Abstract
In Japan, mean birth weight has significantly decreased from 3152 g in 1979 to 3018 g in 2010 and the prevalence of preterm birth (PTB) has risen to 5.7% in the last thirty years. However, the presence and magnitude of geographical differences in low birthweight (LBW) and/or PTB in Japan is not well understood. We implemented spatial analysis to identify localized clusters and hot spots of LBW and/or PTB during 2012–2016. The Japan national birth database was used in this study. A total of 5,041,685 (male: 2,587,415, female: 2,454,270) births were used for spatial analysis using empirical Bayes estimates of the incidence rate of LBW and/or PTB and spatial scan tests to detect hot-spot areas with p values calculated from Monte Carlo iterations. The most and second likely clusters were located in two areas: (1) the small islands in south-west Japan (Amami and Okinawa, Relative risk = 1.09–1.67 with p < 0.001) and (2) the cities on the base of Mt. Fuji, stretching over three neighboring prefectures of Yamanashi, Shizuoka and Kanagawa (Relative risk = 1.10–1.55 with p < 0.001), respectively. We need to optimize the medical resource allocations based on the evidence in geographical clustering of LBW and/or PTB at specific locations in Japan.
Similar content being viewed by others
Introduction
Low birth weight (LBW) is defined by the World Health Organization (WHO) as birth weight less than 2500 g (5.5 lb) irrespective of gestational age. LBW is an important global health issue, having both short- and long-term health consequences. LBW newborns who survive have a higher risk of short-term and long-term morbidities, including non-communicable diseases such as diabetes, hypertension and other cardiovascular diseases1. LBW is the leading cause of death in under-five children worldwide. It accounts for 80% of all neonatal deaths, of which two-thirds are preterm birth (PTB)2,3,4,5,6. Around 15 million PTB and more than 20 million LBW, which is one in seven of all births in the world, were observed globally in 2014 and 2015, respectively7,8. In 2012, a Comprehensive Implementation Plan on Maternal, Infant and Young Child Nutrition was introduced by the World Health Assembly and all member states committed to achieve a global nutrition target of a 30% reduction in the number of infants with LBW by 2025, relative a 2012 baseline9. Recent estimates found that the global LBW incidence has fallen slightly from 17.5% in 2000 to 14.6% in 20157, while the incidence of PTB has increased from 9.8 to 10.6% over the period8. Further, progress toward the target is quite slow in high-income countries such as the UK, Finland, France, Germany, the USA, Australia, and New Zealand7,8. To meet the global LBW target by the year of 2025, progress must more than double, and improved measurement and program investment to address the causes of LBW throughout the lifecycle is urgently required7.
Despite having an advanced healthcare system, Japan has the second highest prevalence of LBW among the Organization for Economic Cooperation and Development (OECD) countries. In Japan, the prevalence of LBW has increased from 8.6% in 2000 to 9.5% in 20157. The mean birthweight of singleton infants in Japan has significantly decreased from 3152 g in 1979 to 3018 g in 201010. PTB prevalence has also increased to 5.7% in the last thirty years8. Several studies reported the trends and maternal risk factors, including parental socio-demographic and economic factors, for LBW and PTB in Japan10,11,12,13,14,15,16,17. However, there is a lack of evidence of identifying the geographical clusters of LBW and/or PTB in Japan, even though geographical disparities have been identified in other countries and may play an important role in the persistence of PTB and LBW in developed nations18,19,20. To the best of our knowledge, only one study explored spatial patterns of LBW in a single administrative area of Japan and reported some LBW prevalent clusters21. Geographical analysis for identifying geographical clusters of LBW and/or PTB helps policymakers to identify priority areas of action as well as boosting public health promotion policies for addressing health issues in those particular areas. This study explores the spatial variation in LBW and/or PTB across all areas in Japan by identifying clusters with statistically significantly elevated or reduced incidence of these events, using a robust national dataset.
Methods
Dataset, exposure and outcomes
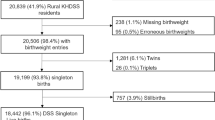
Birth certificate data was obtained from the Ministry of Health, Labor and Welfare in Japan vital registration statistics22. Based on the Guidelines for Statistics Law promulgated by the Ministry of Internal Affairs, our application documents for the secondary use of the Vital statistics were submitted to the MHLW and approved. Data from January 1st, 2012 to December 31st, 2016 was extracted for our analysis. In addition to the individual’s birthweight, maternal age, residential address and gestational age were extracted. We excluded multiple births to avoid the bias of a higher risk of LBW for multiple births10. Every infant was categorized as LBW (birthweight < 2500 g) or normal birthweight (NBW) group (birthweight ≥ 2500 g). In addition, they were also categorized as PTB (gestational age of < 37 weeks) or term birth (gestational age of ≥ 37 weeks). Consequently, the following four groups were considered in this study: LBW, PTB, LBW and term birth (LBW term) and LBW and preterm birth (LBW pre). Since we used national data that the medical profession is required to input at birth, there are essentially no (or at least very small) missing values. If there were missing values, they were dropped.
Statistical/geographical analysis
Japan was divided into 1902 municipalities at the time of the study, organized within 47 prefecture23. Geographical analysis in this study was conducted at the municipality level. Geographical coordinates defined as the centroid of the area (measured by latitude and longitude) were extracted from map data for each municipality (https://nlftp.mlit.go.jp/ksj/jpgis/datalist/KsjTmplt-N03.html). Empirical Bayes estimates of the age-standardized incidence rate (EBSIR) were used to examine how LBW and/or PTB were geographically distributed in Japan during the study period24,25. The age-standardization for each municipality was implemented based on the age structure of Japanese population as a reference: the age categories were ≤ 15, (15,20], (20,25], (25,30], (30,35], (35,40], (40,45], (45,50] and ≥ 50. In addition, when no birth was observed among certain age groups in a small municipality, we imputed a very small value (i.e., 0.0001) for calculating the age-standardized value. We first calculated the age-standardized values using a direct method, and then the Empirical Bayes estimates was calculated. The advantage of EBSIR is that it can incorporate the information of the spatial neighborhood areas to smooth the risk of LBW and/or preterm birth toward the local (spatial) neighborhood mean, stabilizing estimates for municipalities with small numbers of births based on updated information from neighboring municipalities24,25,26. The spatial neighborhood was defined as the queen contiguity where spatial neighbors shared at least a common border. The local adjacency matrix was calculated based on the k-nearest neighborhood method with k = 8.
Spatial clusters were defined following: “a geographically bounded group of occurrences of sufficient size and concentration to be unlikely to have occurred by chance”27. Tango’s statistic for general clustering was used to globally test the existence of clusters in Japan28. Then, to identify the exact locations of geographical clusters of LBW and/or PTB in Japan, the spatial scan statistic and its associated likelihood-based test proposed by Kulldorff and Nagarwalla (1995) were implemented29,30. The test can identify spatial clusters of any size, located anywhere in the study region. Under the assumption of a Poisson distribution for the occurrence of LBW and/or PTB, the most/second/third likely clusters (MLC/SLC/TLC) were defined by maximum likelihood estimation31. To obtain the corresponding p values, 1000 Monte Carlo (resampling with replacement) iterations were implemented29,30. Data management was done by using AWK, SQL and Python and statistical analysis was performed with R version 3.6.1. In this paper, statistical significance was defined as a p value less than 0.05. This study was approved by the Ethics Committee at National Center for Child Health and Development (No. 1274). Informed consent is waived by the Ethics Committee at National Center for Child Health and Development. All methods were carried out in accordance with relevant guidelines and regulations.
Results
Study samples
A total of 5,041,685 (male: 2,587,415, female: 2,454,270) births were reported in Japan from 2012 to 2016. Among them, 8.23%, 4.74%, 5.23%, and 3.00% were categorized as LBW, PTB, LBWat term, and LBWbut premature, respectively. The reported proportions of births by maternal age were 2.21%, 76.64% and 21.1% for the age of ≤ 20, (20, 35] and > 35, respectively. The detailed characteristics are shown in Table 1 and Fig. 1 shows the scatter plot of birthweight and gestational age.
Geographical distributions
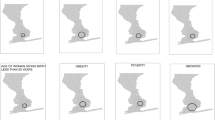
Figure 2 shows the Japanese map and indicates some key locations. The geographical distributions of the EBSIR of LBW and/or PTB were estimated for all women (Fig. 3) and separately by age groups (Fig. 4). The test of the homogeneity of the relative risk measured by Tango’s statistics showed that there was a significant heterogeneity of risk in all groups (p < 0.001 for all sub-groups). Tables 2, 3 and 4 and Supplemental Figs. 1, 2 and 3 show the approximate locations of MLC/SLC/TLC of LBW and/or PTB. As shown in Tables 2, 3, and 4, the most frequently detected area in several sub-groups was an archipelago in the south-west part of Japan (Area 1: A1, A1_1, A2, A2_2, A3, A3_1, A3_2, A3_3, B1_2, and C2_2. Relative risk = 1.09–1.67 with p < 0.001). The second most frequently detected area was highland cities around the north-east part of Mt. Fuji (Area 2: A2_2, B1, B1_2, B1_3, B2, B2_1, B2_3, and C3_3. Relative risk = 1.10–1.55 with p < 0.001). The third frequently detected area was cities around the Kanmon-Kaikyo strait that separates the main island and Kyushu island (Area 3: B2_2, C1, C1_2, C2, C3, and C3_2. Relative risk = 1.08–1.32 with p < 0.001). It is noteworthy that we observed a distinctive difference in the locations of the clusters between LBW term and LBW pre: LBW infants at term were likely to be clustered around the north-east part of Mt. Fuji (A2_2, B2_1, B2_3, and C2, which were partially detected in Area 1), while LBW and premature infants were likely to be clustered around Tohoku or the north part of the Kanto Plain (B3, B3_2, B3_3, and C3_1).
Empirical Bayes estimates of incidence rate of LBW (A, upper), LBW term (B, middle) and LBW pre (C, lower) separately by age groups (20 years or less, 20 and less than 35 years, and over 35 years) in Japan: color scale indicates quantile. This map was created using R version 3.6.1 (https://cran.r-project.org/) and zipangu package.
Discussion
The study revealed the geographical distribution of LBW and/or PTB in Japan from 2012 to 2016 using population-based data. To the best of our knowledge, this is the first study to identify geographical clusters of LBW and PTB among Japanese singleton live-birth infants using spatial analytical techniques at the municipality level. In this study, we identified several characteristic areas, generally called ‘hot spots’, in an archipelago of small islands in south-west Japan (Amami and Okinawa), and a cluster of cities on the base of Mt. Fuji, stretching over three neighboring prefectures of Yamanashi, Shizuoka and Kanagawa. Although we identified more than two geographical clusters, we confine our discussion to these two areas because they were frequently identified in several sub-groups (Tables 2, 3, 4). In Area 1, both term and premature low birth weight incidence were highly clustered. It is well known that Okinawa prefecture, which is the center of Area 1, has had an incidence rate of LBW that is consistently 20% higher than the rest of Japan since the 1970s32. Previous studies have reported that residents in Area 1 have relatively higher values of risks factors for poor birth outcomes, compared with other areas, such as high smoking rates among pregnant women33 and low parental socioeconomic status (SES), including low household income34. Area 1 has the lowest percentage of students attending college or university in Japan, and these low education levels might have a significant impact on the incidence of LBW35. Finally, Area 1 is located far from the main islands of Japan. Residents of Area 1 are genetically isolated from the major island of Japan36, and thus one of the reasons for the regional difference might be a genetic issue.
In contrast, in Area 2 LBW was only highly clustered among pregnancies brought to term. Residents in Area 2, especially the eastern part of Shizuoka prefecture, which is the center of Area 2, have limited access to necessary antenatal care: the number of obstetricians and gynecologists here is relatively small (7.4 in 2012 compared to a Japanese average of 8.6), the number of hospitals or clinics for delivery has reduced from 141 to 93 between 1995 and 201037. Further, there is a military training ground of the Japan Ground Self-Defense Force, which is the largest training ground in the main islands of Japan. It implicates that noise and vibration from the military ground and air crafts might influence maternal health38,39,40. Additionally, Modzelewska et al. (2019) and Okubo et al. (2015) provided the association between green tea intake, which contains high caffeine, and poor birth outcomes (especially PTB)41,42. Green tea is also one of the products with high pesticide residue contain. There is a report that infants with low birth weight have higher contain of pesticide43. It is well known that residents in Area 2 have high green tea intake in Japan. The high intake of green tea might be possible epidemiological explanation for Area 2 although there was no available data about the intake of green tea in pregnant women. Our study is the first evidence that Area 2 is a geographically clustered area of LBW term. However, since our discussion here still includes speculations and not based on the specific data, further research will be needed to identify the characteristic risk factors specific to this area.
The present study is limited by the unavailability of information regarding obstetric, genetic, demographic, cultural, and socioeconomic factors of the parents and their children. Although we used a population-based birth certificate dataset that includes all live births in Japan during the study period, the number of available variables in the dataset was limited to residential addresses, the place of birth, birthweight, sex of infant, maternal age, and gestational age at birth. Therefore, further examination would be required to identify the potential risk factors and predictors that can explain the geographical clusters of LBW and/or PTB in Japan. To address this, we are collecting more detailed information such as maternal BMI, smoking status, alcohol consumption, pregnancy complication, family history, and the quality of hospitals as part of an ongoing study into geographical clustering of birth outcomes. Identification of further risk factors and predictors for the geographical clusters could encourage the future development of effective strategies to promote the prevention of LBW and/or PTB in Japan. In addition, this study excluded multiple births, which are high-risk group and requires special attention. Since these births have different pathology, they are excluded from this study, but the current geographical distribution should be also examined carefully to guarantee the optimal allocation of medical resources. It is our ongoing study.
In conclusion, this spatial analysis identified several geographical clusters of term or preterm low birthweight in Japan during the year of 2012–2016. The results can provide a new insight for future genetic, environmental and epidemiological studies on LBW in Japan. It is important to carefully continue monitor the detected areas to ensure that they can receive all the benefits of Japan’s modern health system, and help infants born here to achieve the same health outcomes as the rest of Japan. Moreover, this study showed that even in Japan, a (relatively) homogeneous country, there exist geographical clusters of low birthweight. In addition to standard measures targeting known risk factors of LBW, we suggest that geographical factors should be assessed more in the future.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Gluckman, P. D., Hanson, M. A. & Beedle, A. S. Early life events and their consequences for later disease: A life history and evolutionary perspective. Am. J. Hum. Biol. 19, 1–19 (2007).
Blencowe, H. et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. The Lancet 379, 2162–2172 (2012).
Katz, J. et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: A pooled country analysis. The Lancet 382, 417–425 (2013).
Lee, A. C. et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob. Health 1, e26–e36 (2013).
Lawn, J. E. et al. Every Newborn: progress, priorities, and potential beyond survival. The Lancet 384, 189–205 (2014).
You, D., New, J. & Wardlaw, T. Levels and trends in child mortality. Report 2015. Estimates developed by the UN inter-agency Group for Child Mortality Estimation. (United Nations Children’s Fund; 2015, 2017).
Blencowe, H., Krasevec, J., de Onis, M. & st al.,. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: A systematic analysis. Lancet Glob. Health 7, e849–e860 (2019).
Chawanpaiboon, S. et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 7, e37–e46 (2019).
McGuire, S. World Health Organization. Comprehensive implementation plan on maternal, infant, and young child nutrition. Geneva, Switzerland, 2014. Adv. Nutr. 6, 134–135 (2015).
Takemoto, Y., Ota, E., Yoneoka, D., Mori, R. & Takeda, S. Japanese secular trends in birthweight and the prevalence of low birthweight infants during the last three decades: A population-based study. Sci. Rep. 6, 31396 (2016).
Tamura, N. et al. Different risk factors for very low birth weight, term-small-for-gestational-age, or preterm birth in Japan. Int. J. Environ. Res. Public Health 15, 369 (2018).
OECD. OECD family database. The structures of families. (2014)
Kamiya, K. Maternal and Child Health Statistics in Japan (Mothers’ and Children’s Health and Welfare Association, 2013).
Goldenberg, R. L., Culhane, J. F., Iams, J. D. & Romero, R. Epidemiology and causes of preterm birth. The Lancet 371, 75–84 (2008).
McCowan, L. & Horgan, R. P. Risk factors for small for gestational age infants. Best Pract. Res. Clin. Obstet. Gynaecol. 23, 779–793 (2009).
Pfinder, M., Kunst, A. E., Feldmann, R., van Eijsden, M. & Vrijkotte, T. G. Preterm birth and small for gestational age in relation to alcohol consumption during pregnancy: Stronger associations among vulnerable women? Results from two large Western-European studies. BMC Pregnancy Childbirth 13, 49 (2013).
Blumenshine, P., Egerter, S., Barclay, C. J., Cubbin, C. & Braveman, P. A. Socioeconomic disparities in adverse birth outcomes: A systematic review. Am. J. Prev. Med. 39, 263–272 (2010).
Insaf, T. Z. & Talbot, T. Identifying areas at risk of low birth weight using spatial epidemiology: A small area surveillance study. Prev. Med. 88, 108–114 (2016).
Tu, W., Tedders, S. & Tian, J. An exploratory spatial data analysis of low birth weight prevalence in Georgia. Appl. Geogr. 32, 195–207 (2012).
Pattenden, S., Casson, K., Cook, S. & Dolk, H. Geographical variation in infant mortality, stillbirth and low birth weight in Northern Ireland, 1992–2002. J. Epidemiol. Community Health 65, 1159–1165 (2011).
Serizawa, K. O. On the regional difference of low birth weight (Tei Syusei Taizyu Zi Syusei Ritu No Chiiki Sa Ni Kansuru Kentou). Kousei No Shihyo 62, 19–24 (2015).
Ministry of Health Labour and Welfare. Vital Statistics in Japan (Ministry of Health Labour and Welfare, 2019).
Ministry of Land I, Transport and Tourism. National Land Numerical Information. Ministry of Land, Infrastructure, Transport and Tourism (2019).
Marshall, R. J. Mapping disease and mortality rates using empirical Bayes estimators. J. R. Stat. Soc. Ser. C Appl. Stat. 40, 283–294 (1991).
Clayton, D. & Kaldor, J. Empirical Bayes estimates of age-standardized relative risks for use in disease mapping. Biometrics 43, 671–681 (1987).
Lawson, A. B. Bayesian Disease Mapping: Hierarchical Modeling in Spatial Epidemiology (Chapman and Hall/CRC, 2013).
Elliot, P. Methodology of enquiries into disease clustering. Small Area Health Statistics Unit, United Kingdom (1989).
Tango, T. A test for spatial disease clustering adjusted for multiple testing. Stat. Med. 19, 191–204 (2000).
Openshaw, S., Charlton, M., Wymer, C. & Craft, A. A mark 1 geographical analysis machine for the automated analysis of point data sets. Int. J. Geogr. Inf. Syst. 1, 335–358 (1987).
Kulldorff, M. & Nagarwalla, N. Spatial disease clusters: Detection and inference. Stat. Med. 14, 799–810 (1995).
Doi, Y. et al. Spatial clusters of Creutzfeldt–Jakob disease mortality in Japan between 1995 and 2004. Neuroepidemiology 30, 222–228 (2008).
Hokama, T. & Binns, C. Trends in the prevalence of low birth weight in Okinawa, Japan: A public health perspective. Acta Paediatr. 98, 242–246 (2009).
Miyake, Y., Tanaka, K. & Arakawa, M. Active and passive maternal smoking during pregnancy and birth outcomes: The Kyushu Okinawa maternal and child health study. BMC Pregnancy Childbirth 13, 157 (2013).
Yoneoka, D., Saito, E. & Nakaoka, S. New algorithm for constructing area-based index with geographical heterogeneities and variable selection: An application to gastric cancer screening. Sci. Rep. 6, 26582 (2016).
Fujiwara, T., Ito, J. & Kawachi, I. Income inequality, parental socioeconomic status, and birth outcomes in Japan. Am. J. Epidemiol. 177, 1042–1052 (2013).
Kato, N., Takimoto, H. & Eto, T. The regional difference in children’s physical growth between Yaeyama Islands of Okinawa Prefecture and national survey in Japan. J. Natl. Inst. Public Health 61, 448–453 (2012).
Mine, Y. & Babazono, A. Regional differences in perinatal mortality rates in Japan: An investigation based on vital statistics. Jpn. J. Hyg. 59, 342–348 (2004).
Matsui, T. et al. Association between the rates of low birth-weight and/or preterm infants and aircraft noise exposure. Nihon Eiseigaku Zasshi 58, 385–394 (2003).
Aydin, Y. & Kaltenbach, M. Noise perception, heart rate and blood pressure in relation to aircraft noise in the vicinity of the Frankfurt airport. Clin. Res. Cardiol. 96, 347–358 (2007).
Brown, A. L. & van Kamp, I. WHO environmental noise guidelines for the european region: A systematic review of transport noise interventions and their impacts on health. Int. J. Environ. Res. Public Health 14, 873 (2017).
Modzelewska, D. et al. Caffeine exposure during pregnancy, small for gestational age birth and neonatal outcome: Results from the Norwegian Mother and Child Cohort Study. BMC Pregnancy Childbirth 19, 80 (2019).
Okubo, H., Miyake, Y., Tanaka, K., Sasaki, S. & Hirota, Y. Maternal total caffeine intake, mainly from Japanese and Chinese tea, during pregnancy was associated with risk of preterm birth: The Osaka Maternal and Child Health Study. Nutr. Res. 35, 309–316 (2015).
Ichikawa, G. et al. LC-ESI/MS/MS analysis of neonicotinoids in urine of very low birth weight infants at birth. PLoS ONE 14, e0219208 (2019).
Funding
This research was partially supported by research grants from Japan Agency for Medical Research and Development (22rea522103h0001).
Author information
Authors and Affiliations
Contributions
D.Y. led the study. D.Y., O.R., Y.M., S.G. conception and design of the study; D.Y., O.R., Y.M., S.G. performed analysis; D.Y., T.Y., H.S., S.G., Y.Y., E.O. wrote the manuscript; D.Y., T.Y., H.S., Y.Y., E.O. draw figures All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rahman, M.O., Yoneoka, D., Murano, Y. et al. Detecting geographical clusters of low birth weight and/or preterm birth in Japan. Sci Rep 13, 1788 (2023). https://doi.org/10.1038/s41598-023-28642-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-28642-9
- Springer Nature Limited