biodegradable hydrogel based on natural polymers: Synthesis, characterization, swelling/reswelling and biodegradability. Eur. Polym. J. 112, 678–687 (2019).
Zhao, Y., Kang, J. & Tan, T. Salt-, pH- and temperature-responsive semi-interpenetrating polymer network hydrogel based on poly (aspartic acid) and poly (acrylic acid). Polymer 47, 7702–7710 (2006).
Barbucci, R., Magnani, A. & Consumi, M. Swelling behavior of carboxymethylcellulose hydrogels in relation to cross-linking, pH, and charge density. Macromolecules 33, 7475–7480 (2000).
Kim, S. J., Shin, S. R., Shin, D. I., Kim, I. Y. & Kim, S. I. Synthesis and characteristics of semi-interpenetrating polymer network hydrogels based on chitosan and poly (hydroxy ethyl methacrylate). J. Appl. Polym. Sci. 96, 86–92 (2005).
Mahdavinia, G. R., Zohuriaan-Mehr, M. J. & Pourjavadi, A. Modified chitosan III, superabsorbency, salt-and pH-sensitivity of smart ampholytic hydrogels from chitosan-g-PAN. Polym. Adv. Technol. 15, 173–180 (2004).
Pass, G., Phillips, G. & Wedlock, D. Interaction of univalent and divalent cations with carrageenans in aqueous solution. Macromolecules 10, 197–201 (1977).
Lee, W. F. & Wu, R. J. Superabsorbent polymeric materials. I. Swelling behaviors of crosslinked poly (sodium acrylate-co-hydroxyethyl methacrylate) in aqueous salt solution. J. Appl. Polym. Sci. 62, 1099–1114 (1996).
Shi, X. & Wang, A. Development of a superporous hydroxyethyl cellulose-based hydrogel by anionic surfactant micelle templating with fast swelling and superabsorbent properties. J. Appl. Polym. Sci. 132, 42027 (2015).
Acknowledgements
This work was carried out with the support Research Council of the University of Isfahan.
Author information
Authors and Affiliations
Contributions
F.J.: Conducting experiments, writing of original draft, formal analysis. N.E.-E.: Investigation, writing of original draft, resources, software, drawing all shapes, drawing tables. A.R.: Review and editing, supervision, conceptualization. All authors reviewed the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jamali, F., Etminani-Esfahani, N. & Rahmati, A. Maleic acid as an important monomer in synthesis of stimuli-responsive poly(acrylic acid-co-acrylamide-co-maleic acid) superabsorbent polymer. Sci Rep 13, 3511 (2023). https://doi.org/10.1038/s41598-023-30558-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-30558-3
- Springer Nature Limited