Abstract
The COVID-19 pandemic outbreak led to a global ventilator shortage. Hence, various strategies for using a single ventilator to support multiple patients have been considered. A device called Ventil previously validated for independent lung ventilation was used in this study to evaluate its usability for shared ventilation. We performed experiments with a total number of 16 animals. Eight pairs of pigs were ventilated by a ventilator or anesthetic machine and by Ventil for up to 27 h. In one experiment, 200 ml of saline was introduced to one subject’s lungs to reduce their compliance. The experiments were analyzed in terms of arterial blood gases and respiratory parameters. In addition to the animal study, we performed a series of laboratory experiments with artificial lungs (ALs). The resistance and compliance of one AL (affected) were altered, while the tidal volume (TV) and peak pressure (Ppeak) in the second (unaffected) AL were analyzed. In addition, to assess the risk of transmission of pathogens between AL respiratory tracts, laboratory tests were performed using phantoms of virus particles. The physiological level of analyzed parameters in ventilated animals was maintained, except for CO2 tension, for which a permissive hypercapnia was indicated. Experiments did not lead to injuries in the animal’s lungs except for one subject, as indicated by CT scan analysis. In laboratory experiments, changes in TV and Ppeak in the unaffected AL were less than 11%, except for 2 cases where the TV change was 20%. No cross-contamination was found in simulations of pathogen transmission. We conclude that ventilation using Ventil can be considered safe in patients undergoing deep sedation without spontaneous breathing efforts.
Similar content being viewed by others
Introduction
In 2020, clinical centers around the world struggled with a shortage of ventilators due to the outbreak of COVID-191,2. In many cases, COVID-19 patients exhibit an atypical acute respiratory distress syndrome (ARDS)3, which leads to the necessity of mechanical ventilation. Thus, the global-scale problem of ventilator shortages is related to the sudden increase in the number of patients requiring long-term ventilatory therapy that has exceeded the number of ventilators available.
In several reports published several years ago, anesthesiologists considered solving the problem of a lack of ventilators by sharing a single ventilator for simultaneous therapy of two or even up to four patients4,5,6. However, this approach has been criticized as insufficient to maintain stable ventilation for each patient in the event of changes in lung mechanics in any of the ventilated patients7. Following the COVID-19 outbreak, various new configurations of ventilator sharing systems have been proposed and tested8,9,10,11,12. These systems provide varying degrees of control over ventilatory branches, delivering airflow to the patients. Most of these solutions use manually titrated pneumatic valves. However, these solutions increase patient monitoring efforts and do not guarantee safe, multiple patient ventilation. The risk associated with such a ventilatory strategy is relatively high; however, it can be considered a potential bridge to full ventilatory support of the patient4,11,13,14,15,16,17,18.
Another concept of shared ventilation is based on continuous automatic control of the tidal volume division between ventilated subjects27. This technical approach was initially applied for independent lung ventilation and validated in a series of laboratory experiments28,29 and clinical tests carried out in patients ventilated during thoracic surgeries30 or undergoing differential ventilation therapy in an intensive care unit31. Based on these studies, we developed a medical device called Ventil that could allow the ventilation of two patients with one ventilator. Therefore, the main goal of this study was to validate the usability of Ventil in shared ventilation using an animal model. The aim of the animal experiments was to evaluate arterial blood gases and respiratory parameters of ventilated subjects with different weights by means of a ventilator/anesthetic machine and Ventil. The shared ventilation approach carries the risk of mechanical ventilation alterations in one patient when the respiratory mechanics in the second patient change (respiratory deterioration or improvement). Therefore, supplementarily to animal experiments, the laboratory investigations were performed using artificial lungs (AL). The aim of laboratory tests was to evaluate alterations in tidal volume and inspiratory peak pressure of an unaffected ventilated AL when the mechanical properties of the respiratory system (resistance and compliance) were altered in the second (affected) AL.
Another clinical problem of shared ventilation is the risk of transmission of pathogens in the respiratory tract. Then, a research problem arises, resulting from a lack of fully defined methods that can be used to assess the transport of pathogens in the ventilation duct under laboratory conditions in a short time and from not considering the risk of biohazards. For pathogens such as viruses, the term “viral load”, expressed as a titer, is a numerical representation of the quantity of virus in a given volume of fluid in a sample, e.g., saliva, blood, plasma, etc. For viruses transmitted via airborne droplets, the most common method for viral titer estimation is quantitative PCR (qPCR) performed on nasopharyngeal swab samples. Despite the large body of evidence concerning SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness19 published to date, there are few or no data on the virus load required for human infection. Nonetheless, Han et al. showed that the average titer of the initial viral load obtained from swab specimens taken from asymptomatic children was ca. 2 × 106 genomic RNA copies per mL, whereas symptomatic children had an initial viral load as high as 1 × 10920.
Moreover, according to Chu et al., in the culture medium of Caco2 and Calu3 cells infected with SARS-CoV-2 virus, the number of virus genome copies 120 h after infection was determined to be 109 in 1 mL of supernatant21. The multiplicity of infection (MOI) was 0.1. In our studies using the viral phantom in the form of fluorescently labeled nanospheres, the estimated phantom titer was determined from an exponential pattern of viral reproduction in the respiratory system. Another published study showed that the minimal dose of MERS-CoV required to effectively infect a cell culture was 107 copies per mL22. In the case of SARS-CoV-2, no infection occurred with a titer below 106 copies per mL23, cycle threshold values higher than 24 in another study24 or cycle threshold values higher than 34 in other studies25,26. Thus, laboratory tests of pathogen transmission were performed. For the investigation of pathogen spread along the respiratory tract and contamination of the second tract, fluorescent nanospheres were used as a phantom of virus particles.
Methods
Ventil device
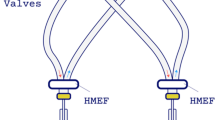
Ventil is a device initially developed for differential mechanical lung ventilation and can be used in one-lung ventilation as well as in the case of asymmetric lung pathologies. The apparatus splits the flow according to the selected ratio (by a division knob). However, it must be emphasized that Ventil is not a simple splitter of the flow from the ventilator. Rather, it is a device that can adjust minute ventilation, keeping the parameters stable regardless of changes in the air duct properties of the lungs. The division of the flow is stabilized by two flowmeters, continuously measuring the flows in output ports and sending these flow signals to the control system, which then corrects the splitting to maintain the flow division according to the selected ratio. External mechanical or electromagnetic (fixed or adjustable) positive end-expiratory pressure (PEEP) valves can be used with the Ventil. Therefore, the PEEP for both lungs can also be regulated. Ventil requires one-way valves to separate tracks (particular lung circuits) and protect them from gas mixing. The Ventil apparatus is placed in the breathing circuit between a ventilator and the ventilated subjects (as shown in Fig. 1 and S1). It does not communicate with the ventilator; thus, any kind of ventilating machine can be used without its modification. We manufactured a short series of 200 Ventil devices. Moreover, Ventil was certified for independent ventilation of two lungs in a single patient (manufacturer Łukasiewicz-ITAM, Zabrze, Poland). Technically, Ventil can be used to ventilate two patients instead of two lungs.
Ventilation of two pigs was performed with a Ventil and a ventilator. Animal experiment test stand. A, Ventil; B, ventilator or anesthetic machine; C, ventilated subject; D, patient monitor; E, adult polypropylene extendible limb 22F-22F; F, adult polypropylene anesthesia extendible breathing circuits with Y-pieces; G, antibacterial and antiviral filter; H, positive end-expiratory valve with one-way valve and set of connectors; green arrows—the flow direction during the inspiration; dark red arrows—the flow direction during the expiration.
The test stand during experiments on animals
Two Puritan Bennett 980 and 840 ventilators (Medtronic, Minneapolis, MN) and two Anastazja 7700 anesthesia ventilators (Farum Ltd., Warsaw, Poland) were used with two Ventil devices (serial numbers SN0022 and SN0024). Four patient monitors (Datex-Ohmeda S/5, GE Healthcare, Boston, MA) were used with the E-PRESTN module (GE Healthcare, Boston, MA). Three of them were also equipped with an E-CAIOV module (GE Healthcare, Boston, MA). Data from patient monitors were collected by Windows 10 PC-based computers. We calculated the median and interquartile range (IQR) values for the following continuously recorded signals during the experiments: oxygen saturation (SpO2), oxygen inspiratory fraction (FiO2), end-tidal carbon dioxide partial pressure (EtCO2), peak inspiratory pressure (PIP), positive end-expiratory pressure (PEEP), respiratory rate (RR), and tidal volume (TV) and calculated the following parameters: driving pressure (ΔP), minute ventilation (MV) and static compliance (Cst). The MV was an inspired tidal volume times RR. The driving pressure was calculated as the difference between the plateau pressure (Pplat) and the positive end-expiratory pressure (Pplat – PEEP). The static compliance was the ratio of the expired TV and the driving pressure. These 3 parameters were calculated per recorded sample. The statistics were processed for all pigs used in the experiments. The spirometric variables were not recorded by the fourth patient monitor due to the E-CAIO (GE Healthcare, Boston, MA) instead of the E-CAIOV module implemented (the E-CAIO module does not measure spirometric variables, in contrast to the E-CAIOV module). All recorded data were postprocessed by MATLAB R2019b and MS Excel 2019 software.
Two Ventil flowmeters (Ventil outputs) were connected with inspiratory arms of two adult polypropylene (PP) anesthesia extendible 60/180 cm breathing circuits with Y-pieces (Medtronic, Minneapolis, MN) by Fixed elbow 22 M—7.6 mm port—22 M/15F connectors (Intersurgical, New York, NY) and electrostatic antibacterial and antiviral Barrierbac S 22 M/15F filters (Medtronic, Minneapolis, MN) in series. The Y-pieces were connected to the animals by electrostatic filters (various vendors) and the patient-monitored probes. The animals' expiratory limps were connected to the electrostatic antibacterial and antiviral Barrierbac S 22 M/15F filters (Medtronic, Minneapolis, MN), one-way valves (Intersurgical, New York, NY), and 2.5 cmH2O PEEP valves (Intersurgical, New York, NY or Flexicare, Mountain Ash, Great Britain). The PEEP valve output was connected (through 30 M-22 M [Intersurgical, New York, NY] or 22 M-22 M/15F [R-Vent Medikal, İzmir, Turkey] connectors) by third adult PP anesthesia extensible 60/180 cm breathing circuits (Medtronic, Minneapolis, MN) to the ventilator (Y-piece site) by a 22F-22F connector (Intersurgical, New York, NY). The configuration is presented in Fig. 1.
Animal use protocol
We investigated 16 pigs in 8 pairs ventilated by the Ventil and a ventilator or an anesthetic machine (Fig. 1) set to volume-controlled ventilation modes. This animal study was approved by the First Bioethical Commission in the Ethics Committee for Animal Experiments in Warsaw (agreement no WAW2/047/2020). All methods were carried out in accordance with relevant guidelines and regulations and reported in accordance with ARRIVE guidelines (https://arriveguidelines.org). After obtaining approval, the following animals (pigs) were classified for testing: males and females of the Great White Polish Breed, aged approximately 3–6 months. The choice of pigs was based on the assumption of the similarity of body and lung mass and therefore ventilation parameters to those of humans, with a tidal volume of 6–8 ml/kg. Preparation for qualified animals was carried out according to an established schedule. Prior to intubation and mechanical ventilation, the animals received premedication: medetomidine 0.05–0.1 mg/kg (Cepetor 1 mg/ml, ScanVet Ltd., Warsaw, Poland), butorfanol 0.1–0.2 mg/kg (Butomidor 10 ml, Richter Pharma AG, Wels, Austria), and ketamine 5–10 mg/kg (Bioketan, Vetoquinol Biowet Ltd., Gorzów Wielkopolski, Poland) as an intramuscular injection (buttock muscles). After securing an intravenous line (BD Venflon 1.2 mm, Becton Dickinson, Franklin Lakes, NJ) with access to the posterior ear vein (vena auricularis posteriori), the animals were induced into general anesthesia with propofol (Scanofol 10 mg/ml, ScanVet Ltd., Warsaw, Poland) at a dose based on the body weight (4.5 mg/kg). After intubation (the size of the endotracheal tubes was visually and manually determined based on the weight of the animals and was in the range of 6–11), general anesthesia was conducted with isoflurane (Aerrane Baxter Healthcare, Baxter Inc., Warsaw, Poland) in a volumetric percentage adequate to the induced clinical effect, starting with a concentration of 5 vol%, with a continuation of 2 vol%. Subsequently, the anesthetized animals underwent a computer tomography (CT) scan of the thorax (lungs were inflated with air) and then were connected to the anesthesia machine or ventilator by Ventil. All animals under this experiment had artery cannulas placed in the iliac artery and a central intravenous line in the iliac vein, and both were used to assess cardiovascular indices and to obtain blood samples for laboratory tests. For all subjects, a bladder catheter was also placed to assess diuresis. Anesthesia was conducted with isoflurane during the experiment when the animals were ventilated by an anesthetic machine, while propofol (2 mg/kg/h) was applied in those experiments when the ventilator was used. During all experiments, we adjusted the tidal volume, RR, maximal flow (V̇max), FiO2, inspiratory-to-expiratory ratio (I:E) and PEEP in the ventilator and the Ventil division knob to keep animal respiratory variables in the physiological range. We changed the position of the ventilated animals at least every 4 h. Arterial blood gas (ABG) samples were collected and analyzed by a blood analyzer (epoc® Blood Analysis System, Siemens Healthineers, Erlangen, Germany) in terms of the activity of hydrogen ions (pH), carbon dioxide partial pressure (pCO2), oxygen partial pressure (pO2), bicarbonate concentration (HCO3−), base excess in the extracellular fluid (BE (ecf)), oxygen saturation of hemoglobin (SaO2) and the lactate level.
In all experiments except No. 3, ABG samples were collected at 0, 5, 12, 17, and 24 h of the experiment from all subjects. In these experiments, we ventilated the animals for at least 24 h. In experiment No. 3, ABG was recorded at 0, 2, 4, 6, 8, and 10 h, and this experiment was run for 11 h. In this experiment, in the 3rd and 8th hours, we introduced 100 ml of bronchopulmonary saline (in a total of 200 ml) to pig #1 to reduce lung compliance (as in ARDS). The purpose of this assessment was to assess the influence of deterioration of mechanical properties of the lungs/respiratory track in one subject on the ventilatory variables of the second subject.
After the experiment, a CT scan of the thorax was repeated for all animals. At the end of the experiment, when the first ventilated animal was undergoing a CT scan , a 2 L respiratory bag was used instead. In experiment no 3, CT scans were additionally performed during ventilation. When one animal was undergoing a CT scan, a 2 L respiratory bag was used instead while the experiment continued. We did not analyze these additional CT scans in this study. We investigated all CT scans at the beginning and end of the experiments in terms of the presence of emphysema, pneumothorax and pleural effusion.
Statistical analysis
The pairs of pigs were divided into two groups in terms of weight: the WS group included pigs with equal or similar weights, and the WD group included pigs with different weights. We examined arterial blood gas parameters. The Mann–Whitney U test was used to compare the differences between all values of ABG parameters in pairs with WS and WD groups acquired across the experiments because the distribution of these parameters differed from the normal distribution and because for most of them, the homogeneity of variances was not fulfilled. For all experiments except no. 3, the Friedman test and Spearman's rank correlation were performed to analyze the repeatable AGB parameters measured at fixed intervals of time (at 0, 5, 12, 17, and 24 h). The analyses were performed separately for each of the parameters for the WS and WD groups. The value for a statistically significant difference was set at α = 0.05 for all statistical analyses. The Statistica v.13.3 software package was used for the calculations.
Laboratory test bench
A Ventil with serial number SN0003 equipped with two flowmeters (SpiroQuant H, EnviteC, Germany) was used. Two elbow connectors with luer-lock ports (22 M/15F- and straight connectors (22 M-22 M and 22F-22F) were connected the Ventil flowmeters and the air-gas flowmeters SFM3000 (Sensirion, Switzerland). Two adult PP anesthesia extendible 60/180 cm breathing circuits with Y-pieces (Medtronic, Minneapolis, MN) were connected to SFM 3000 (inspiratory arms) with artificial test lungs SmartLung 2000 (IMT Analytics, Switzerland) through HMEF filters. Expiratory limbs were connected to electrostatic antibacterial and antiviral filters Barrierbac S 22 M/15F (Medtronic, Minneapolis, MN), one-way valves (Intersurgical, New York, NY), and 2.5 cmH2O PEEP valves (Intersurgical, New York, NY). The PEEP valve output was connected (through 30 M-22 M connectors (Intersurgical, New York, NY)) by third adult PP anesthesia extensible 60/108 cm breathing circuits (Medtronic, Minneapolis, MN) to the ventilator (Y-piece site) by a 22F-22F connector (Intersurgical, New York, NY). Luer-lock ports were connected with 143SC01D-PCB pressure sensors (Sensortechnics GmbH, Germany). These pressure sensors and SFM3000 flow sensors are part of the measurement system. Pressure signals were recorded by the real-time NI PXI-1042 system with an NI PXI-6289 data acquisition board installed (both of them from National Instruments, Austin, TX). Flow data from SFM3000 flow sensors were recorded by the STM32VLDISCOVERY board (STMicroelectronics, France-Italy). All pressure and flow data were collected by a Windows 7 PC laptop with its own developed software in LabVIEW™ 2013 (National Instruments, Austin, TX) for data storage and visualization. The configuration is presented in Fig. S1. The picture with the given setup is shown in Fig. S2.
Laboratory experiments: effects of changing resistance and compliance in one artificial lung (AL) on flow and pressure in the second lung
We changed the resistance (R, all values expressed in mbar/L/s) from the baseline value R = 5 to values 20 (R5 to R20), 50 (R5 to R50) and 200 (R5 to R200) and compliance (C, all values expressed in mL/mbar) from baseline value C = 75 to values 60 (C75 to C60) and 25 (C75 to C25) as well as from C = 60 (for both AL) to C = 75 (C60 to C75). We also performed breathing circuit disconnection (in the patient's filter point – P1 marker in Fig. S1) tests (R5 to R0) and replaced the first AL (R = 5, C = 75) with the 2-L respiratory bag (Medtronic, Minneapolis, MN) with R ~ 0 and C ~ 15 (AL to Bag). The second AL parameters were not affected during the experiments. For the ‘R5 to R20’, ‘R5 to R50’, ‘R5 to R200’ and ‘R5 to R0’ events, a baseline C = 75 value was used. For the ‘C75 to C60’, ‘C75 to C25’ and ‘C60 to C75’ events, a baseline R = 5 value was used. Baseline R = 5 and C = 75 values were used for AL in the ‘AL to Bag’ event. We investigated how the pressure and delivered tidal volume were changed in AL #2 (expressed by index 2 for pressures and volumes in Table 4) when the parameters of AL #1 were changed (pressures and volumes in Table 4 for this AL are expressed by index 1). All tests were performed for three respiratory rate values of 12, 18, and 24 breaths/min.
Simulation of cross-contamination
The experimental measurement system and conditions are described in supplement R1—Technical Report of IBBE PAS on the transmission of nanoparticles/solutions in two respiratory branches of the Ventil system for experiments carried out in the period April 4–29, 2020. Figure R1 (technical report R1) shows a diagram of the test system with the Ventil apparatus supplying the respiratory tract during independent ventilation of two artificial lungs (right and left). Left: Right flow ratio 6:4. To test the possibility of transmission between the two airways, the test solutions and phantom suspensions were administered to the expiratory tract of the artificial L lung using an Areogen nebulizer (N) in 3 mL portions repeated several times. The checkpoints for the presence of test fluorescent substances and nanoparticles were at test points T1, T2, and T3. In the experiments, fluorescent compounds, such as sodium fluorescein (fluorescein) and methylene blue, purchased from Sigma and fluorescent red and green polystyrene nanospheres (red fluorescent polystyrene microspheres—EPRUI-RF-100C and green fluorescent polystyrene microspheres—EPRUI-GF-100C) with a diameter of 100 nm were used as virus phantoms. The stable fluorescence of the nanospheres was ensured by the incorporation of the dye inside them (so-called internal labeling). Fluorescein and methylene blue solutions at a concentration of 1 mM were used. In contrast, fluorescent nanospheres were administered as a 60 µL suspension and 240 µL stock suspension (provided by the manufacturer) per 3 mL of deionized water. Then, the estimated number of nanospheres in the prepared suspensions was 6.5.1012 and 24.1012 particles per 3 mL, respectively.
Results
Animal experiments
Of 8 experiments, one was abandoned after 4 h due to decreasing saturation and cardiorespiratory disorders in both animals. Asystole was found in one animal along with no response to administered drugs during resuscitation. In addition, dysfunction of unidirectional mechanical positive end-expiratory pressure (PEEP) components in the circuit was found, possibly causing mixing of exhaled gases in the respiratory circuits of the ventilated subjects. Another experiment was aborted after approximately 8–9 h due to carbon dioxide accumulation, and the body temperature increased up to 43 °C in one pig. The animal died as a result of asystole. Malignant hyperthermia was considered the cause of death. These experiments were excluded from the analyses. The configuration of the other 6 successfully completed experiments is summarized in Tables 1 and 2.
The median and lower IQR bound of SpO2 for all animals were higher than the clinically accepted level of 90%. The median inspiratory oxygen fractions, in most cases, were below 0.5. The median EtCO2 level was above the range considered normal (35–45 mmHg). The level of permissive hypercapnia in the ventilated subjects was up to 65.6 mmHg (median EtCO2 values during the experiments). The median driving pressure was below 10 cmH2O for all animals except 2 cases: Experiment 5, pig #1 with chronic emphysema and pneumothorax of the left lung and pig #1 in Experiment 3 with reduced compliance. These two pigs are characterized by lower (median) static lung compliance than the others. They also have the highest PIP values. The median TV (expressed in ml per kg) of 8 animals was in the range of 4–6 ml/kg recommended for mechanical ventilation in ARDS subjects, while in 3 animals, the TV was higher than 6 ml/kg up to 7.17 (in one animal, the TV was not measured).
Computer tomography scan analyses
We did not find emphysema in any subject before and after ventilation, except subject #1 (Experiment 5), in which right-sided emphysema was indicated before and after the experiment. We did not find pneumothorax in any subject before and after ventilation, except the same animal that presented signs of emphysema, in which left-sided pneumothorax was indicated before and both-sided pneumothorax after the experiment. We did not find pleural effusion in any subject before the experiment and only trace amounts on both sides in subject #2 (Experiment 1) after ventilation.
The analysis of CT scans showed that, generally, Ventil together with a ventilator or anesthetic machines did not cause any severe lung injuries in ventilated animals, with one exception, namely, subject #1 in Experiment 5, which was characterized by chronic emphysema and pneumothorax before the experiment and then very low lung compliance. This animal had the highest median PIP value and ΔP value over 10 cmH2O.
Time-trend analysis of the selected animal experiments
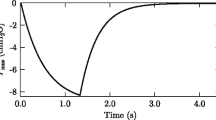
In Fig. 2A,B, we present the time trends of Experiment 6. In the time trends, we marked m1–m4 symbols indicating exemplary events related to the experiment. Marker m1 indicates the time when the Ventil flow division ratio was significantly changed to decrease EtCO2 in subject #2 and increase it in subject #1. Marker m2 indicates the time when RR and FiO2 were decreased. Marker m3 indicates the time when the side on which the pigs were lying was changed. Before this, we found a rapid increase in PIP and then ΔP and a reduction in intrinsic PEEP and the static compliance Cst of subject #1. The body temperature of this pig tended to be distinctly elevated on the side it was lying on during all the experiments. This temperature elevation probably accompanied the Cst decrease, as these rapid Cst changes disappeared after the body position was changed. The rapid decrease in Cst on #1 did not cause significant changes in the ventilation of subject #2. Marker m4 shows the end of the experiment and the moment pig #1 was disconnected, and a 2-L respiratory bag was terminated to the breathing circuit. This resulted in a tidal volume increase for pig #2, which was followed by an increase in PIP and a decrease in EtCO2. This phenomenon we also found in a second experiment is described in the next paragraph.
Time traces for the selected animal experiments. (A, B) Ventilatory parameters over time for Experiment (pair) 6. (C) Ventilatory parameters for Experiment 3. Blue traces denote object #1, while red traces denote object #2 (except TV1/TV2). Markers: m1, Changing the Ventil flow division knob; m2, Respiratory rate reduction; m3, Animal position change from the left side to the right side; m4, One object being exchanged with a 2 L respiratory bag; S1, Injection of the first 100 ml of saline to pig #1; p1, Flow division changes by the Ventil; p2 and p3, The times when a 2-L respiratory bag was connected instead of pig #1 and #2, respectively; s2, Injection of the second 100 ml of saline to pig #1; p4, Animal position change from the right side to the left side; p5, Exchange of the #1 object with a 2 L respiratory bag; MV, Minute ventilation; TV, Tidal volume; FiO2, Oxygen fraction in the inhaled gas; SpO2, Arterial blood saturation; EtCO2, End-tidal carbon dioxide; TV1/TV2, Tidal volume ratio—ventilation ratio; PIP, Peak inspiratory pressure; Pplat, Plateau pressure; PEEPi, Intrinsic positive end-expiratory pressure; ΔP, Driving pressure (Pplat–PEEPi); TVe, Expiratory tidal volume, Cst, Static lung compliance.
The time trends of experiment no. 3 are presented in Fig. 2C. Marker S1 indicates the injection of the first 100 ml of saline into the lungs of pig #1. It caused a gradual increase in PIP in pig #1, while in pig #2, the ventilatory parameters were not changed significantly. Marker p1 indicates the time when the ventilatory parameters were changed. Markers p2 and p3 indicate the time periods when a 2-L respiratory bag was connected instead of pigs #1 and #2, respectively. Marker S2 indicates the injection of the second 100 ml portion of saline into pig #1. This caused the next gradual increase in PIP in this subject. However, it did not affect the ventilation of pig #2. Marker p4 is the time when the side on which the pigs were lying was changed from right to left. Marker p5 indicates the disconnection of pig #1 and the connection of the 2-L respiratory bag into the breathing circuit.
Arterial blood gas analysis in animal experiments
The results of the ABG parameters examined for the Friedman ANOVA test are presented in Fig. 3. The comparison of all ABG samples in the WS and WD groups showed that statistically significant differences between groups were found in relation to pH, pO2, and SaO2 (Table 3). The presented results show the connection between those parameters according to the well-known acid–base equilibrium model, and there is confirmation of the reliability and validity of the research approach. The observations concerning steady changes in the time of AGB parameters in groups WS and WD showed that there was no statistically significant difference between the values of these parameters over time (independent variable) (Table 3). Despite the lack of these statistically significant differences, in the WD group, Be(ecf) showed a positive Spearman correlation coefficient (r = 0.433), which means that the Be(ecf) values increased over time during the experiments. In relation to the WS group, the Spearman correlation coefficients were also positive for cHCO3− and Be(ecf) (0.509 and 0.561, respectively). In the WS group, the opposite trend was found for lactate levels (r = − 0.393), which means that lactate values decreased over time.
Arterial blood parameters were recorded at 0, 5, 12, 17 and 24 h in Experiments 1 and 2 (WS group) and Experiments 4, 5, and 6 (WD group) for ANOVA Friedman’s test. Squares indicate the median values. Boxes indicate the IQR. Whiskers indicate the nonoutlier range. Circles indicate outliers, and asterisks indicate extreme values—the values beyond the nonoutlier range. pH, The activity of hydrogen ions; pCO2, Carbon dioxide partial pressure; pO2, Oxygen partial pressure; cHCO3−, Bicarbonate concentration; Be(ecf), Base excess in the extracellular fluid; SaO2, Oxygen saturation of hemoglobin; lactate, Lactate level.
Laboratory experiments: effects of changing resistance and compliance in one artificial lung on flow and pressure in the second lung
We performed the tests of mechanical property changes (named later 'events') in a first artificial lung (AL) to the flow and pressure changes in a second AL (the scheme of the laboratory setup is presented in Fig. S1, and its picture is presented in Fig. S2). The results are presented in Table 4 and Figs. S3–S8.
The pressure change (dP), defined as the difference between the PIP in one of the Ventil system’s output channels before and after the event, was obviously changed in the affected AL (dP2). The percentage relative error for dP2 (dP2%) was proportional to the simulated R and C changes. For the tested (not affected) AL, this pressure change (dP1), expressed as a percentage relative error (dP1%), was less than 10% for all cases, except 'R5 to R200' for the RR = 12 event, where it was 11%. The TV change (dV) for affected and tested AL (dV1 and dV2, respectively) expressed as the percentage relative errors (dV1% and dV2%, respectively) were also less than 10% with two exceptions. One exception was for 'R5 to R200' for RR = 12. In this case, the inspiration was finished early by the ventilator due to the very high pressure in the ventilator output port (Fig. S3). Therefore, dV1% and dV2% were at the level of − 20%. The second exception was for ‘R5 to R200’ for RR = 24. In this case, the tidal volumes were 13% and 20% higher (dV1%, dV2%) for the tested and affected AL, respectively. Because the Ventil apparatus only splits the gas flow, the TV increase for both ALs in the last case could have been caused only by the work of the ventilator under this high-pressure condition in the breathing circuit.
These experiments show the strong Ventil endurance in maintaining division of the minute ventilation when the respiratory system R and C parameters are changed (i.e., respiratory deterioration or improvement in a patient). Ventil stabilized the ventilation within a few respiratory cycles after an adverse event had occurred.
Other laboratory experiment results using the same setup (Figs. S1, S2) are described in the supplementary materials and are presented in Figs. S9–S15.
Simulation of cross-contamination: laboratory tests
Research on the transmission of particles in the dual tract respiratory system with Ventil was carried out in two stages with the use of (1) aqueous solutions of fluorescent compounds and (2) suspension of the SARS-CoV-2 coronavirus phantom in the form of fluorescent nanospheres. The presence of test substances and nanoparticles was examined at test points T1, T2 and T3. The results are presented in Supplement R1 in the form of a technical report on the transmission of solutions/nanoparticles in two respiratory branches of the Ventil system. There were no fluorescein traces on the postfiltering side of the filters located at test points T1, T2 and T3. No traces of fluorescein were observed on the postfiltering surface filter surface for the filter placed at test point T3. Furthermore, in our experiments under most critical conditions, when electrostatic filter F and the PEEP valve were removed, viral phantoms in the form of fluorescently labeled nanospheres with a diameter of 100 nm in the stream of air exhaled from the left lung did not spread further than the filtering surface of the filter at test point T3.
In summary, based on the conducted technical studies related to the assessment of the possibility of transmission of solutions/suspensions in two respiratory branches of the Ventil system, it was found that under the conditions of the experiment with the use of fluorescent solutions and suspensions, no transmission occurred.
Discussion
The results in the animal model show that the ventilation of two animals can be effectively continued for at least 24 h by the ventilator and the Ventil system, independent of the body weight difference in the pair. The oxygenation, ventilatory status and acid–base balance of the animals were stable during the experiments. The animals’ weight difference was effectively compensated using Ventil use by adjusting the TV according to their need. Subject-specific minute ventilation was maintained by changing the ventilator’s MV and the Ventil device’s division ratio. The EtCO2 levels were elevated due to the lung-protective strategy applied in the experiments.
Evaluating ABG parameters, we focused on the direction and range of changes over time. SaO2, pH and lactate concentrations were maintained in the normal range. The results of pO2 exceeded the normal level of 100 mmHg in both groups. Our results in the WS group are comparable with the results reported by Bitelia et al. for the group of pigs ventilated with oxygen and compressed air FiO2 0.5/air32. The WD group showed lower values than the WS group but within the normal range for all time points. The pCO2 showed high values that are comparable with the values reported by others32,33. The value of cHCO3− remained at a high level, with a trend of changes similar to pCO2. The high values of cHCO3− and the same trend as pCO2 may indicate that elevated cHCO3− is a metabolic response to pCO2, especially when changes in blood oxygenation and a low lactate concentration indicate adequate tissue oxygenation. Taking into account the time of observation, we presume that two of three compensatory systems, extracellular and respiratory, can be responsible for this finding. The renal compensatory system acts slowly to compensate for acid–base balance34. Among several factors that can be responsible for the obtained results of pCO2 and cHCO3−, the primary cause should be sought in disorders of CO2 exchange due to changes in the position of the pigs from the side to the back, which can induce disturbances in gas exchange between perfusion and ventilation.
The CT scan analysis carried out before and after long-term ventilation did not reveal serious injuries, such as emphysema, pneumothorax and pleural effusion caused by the ventilation procedure, with the exception of the animal with emphysema in which left-sided pneumothorax and both-sided pneumothorax were indicated before and after the experiment, respectively. This animal had ΔP equal to 12, while other animals had ΔP equal to or less than 10. This result suggests that the safe ΔP indicator for ventilated pigs is < 10 cmH2O.
In animal experiment 3, the progressive changes in the lung compliance of subject #1 due to saline instillation did not affect the ventilation of unaffected subject #2; however, these changes were not strong because 200 ml of saline was not a large volume in comparison to the affected subject’s weight. In Experiments 3 and 6, replacing an animal with a 2 L respiratory bag (no resistance, very small compliance) increased the PIP up to 17% in unaffected subjects and changed the TV as much as 25% in ventilated subjects. Because the MV of the ventilator was constant, these changes are related to Ventil’s division ratio. In laboratory experiments for ‘AL to Bag’ events, PIP changes in the unaffected AL were less than 1%, and TV changes for both ALs ranged from 0.7 to 6.3%. However, in the animal experiments, the inspiratory flow of the ventilator was 80 L/min, while it was 40 L/min in the laboratory experiments. This means that Ventil is more accurate and more load independent for lower inspiratory flows. Less performance for higher flows can be related to the higher dispersion of two analog flow sensors in the Ventil. If these sensors’ pressure-flow characteristics are load impedance dependent, then flow level inequality is produced, and the volume division is not exactly as expected. However, the laboratory results in maintaining MV in both channels (when R and C are changed) are still acceptable and can be somewhat corrected by Ventil’s flow division knob.
Limitations of the shared ventilation by Ventil
As in other solutions, in the case of ventilation by Ventil, subjects share the respiratory rate, inspiratory time, pause time and oxygen content set in the ventilator, which limits the respiratory therapy capabilities. Moreover, the respiratory activities of both ventilated subjects may lead to dynamic changes in the pressure of the airways and significant disturbances in the ventilation mechanics of one or both subjects. Thus, subjects with maintained respiratory function need deep sedation10,11,16.
Additional mechanical PEEP valves can be used with Ventil to differentiate intrinsic PEEP (PEEPi) levels during therapy. In animal experiments, mechanical, fixed PEEP valves were used. However, neither ventilator that we used cooperated when fixed PEEP valves of 5 cmH2O or higher were used. The occlusion alarm was indicated by both ventilators immediately. The 2.5 and 0 cmH2O PEEP valves (only one-way valves) worked correctly. Therefore, extrinsic PEEP (PEEPe) values were also set in the ventilator. PEEPi in the lungs of the subject at the end of the expiration is a sum of set PEEPe and mechanical PEEP valves. Other authors in their studies neglected differential PEEP levels12 and used only a shared PEEP set in a ventilator. However, various PEEP levels for ventilated patients in shared ventilation systems are an open issue.
Safety indications
The shared ventilation in the context of the treatment of COVID-19 patients provoked a global discussion on the limitations and safety of such approaches in emergencies38,39. Most of the studies of shared ventilation were based on laboratory experiments in which only test lungs were used4,7,12,16,36. Only a few animal experiments5,8,9,10 or preliminary tests on a few pairs of patients35,37 were carried out. Further clinical studies focused on safety and effectiveness are necessary. Below, we discuss some safety indications.
Ventilator alarm and monitor management in the case of shared ventilation is a challenge10,16,35. External monitoring systems are necessary for ventilated subjects, as has also been confirmed by other authors10,11,12. For 1:1 division, the peak pressure difference between the Ventil outputs and that measured by the ventilator can be as high as 20 cmH2O for a flow level of approximately 100 L/min (Fig. S15). Peak pressure values in the ventilator can then be elevated, and the high-pressure alarm must be corrected. The Ventil displays only the mean pressure in the output ports and the mean flow for both channels. A low mechanical ventilation alarm is also essential to prevent leakage in the whole respiratory circuit35.
In comparison to other shared ventilation solutions, Ventil is an active system. However, its malfunction should not introduce rapid mechanical ventilation changes for ventilated subjects (Figs. S9–S14). A dividing head in the Ventil is constructed to make it mechanically impossible to close one of the channels or both of them, even in the case of electronic and control system failure. Moreover, we did not use pressure relief valves in our animal experiments due to continuous supervision and external subject monitoring. However, they must be applied in the breathing circuit in clinical settings10. These valves prevent excessive ventilation in patients. Using Ventil, the operator must also carefully manage when an extensible, flexible tube between the ventilator and the Ventil is used. In our laboratory experiments, too-rapid stretching of this tube caused a ventilator (Bennett 840) malfunction. The ventilator’s safety valve was activated, and the ventilator was switched into the service mode. This was probably due to the negative pressure created between the ventilator and the Ventil apparatus.
The complexity of the setup (Fig. 1) can also be problematic for medical staff. An additional device with a set of additional tubes and connectors increases the risk of leakage or a wrong connection. Some components in the setup can be reduced by modifying the Ventil’s design, but the setup in shared ventilation will always be more complex than “one patient – one ventilator”.
Cross-contamination in shared ventilation by Ventil
Similar to the results obtained by other authors, there was no transmission of fluorescent dye solutions—trypan blue10, fluorescein or methylene blue—as indicated in our study presented in Report R1 in the supplementary materials. Concerning the studies described in10, which include continuous nebulization of 5 ml of trypan blue solution for 10 min and visual observation of the filters, in our experiment, in addition to the use of fluorescein and methylene blue solutions, we proposed a pathogen transmission study method using phantoms of the virus in the form of nanospheres with a diameter of 100 nm internally labeled with fluorescent dyes, the concentration of which in the administered suspension was approximately 24.1012 particles/3 mL, which corresponds to a viral titer of ca. 1000-fold excess with respect to SARS-CoV-2 titer after a 5-day incubation of infected Calu3 cells[21]. The proposed method using nanoparticles can be safely carried out in the laboratory environment, obtaining more specific (in relation to the acquisition from experiments with solutions) preliminary information about the possibility of cross-transmission in the respiratory tract during independent ventilation of artificial lungs.
Conclusions
In conclusion, Ventil might be considered to ventilate two patients by one ventilator with an acceptable safety level. It meets the same challenges as other shared ventilation systems. However, Ventil automatically stabilizes the patients' minute ventilation and is highly invulnerable to their respiratory resistance and compliance changes. Notwithstanding, the Ventil system is feasible for differential lung ventilation and can still be applied for respiratory therapy in cases involving asymmetrical lung pathology or thoracic surgery. Additionally, it can be used in emergency situations when the lack of ventilatory support for a large number of patients is expected, such as during terrorist attacks or widespread diseases affecting respiratory function.
Data availability
All data, except CT scans, are available in the main text or the supplementary materials. The CT scans are available from the corresponding author upon reasonable request.
References
Ranney, M. L., Griffeth, V. & Jha, A. K. Critical supply shortages—The need for ventilators and personal protective equipment during the Covid-19 pandemic. N. Engl. J. Med. 382, e41 (2020).
Grasselli, G., Pesenti, A. & Cecconi, M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: Early experience and forecast during an emergency response. JAMA 323, 1545–1546 (2020).
Marini, J. J. & Gattinoni, L. Management of COVID-19 respiratory distress. JAMA 323, 2329–2330 (2020).
Neyman, G. & Irvin, C. B. A single ventilator for multiple simulated patients to meet disaster surge. Acad. Emerg. Med. 13, 1246–1249 (2006).
Paladino, L. et al. Increasing ventilator surge capacity in disasters: Ventilation of four adult-human-sized sheep on a single ventilator with a modified circuit. Resuscitation 77, 121–126 (2008).
Sommer, D. D., Fisher, J. A., Ramcharan, V., Marshall, S. & Vidic, D. M. Improvised automatic lung ventilation for unanticipated emergencies. Crit. Care Med. 22, 705–709 (1994).
Branson, R. D., Blakeman, T. C., Robinson, B. R. & Johannigman, J. Use of a single ventilator to support 4 patients: Laboratory evaluation of a limited concept. Respir. Care 57, 399–403 (2012).
Paulsen, M. J. et al. FDA emergency use authorization-approved novel coronavirus disease 2019, pressure-regulated, mechanical ventilator splitter that enables differential compliance multiplexing. ASAIO J. 68, 1228–1230 (2022).
Stiers, M. et al. Successful ventilation of two animals with a single ventilator: Individualized shared ventilator setup in an in vivo model. Crit. Care 24, 523 (2020).
Srinivasan, S. S. et al. A rapidly deployable individualized system for augmenting ventilator capacity. Sci. Transl. Med. 12, eabb9401 (2020).
Tonetti, T. et al. One ventilator for two patients: Feasibility and considerations of a last resort solution in case of equipment shortage. Thorax 75, 517–519 (2020).
de Jongh, F. H. C. et al. Ventilating two patients with one ventilator: Technical setup and laboratory testing. ERJ Open Res. 6 (2020).
Levin, M. A. et al. Differential ventilation using flow control valves as a potential bridge to full ventilatory support during the COVID-19 crisis. Anesthesiology 133, 892–904 (2020).
Castañón-González, J. A. et al. Simultaneous mechanical ventilation of several patients with a single ventilator. Gac Med. Mex. 156, 249–252 (2020).
Chatburn, R. L., Branson, R. D. & Hatipoğlu, U. Multiplex ventilation: A simulation-based study of ventilating two patients with one ventilator. Respir. Care 65 (2020).
Han, J. S. et al. Personalized ventilation to multiple patients using a single ventilator: Description and proof of concept. Crit Care Explor. 2, e0118 (2020).
Laffey, J. G., Chikhani, M., Bates, D. G. & Hardman, J. G. Supporting more than one patient with a single mechanical ventilator: Useful last resort or unjustifiable risk?. Br. J. Anaesth. 125, 247–250 (2020).
Cherry, A. D., Cappiello, J., Bishawi, M., Hollidge, M. G. & MacLeod, D. B. Shared ventilation: Toward safer ventilator splitting in resource emergencies. Anesthesiology 133 (2020).
Cevik, M. et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: A systematic review and meta-analysis. Lancet Microbe 2, e13–e22 (2021).
Han, M. S. et al. Viral RNA load in mildly symptomatic and asymptomatic children with COVID-19, Seoul, South Korea. Emerg. Infect. Dis. 26, 2497–2499 (2020).
Chu, H. et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe 1, e14–e23 (2020).
Muth, D. et al. Infectious middle east respiratory syndrome coronavirus excretion and serotype variability based on live virus isolates from patients in Saudi Arabia. J. Clin. Microbiol. 53, 2951–2955 (2015).
Wölfel, R. et al. Virological assessment of hospitalized patients with COVID-2019. Nature 581, 465–469 (2020).
Bullard, J. et al. Predicting infectious severe acute respiratory syndrome coronavirus 2 from diagnostic samples. Clin. Infect. Dis. 71, 2663–2666 (2020).
Arons, M. M. et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N. Engl. J. Med. 382, 2081–2090 (2020).
La Scola, B. et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur. J. Clin. Microbiol. Infect. Dis. 39, 1059–1061 (2020).
Darowski, M. & Englisz, M. Artificial ventilation of the lungs for emergencies. Front. Med. Biol. Eng. 10, 177–183 (2000).
Darowski, M., Hedenstierna, G. & Baehrendtz, S. Development and evaluation of a flow-dividing unit for differential ventilation and selective PEEP. Acta Scandinavica 29, 61–66 (1985).
Hedenstierna, G., Baehrendtz, S., Darowski, M. & Frostell, C. Practical aspects of differential ventilation with selective peep in acute respiratory failure. Int. J. Clin. Monit. Comput. 1, 5–11 (1984).
Kowalczyk, M. et al. Successful 1:1 proportion ventilation with a unique device for independent lung ventilation using a double-lumen tube without complications in the supine and lateral decubitus positions. A pilot study. PLoS ONE 12, e0184537 (2017).
Sawulski, S. Independent lung ventilation for treatment of post-traumatic ARDS. Anaesthesiol. Intensive Ther. 44, 96–99 (2019).
Biteli, E. G. F. et al. Blood gas analysis in pigs submitted to different concentrations of nitrous oxide or oxygen, under different ventilatory modalities. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 71, 35–43 (2019).
Bekheit, M., Bucur, P., Vibert, E. & Anders, C. The reference values for hepatic oxygen consumption and net lactate production, blood gasses, hemogram, major electrolytes, and kidney and liver profiles in anesthetized large white swine model. Transl. Surg. 1, 95–100 (2016).
Edwards, S. L. Pathophysiology of acid base balance: The theory practice relationship. Intensive Crit. Care Nurs. 24, 28–40 (2008).
Beitler, J. R. et al. Ventilator sharing during an acute shortage caused by the COVID-19 pandemic. Am. J. Respir. Crit. Care Med. 202, 600–604 (2020).
Rodríguez-Villar, S. Sharing a single ventilator (“In vitro”). Med. Intensiva. 44, 514–516 (2020).
Milner, A., Siner, J. M., Balcezak, T. & Fajardo, E. Ventilator sharing using volume-controlled ventilation during the COVID-19 pandemic. Am. J. Respir. Crit. Care Med. 202, 1317–1319 (2020).
Mancebo, J., Richard, J.-C. & Brochard, L. Ventilator sharing during shortages. A Siren’s song?. Am. J. Respir. Crit. Care Med. 202, 490–491 (2020).
Chase, J. G. et al. In-parallel ventilator sharing during an acute shortage: Too much risk for a wider uptake. Am. J. Respir. Crit. Care Med. 202, 1316–1317 (2020).
Acknowledgements
We would like to thank Dr K.J. Pałko from IBBE PAS and the Ł-ITAM team for Ventil development and commercialization, including its certification process.
Funding
This study was supported by the Polish Ministry of Science and Higher Education (Agreement No. 21/WFSN/2020 from 18th of March 2020).
Author information
Authors and Affiliations
Contributions
Conceptualization: K.Z., D.P., A.L., M.D., P.Ł. Methodology: K.Z., B.L., A.L., Z.G., P.Ł. Investigation: K.Z., B.L., K.S., M.S., K.F., M.B., J.O., K.A., A.J., M.G., P.O., M.M., D.P., K.P., E.R., R.Z., K.K.R., A.S., and R.P. Visualization: K.Z., B.L. Funding acquisition: A.L., P.Ł. Project administration: P.Ł. Supervision: Z.G., P.Ł. Writing—original draft: K.Z., B.L., D.P., M.D., A.L., Z.G., P.Ł. Writing—review and editing: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zieliński, K., Lisowska, B., Siewruk, K. et al. Automatic air volume control system for ventilation of two patients using a single ventilator: a large animal model study. Sci Rep 12, 22591 (2022). https://doi.org/10.1038/s41598-022-26922-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26922-4
- Springer Nature Limited