Abstract
The placenta is critical to human growth and development and has been implicated in health outcomes. Understanding the mechanisms through which the placenta influences perinatal and later-life outcomes requires further investigation. We evaluated the relationships between birthweight and adult body mass index (BMI) and genetically-predicted gene expression in human placenta. Birthweight genome-wide association summary statistics were obtained from the Early Growth Genetics Consortium (N = 298,142). Adult BMI summary statistics were obtained from the GIANT consortium (N = 681,275). We used S-PrediXcan to evaluate associations between the outcomes and predicted gene expression in placental tissue and, to identify genes where placental expression was exclusively associated with the outcomes, compared to 48 other tissues (GTEx v7). We identified 24 genes where predicted placental expression was significantly associated with birthweight, 15 of which were not associated with birthweight in any other tissue. One of these genes has been previously linked to birthweight. Analyses identified 182 genes where placental expression was associated with adult BMI, 110 were not associated with BMI in any other tissue. Eleven genes that had placental gene expression levels exclusively associated with BMI have been previously associated with BMI. Expression of a single gene, PAX4, was associated with both outcomes exclusively in the placenta. Inter-individual variation of gene expression in placental tissue may contribute to observed variation in birthweight and adult BMI, supporting developmental origins hypothesis.
Similar content being viewed by others
Introduction
Between 1999 and 2018, the prevalence of adult obesity has increased by almost 12%, with over 40% of Americans over the age of 20 currently being classified as obese1. Obesity, commonly assessed using body mass index (BMI), has a significant impact on individuals’ health, with obese individuals at increased risk for numerous diseases and conditions, including all-cause mortality, hypertension, dyslipidemia, Type 2 diabetes, and heart disease2. In 2016, the health consequences of obesity were estimated to account for $1.72 trillion dollars in total health care costs in the United States3. Though a major public health issue, existing strategies for prevention and intervention in adults have had limited to moderate success, suggesting that the understanding of the mechanisms contributing to the variation in BMI and underlying development of obesity is incomplete4. Given that the majority of obesity interventions are based on modifying known risk factors, further research investigating determinants of obesity could provide key insights into etiology and additional actionable targets.
BMI and obesity are complex traits with etiologies that are multifactorial, involving genetic and environmental factors as well as the interaction between these factors5. Several factors during the preconception, fetal, and infant periods possess substantial impact on adult obesity, such as gestational weight gain, exposure to diabetes in utero, and genetic variation. Weight, BMI, and obesity status earlier in life are significant predictors risk factors for adult BMI and obesity, as BMI tends to track from early childhood and adolescence into adulthood6,7. Specific growth trajectories in infancy and early childhood also increase the risk of obesity in later life8,9,10,11,12,13. Birthweight is also consistently associated adult BMI and obesity throughout the life course14. Most observational studies linking birthweight with later anthropomorphic measures have found a linear relationship between birthweight and adult BMI, overweight and obesity status of offspring. Recent investigations suggest those born at low birthweight (< 2500 g) have a decreased risk of later overweight status (odds ratio (OR): 0.73, 95% confidence interval: 0.63–0.84) while overweight adults are 1.60 (95% confidence interval: 1.45–1.77) times more likely to have high birthweight (> 4000 g) compared to individuals who are not obese in adulthood15.
Though a strong relationship between birthweight and adult BMI or obesity has been consistently found in observational epidemiologic research, the causal mechanisms leading to the variation in birthweight and later-life BMI, as well as the etiologic mechanisms underlying their association remains unclear. New approaches are necessary because classical, observation epidemiologic research has not provided a complete explanation for these outcomes. With growing evidence that both have origins in the perinatal period, the Developmental Origins of Health and Disease (DOHaD) theory, a model for disease causation that states that an individual’s health throughout their life course is significantly influenced by exposures during pregnancy16,17, may provide a framework that can guide research on causal mechanisms. The theory suggests the environment in utero and early postnatal life programs individuals’ characteristics and contributes to disease(s) later in life. Programming in this context largely occurs through epigenetics, resulting in changes in gene expression. Thus, this framework moves beyond environmental and genetic risk factors for these outcomes and steers research toward potential biologic and molecular mechanisms.
Under the DOHaD framework, the placenta is an important biological conduit to mediate genomic and non-genomic transmission of risk for noncommunicable diseases and traits like birthweight and BMI. As the physical and functional connection between a mother and developing fetus, the placenta is critical to fetal growth and development18. The genetic regulation of placental gene expression has recently been described through expression quantitative trait loci (eQTL) studies19, which have identified associations with childhood phenotypes20. Because germline DNA is unvarying from conception, gene expression in placental tissue can be predicted in newborns and adult individuals using genome-wide association (GWAS) data.
The objective of this study was to dissect the relationship between adult BMI and genetically-predicted gene expression (hereby referred to only as gene expression) in the placenta, with the underlying premise that alterations in placental-specific gene expression would be associated with individuals’ BMI later in life. Given that birthweight is consistently associated with later life BMI and the placenta plays a key role in fetal growth, we also evaluated the association between gene expression and birthweight. As there is some overlap between known birthweight and adult BMI loci21,22, we compared the results from the birthweight analysis to the results of the BMI analysis. Since the existence of the placenta is considerably closer to the birthweight phenotype, we hypothesized that there would be substantial differences in the genes where expression in the placenta associates with birthweight versus adult BMI.
Results
Birthweight z-score gene expression results
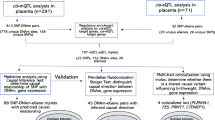
Across the 49 tissues, we tested the association between 263,683 genes’ expression and birthweight z-score (Fig. 1; Supplementary Table S1). More than 40,000 (15.19%) of the tested expression-birthweight z-score associations had suggestive significance at the 0.05 level; however, 804 (0.30%) gene expression results were significant after Bonferroni correction. The most significant result was with predicted HMGA2 expression in transformed fibroblast cells (Supplementary Table S2). Increased HMGA2 expression in this tissue was associated with increasing birthweight z-score (effect size: 0.35 standard deviations [SD] of birthweight per SD of HMGA2 expression, p-value: 3.58 × 10−57). Increased expression of this gene was also highly significant in the placenta (effect size: 0.04 SD of birthweight per SD of HMGA2 expression, p-value: 4.32 × 10−16). The second most significant result was a positive association between expression of RPSAP52 and birthweight z-score in the placenta (effect size: 0.11 SD of birthweight per SD of RPSAP52 expression, p-value: 7.49 × 10−44). Expression of ADCY5 was also associated with birthweight z-score in the placenta (effect size: -0.09 SD of birthweight per SD of ADCY5 expression, p-value: 4.49 × 10−41). Both HGMA223,24,25,26 and ADCY523,24,25,26,27 have been previously implicated in GWAS studies for birthweight. RPSAP52, which was only significantly associated with birthweight in placental tissue, has not been previously associated with birthweight before but has been associated with Type 2 diabetes28. Within the 804 significant associations, there were 148 genes where expression was only significant in a single tissue (Supplementary Table S3).
Birthweight Miami Plot. The bottom of the graphic is a Manhattan plot which displays significant SNPs from GWAS. The top of the graphic is the results from S-PrediXcan, with symbols now representing entire genes and their genetically determined expression levels. The x-axis are chromosomes. The y-axis is log and negative log p-values from the GWAS and S-PrediXcan analyses. Colors correspond to specific tissues.
Among the statistically significant gene expression association results, there were 24 genes (Supplementary Table S4) for which placental expression was associated with birthweight z-score. Fifteen of these 24 associations were specific to the placenta, as they were not significantly associated with birthweight z-score in any other tissue (Table 1). Six of the genes where expression was significantly associated with birthweight z-score in the placenta have been previously associated with birthweight: ADCY523, HMGA223, TNFSF1223 PLEKHA124,26, FES26, and SLC38A123. SLC38A1 was the only one of these genes where expression was only associated with birthweight in placental tissue (Table 1).
Adult BMI gene expression results
The adult BMI analysis consisted of 258,869 association tests (Fig. 2; Supplementary Table S5). More than 30% (78,679 associations) of the tested expression-BMI associations had suggestive significance at the 0.05 level, 8,834 (11.23%) of which were significant after correction for multiple testing (Supplementary Table S6). The most significant gene was FTO-IT1, with increasing expression in the placenta associated with increasing BMI (effect size: 0.12 kg/m2 per SD of FTO-IT1 expression, p-value: 8.20 × 10−181). The second most significant result was a positive association between increasing predicted expression of FTO and BMI in the skeletal muscle tissue (effect size: 0.20 kg/m2 per SD of FTO expression, p-value: 4.38 × 10−171). Previous GWAS studies have tied variants in FTO to BMI in infancy, childhood, and adulthood. The association between FTO variants and BMI is one of the strongest associations documented and has been widely replicated in numerous populations29,30,31,32,33. One thousand one hundred and forty-eight genes’ expression was only significant in a single tissue (Supplementary Table S7).
Adult Body Mass Index Miami Plot. The bottom of the graphic is a Manhattan plot which displays significant SNPs from GWAS. The top of the graphic is the results from S-PrediXcan, with symbols now representing entire genes and their genetically determined expression levels. The x-axis are chromosomes. The y-axis is log and negative log p-values from the GWAS and S-PrediXcan analyses. Colors correspond to specific tissues.
Of the significant associations, the placental expression of 182 genes was linked to BMI (Supplementary Table S8). When comparing against predicted expression models from 48 other tissues from GTEx, 110 of the significant genes in the placenta were unique and not associated with BMI when analyzed in any other tissue (Table 2). FTO-IT1, the most significant gene of all tested associations, was one of the genes where placental expression was exclusively associated with adult BMI. Many significant genes have been previously tied to BMI through their association with regulatory, intergenic, or genic variants. Within these genes, 50 were significant in placental tissue, with 11 genes significantly associated with BMI exclusively in placental tissue. These 11 genes are: TOMM4032,34,35,36,37,38, LINC0097137, RBBP635,36,37,39,40,41, FAM169A37, RPL21P11940, ZZZ336,38,42, CREB141, GRIA137,40, POU6F2-AS140, LIMD137, and ZFYVE137 (Table 2; Supplementary Table S8).
Overlapping results across adult BMI and birthweight for the placenta
A single gene was significantly associated with both birthweight and adult BMI in placental tissue: PAX4. For both outcomes, PAX4 was only significant in the placental tissue. The direction of effect was consistent between both phenotypes (birthweight effect size: 0.02 SD of birthweight per SD of PAX4 expression, p-value: 2.89 × 10−9; BMI effect size: 0.03 kg/m2 per SD of PAX4 expression, p-value: 7.00 × 10–8) but it has not been tied to either outcome previously.
Gene enrichment
Evaluation of gene set enrichment among genes associated with birthweight in the placenta identified a significantly enriched reactome pathway: transmission across chemical synapses, which featured four birthweight-placental gene expression genes among the 269 involved in the pathway (p-valueadj = 0.027) (Supplementary Fig. S1). There were no GO biological processes, GO cellular components, or molecular functions that were enriched. Evaluation of gene set enrichment among genes associated with adult BMI in the placenta did not identify enriched GO biologic processes, GO cellular components, or reactome pathways.
Discussion
This study evaluated associations between gene expression and body weight traits, in the context of large-scale GWAS. We observed evidence that specific genes exert effects on these traits through expression levels in placenta and did not observe evidence of effects from these genes from adult tissues. This suggests that the process leading to birthweight and adult body composition that begins with genetic factors has intermediate steps that occur as early as the placenta, and that do not have effects in other tissues. This supports the DOHaD hypothesis, and these studies that identify genes and tissue context may provide biomarkers or drug targets for adverse outcomes.
Expression of 804 genes was significantly associated with birthweight, while 8,834 genes’ predicted expression was associated with adult BMI. Predicted expression of 24 and 182 of these genes was significantly associated with birthweight and BMI in placenta tissue, respectively. However, placental expression of only 15 of the genes associated with birthweight and 110 of those associated with BMI were significantly and exclusively associated with these outcomes. Many of the genes where expression levels were only linked to the outcome in the placenta have not been previously associated with birthweight or BMI, as single nucleotide polymorphisms (SNPs) in only one (SLC38A1)23 and 11 (TOMM4032,34,35,36,37,38, LINC0097137, RBBP635,36,37,39,40,41, FAM169A37, RPL21P11940, ZZZ336,38,42, CREB141, GRIA137,40, POU6F2-AS140, LIMD137, ZFYVE137) of these genes have been associated with birthweight and adult BMI in previous GWAS, respectively. Thus, our study replicates the association between these previously reported genes and birthweight and BMI, providing a tissue-specific mechanism of action. Importantly, this study identified numerous novel associations, tying previously unknown genes to these outcomes through their expression in various tissues.
ADCY5 was significantly associated with birthweight in the placenta. It has been consistently associated with birthweight and Type II diabetes27,43. Though the causal mechanisms are unknown, a previous EGG Consortium study noted its pleiotropic effects on glucose regulation and Type II diabetes in adulthood. One potential mechanism explaining the association between birthweight, diabetes, and the gene is the fetal insulin hypothesis. Under this hypothesis, ADCY5 could impact insulin secretion and its risk allele would have a direct effect on fetal growth via reduced insulin secretion27,44. Of the genes associated only with birthweight in the placenta, SLC38A1 was the only one previously associated with birthweight23. The protein encoded by this gene is an amino acid transporter and plays critical roles in the uptake of nutrients, energy production, and chemical metabolism. The other 14 genes with expression levels only associated with the outcome in the placenta have not been previously tied to birthweight. The only enrichment among the genes associated with birthweight in the placenta was for a reactome pathway (transmission across chemical synapses). No known biological process or molecular function was over- or under-represented in this collection of genes. However, variants in these genes have been associated with related phenotypes. Several variants nearby implicated genes are associated with metabolic and hormonal traits, such as fasting blood glucose (VARS)45, triglyceride measurement (KDM4B)46, and 17-hydroxyprogesterone (MDC1)47, and may represent diverse molecular pathways involved in regulation of birthweight. Polymorphisms in these genes have also been tied to Type II diabetes (PAX4)48, height (KDM4B)40, and growth retardation and diabetic complications in mammalian knockout models (PAX4)49. Furthermore, two genes which were not previously reported as associated with birthweight (MDC1, PAX4), as well as SLC38A1, exist in topologically associated domains with SNPs that were previously associated with birthweight23. These observations from Warrington et al. support the validity of our findings that genetically predicted gene expression of these genes is associated with inter-individual variation in birth weight.
We found a larger number of genes where placental expression, specifically, was associated with BMI. Again, the biological processes and molecular functions of the genes varied widely. Notable findings include the negative association between SIGLEC6 expression and BMI (p-value: 1.75 × 10−8). This gene is expressed nearly exclusively in the placenta and nearby associations with BMI have previously been attributed to other genes. However, there is a clear potential causal mechanism linking SIGLEC6 and BMI. The gene encodes a transmembrane receptor that binds sialyl-TN glycans and leptin, the latter of which is a hormone predominantly made by adipose cells and functions in energy homeostasis, neuroendocrine function, and metabolism. SIGLEC6 is also involved in sialic acid biology and its increased expression has been found in placentas from pregnancies complicated by preterm preeclampsia50. The most significant gene of all tested associations was FTO-IT1. Its expression was exclusively associated with adult BMI in the placenta with increased expression associated with an increase BMI. Though the exact physiological function of the gene is unknown, as a long noncoding ribonucleic acid it likely functions in transcription regulation, potentially of the FTO gene which has a well-known association with weight and BMI and is hypothesized to play a role in regulation of appetite51.
Gene expression of PAX4 was the only commonality between the birthweight and BMI analyses. For both birthweight and BMI, this gene’s expression was significantly tied to the outcomes exclusively in the placenta. PAX4, a member of the paired-box family of transcription factors, plays a critical role in fetal development, particularly in the differentiation and development of pancreatic islet beta cells. This gene has not been previously tied to birthweight or BMI but has been linked to Type II diabetes, a phenotype closely linked to increasing BMI, as well as energy storage, metabolism, and homeostasis.
In our study, birthweight and adult BMI had limited similarities in gene expression patterns in the placenta. This could be due to the timing of the phenotypes in relation to the existence of the placenta. Differences may occur due to biological proximity of the placenta to birthweight compared to adult BMI. The small overlap between the two phenotypes’ genes where expression was significantly associated with the outcome exclusively in placental tissue is in line with other studies of weight and BMI, which have found differing genetic factors influence these outcomes at different periods in life52,53. Another likely contributor to the differences in placental gene expression associations with birthweight and adult BMI is their phenotype definitions. The neonatal outcome of birthweight is the sum of both fat-free mass and fat mass54. It does not discriminate between these two masses. Changes in weight could represent alterations in fat, muscle, fluids, bone, or combinations of these components54. Thus, body weight, though often used to assess nutritional and health status, is unable to quantify body composition and is a poor indicator of obesity. In comparison, BMI is a measure of weight adjusted for height55. It incorporates components of both body structure (e.g., skeletal mass, limb length, etc.) and body composition (e.g., fat mass, etc.)56. Differences in placental expression patterns may be due to BMI accounting for height; whereas, the neonatal phenotype does not account for body shape. Regardless of the small overlap in significant placental gene expression, these results highlight the importance of the gestational period in defining disease risk57.
As this study utilized genetically-predicted gene expression, expression level estimates did not account for components of expression attributable to environment and other factors. However, our approach of estimating genetically-predicted gene expression and its associations with outcomes is not subject to confounding due to trait- or outcome-altered expression. Direct measurement of expression levels in such large samples would also require prohibitive resources. Thus, the use of S-PrediXcan offers the advantage of estimation of transcriptome measurement and its association with the outcomes without requiring actual transcript measurements. S-PrediXcan also takes GWAS summary statistics as input, allowing us to use publicly available datasets, leverage larger populations for both birthweight and BMI analyses, and achieve higher power. This study may have limited generalizability as both populations contained only those of European ancestry individuals. Future analyses should replicate this approach in more diverse, trans-ancestry populations as the data becomes available. Since birthweight and BMI have been linked to numerous outcomes, including gestational age, childhood BMI, and obesity, future studies should compare these results to results from S-PrediXcan analyses of related phenotypes. Studies directly measuring expression levels in the placenta and relating them to birthweight and BMI are also necessary and would aid in furthering the understand of environmental and trait-related effects on transcription.
We were unable to investigate potential impact or confounding effects caused by correlation between maternal and fetal genotypes. However, our results rely entirely on fetal genotypes and the placenta model is built using expression data collected exclusively from the neonatal side of the placenta. Therefore, it is unlikely that the results are due to this path of confounding. Future studies should aim to collect maternal data to account for correlation between maternal and fetal genotypes and compare results between them. Sample sizes for the placenta and 48 GTEx tissues varied (Supplementary Table S9). Variable sample size may have impacted discovery. In cases of drastically different sample sizes between the placenta and non-placental tissue, caution should be taken when comparing and interpreting results due to the potential differences in power. In these cases, further comparison based on the relative effect size might be more appropriate.
This study found hundreds of genes with expression associated with birthweight and adult BMI, several of which were only significantly associated in placental tissue. Many of the genes where expression was exclusively associated with the outcomes in the placenta have not been previously linked to birthweight or BMI. However, there were several genes, where expression was associated with the outcomes in only the placenta, which contain polymorphisms previously implicated in birthweight and BMI. This furthers the mechanistic understanding of the discoveries of previous studies as this analysis tested the molecular mechanisms through which genetic variation affects outcomes. Interestingly, limited overlap in the genes that were only associated with the outcomes in the placenta was observed between birthweight and adult BMI. This is in line with previous studies of weight and BMI, which have suggested that different genetic factors influence these outcomes at different periods in life. In conclusion, inter-individual variation of gene expression in placental tissue may contribute to the observed variation observed in short-term outcomes, like birthweight, as well as long-term outcomes, like adult BMI. Results of this study further support a developmental origin of both birthweight and adult BMI, with placental gene expression providing a possible mechanistic link between exposures in utero and later life health.
Methods
Birthweight summary statistics
Birthweight summary statistics were obtained from the most recent Early Growth Genetics Consortium (EGG) GWAS24. The EGG Consortium birthweight summary statistics were downloaded at www.egg-consortium.org. The EGG summary statistics were obtained from a genome-wide meta-analysis of birthweight z-score, which combined data from the EGG Consortium and the UK Biobank23,58. The summary statistics contained up to 298,142 European ancestry participants.
Body mass index summary statistics
Adult BMI GWAS summary statistics were obtained from the most recent Genetic Investigation of ANthropometric Traits (GIANT) consortium publication22. Up to 681,275 individuals, obtained from the UK Biobank and 114 other GWAS studies, were included in the analyses of roughly 2.4 million SNPs.
Placental gene expression source data
Placental gene expression and eQTLs were evaluated and computed using published gene expression data from the Rhode Island Child Health Study19. Gene expression data on 150 samples were derived from placenta tissue excluding maternal decidua and processed using whole transcriptome RNAseq. Whole genome genotyping (Illumina MEGAex Array, Illumina Inc., San Diego, CA) was used for generating eQTLs, which have been previously published19.
Construction of placental gene expression models
The eQTL summary statistics were processed into genetically predicted expression models. Total eQTLs were filtered within each gene for false discovery rate (FDR)-adjusted p-value less than 0.1 and linkage disequilibrium (LD) clumping was performed (0.1 r2 and 250 kilobase window). To retain only those genes with substantial genetic regulation in the placenta, the variance explained by each eQTL SNP was calculated as 2pqβ2, where β is the effect size for eQTL association from the original summary statistics file, p is the frequency of allele 1, and q is the frequency of allele 2. The sum of SNP variances was computed for each gene and genes were ordered by expression variance explained. Genes with variance of greater than two and less than 0.01 were excluded from the final prediction models. Final models utilized 25,885 genetic variants associated with expression of 15,154 genes.
Genetically-predicted gene expression analyses
Gene expression was estimated in placental tissue using birthweight and adult BMI summary GWAS statistics separately with S-PrediXcan. S-PrediXcan calculates effects and tests for association between outcomes and the genetically determined component of expression for genes in each tissue using SNP-level association and eQTL summary statistics59. To identify genes where expression levels are only associated with the outcomes in the placenta, S-PrediXcan was also used to obtain gene expression estimates in 48 other tissues, for both phenotypes, using existing models from the Genotype Tissue Expression (GTEx v7) project (predictdb.org). GTEx and prediction models based on the data from it utilized samples from non-diseased tissues collected from both male and female donors from a variety of races and ages. Sample sizes varied based on tissue and ranged from 70 to over 400 for version 7. Gene associations with p-values less than 0.05 were considered nominally significant. All effect sizes are presented with regard to increasing predicted gene expression. Genes significantly associated with the outcomes in the placenta were compared to results in the GWAS Catalog. Genes reported and mapped using the GWAS Catalog rules for each outcome were considered and discussed in results as previously implicated in GWAS studies. All results are based on publicly available summary statistics and do not constitute human subjects research.
Functional annotation
Significant genes from the birthweight and BMI analysis were compared. The web-based annotation tool Functional Mapping and Annotation of Genome-Wide Association Studies (FUMA) gene2func was used to test for their enrichment in cell types and pre-defined biological pathways for all significant associations in the placenta60,61.
Data availability
Publicly available data analyzed during this study are included in published studies from the Early Growth Genetics (EGG) Consortium23 and the Genetic Investigation of ANthropometric Traits (GIANT) consortium22. Any additional information and data are available upon reasonable request. The data and materials can be shared by Dr. Digna R. Velez Edwards (Digna.r.velez.edwards@vumc.org) upon reasonable request.
References
Hales, C. M., Carroll, M. D., Fryar, C. D. & Ogden, C. L. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief 10, 1–8 (2020).
National Heart Lung and Blood Institute (NHLBI). (ed NHLBI) (2013).
Waters, H. & Graf, M. America’s Obesity Crisis: The Health and Economic Costs of Excess Weight (Santa Monica, 2018).
Tseng, E. et al. Effectiveness of policies and programs to combat adult obesity: A systematic review. J. Gen. Intern. Med. 33, 1990–2001. https://doi.org/10.1007/s11606-018-4619-z (2018).
Chan, R. S. & Woo, J. Prevention of overweight and obesity: How effective is the current public health approach. Int. J. Environ. Res. Public Health 7, 765–783. https://doi.org/10.3390/ijerph7030765 (2010).
Kvaavik, E., Tell, G. S. & Klepp, K. I. Predictors and tracking of body mass index from adolescence into adulthood: Follow-up of 18 to 20 years in the Oslo Youth Study. Arch. Pediatr. Adolesc. Med. 157, 1212–1218. https://doi.org/10.1001/archpedi.157.12.1212 (2003).
Rundle, A. G. et al. Tracking of obesity in childhood into adulthood: Effects on body mass index and fat mass index at age 50. Child Obes. 16, 226–233. https://doi.org/10.1089/chi.2019.0185 (2020).
Liu, J. X. et al. Body mass index trajectories during infancy and pediatric obesity at 6 years. Ann. Epidemiol. 27, 708–715. https://doi.org/10.1016/j.annepidem.2017.10.008 (2017).
Munthali, R. J., Kagura, J., Lombard, Z. & Norris, S. A. Early life growth predictors of childhood adiposity trajectories and future risk for obesity: Birth to twenty cohort. Child Obes. 13, 384–391. https://doi.org/10.1089/chi.2016.0310 (2017).
Pryor, L. E. et al. Developmental trajectories of body mass index in early childhood and their risk factors: An 8-year longitudinal study. Arch. Pediatr. Adolesc. Med. 165, 906–912. https://doi.org/10.1001/archpediatrics.2011.153 (2011).
Roy, S. M. et al. Body mass index (BMI) trajectories in infancy differ by population ancestry and may presage disparities in early childhood obesity. J. Clin. Endocrinol. Metab. 100, 1551–1560. https://doi.org/10.1210/jc.2014-4028 (2015).
Stuart, B. & Panico, L. Early-childhood BMI trajectories: Evidence from a prospective, nationally representative British cohort study. Nutr. Diabetes 6, e198. https://doi.org/10.1038/nutd.2016.6 (2016).
Willers, S. M. et al. BMI development of normal weight and overweight children in the PIAMA study. PLoS ONE 7, e39517. https://doi.org/10.1371/journal.pone.0039517 (2012).
Belbasis, L., Savvidou, M. D., Kanu, C., Evangelou, E. & Tzoulaki, I. Birth weight in relation to health and disease in later life: An umbrella review of systematic reviews and meta-analyses. BMC Med. 14, 147. https://doi.org/10.1186/s12916-016-0692-5 (2016).
Schellong, K., Schulz, S., Harder, T. & Plagemann, A. Birth weight and long-term overweight risk: Systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PLoS ONE 7, e47776. https://doi.org/10.1371/journal.pone.0047776 (2012).
Barker, D. J. The fetal and infant origins of adult disease. BMJ 301, 1111 (1990).
Barker, D. J. The origins of the developmental origins theory. J. Intern. Med. 261, 412–417. https://doi.org/10.1111/j.1365-2796.2007.01809.x (2007).
Gude, N. M., Roberts, C. T., Kalionis, B. & King, R. G. Growth and function of the normal human placenta. Thromb. Res. 114, 397–407. https://doi.org/10.1016/j.thromres.2004.06.038 (2004).
Peng, S. et al. Expression quantitative trait loci (eQTLs) in human placentas suggest developmental origins of complex diseases. Hum. Mol. Genet. 26, 3432–3441. https://doi.org/10.1093/hmg/ddx265 (2017).
Peng, S. et al. Genetic regulation of the placental transcriptome underlies birth weight and risk of childhood obesity. PLoS Genet. 14, e1007799. https://doi.org/10.1371/journal.pgen.1007799 (2018).
Tekola-Ayele, F. et al. Genetic overlap between birthweight and adult cardiometabolic diseases has implications for genomic medicine. Sci. Rep. 9, 4076. https://doi.org/10.1038/s41598-019-40834-w (2019).
Yengo, L. et al. Meta-analysis of genome-wide association studies for height and body mass index in approximately 700000 individuals of European ancestry. Hum. Mol. Genet. 27, 3641–3649. https://doi.org/10.1093/hmg/ddy271 (2018).
Warrington, N. M. et al. Maternal and fetal genetic effects on birth weight and their relevance to cardio-metabolic risk factors. Nat. Genet. 51, 804–814. https://doi.org/10.1038/s41588-019-0403-1 (2019).
Plotnikov, D., Williams, C. & Guggenheim, J. A. Association between birth weight and refractive error in adulthood: A Mendelian randomisation study. Br. J. Ophthalmol. 104, 214–219. https://doi.org/10.1136/bjophthalmol-2018-313640 (2020).
Horikoshi, M. et al. Genome-wide associations for birth weight and correlations with adult disease. Nature 538, 248–252. https://doi.org/10.1038/nature19806 (2016).
Horikoshi, M. et al. New loci associated with birth weight identify genetic links between intrauterine growth and adult height and metabolism. Nat. Genet. 45, 76–82. https://doi.org/10.1038/ng.2477 (2013).
Freathy, R. M. et al. Variants in ADCY5 and near CCNL1 are associated with fetal growth and birth weight. Nat. Genet. 42, 430–435. https://doi.org/10.1038/ng.567 (2010).
Vujkovic, M. et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat. Genet. 52, 680–691. https://doi.org/10.1038/s41588-020-0637-y (2020).
Kilpelainen, T. O. et al. Genetic variation near IRS1 associates with reduced adiposity and an impaired metabolic profile. Nat. Genet. 43, 753–760. https://doi.org/10.1038/ng.866 (2011).
Li, H. et al. Association of genetic variation in FTO with risk of obesity and type 2 diabetes with data from 96,551 East and South Asians. Diabetologia 55, 981–995. https://doi.org/10.1007/s00125-011-2370-7 (2012).
Helgeland, O. et al. Genome-wide association study reveals dynamic role of genetic variation in infant and early childhood growth. Nat. Commun. 10, 4448. https://doi.org/10.1038/s41467-019-12308-0 (2019).
Justice, A. E. et al. Genome-wide meta-analysis of 241,258 adults accounting for smoking behaviour identifies novel loci for obesity traits. Nat. Commun. 8, 14977. https://doi.org/10.1038/ncomms14977 (2017).
Yang, J. et al. FTO genotype is associated with phenotypic variability of body mass index. Nature 490, 267–272. https://doi.org/10.1038/nature11401 (2012).
Locke, A. E. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 518, 197–206. https://doi.org/10.1038/nature14177 (2015).
Pulit, S. L. et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum. Mol. Genet. 28, 166–174. https://doi.org/10.1093/hmg/ddy327 (2019).
Hoffmann, T. J. et al. A large multiethnic genome-wide association study of adult body mass index identifies novel loci. Genetics 210, 499–515. https://doi.org/10.1534/genetics.118.301479 (2018).
Zhu, Z. et al. Shared genetic and experimental links between obesity-related traits and asthma subtypes in UK Biobank. J. Allergy Clin. Immunol. 145, 537–549. https://doi.org/10.1016/j.jaci.2019.09.035 (2020).
Winkler, T. W. et al. The influence of age and sex on genetic associations with adult body size and shape: A large-scale genome-wide interaction study. PLoS Genet. 11, e1005378. https://doi.org/10.1371/journal.pgen.1005378 (2015).
Akiyama, M. et al. Genome-wide association study identifies 112 new loci for body mass index in the Japanese population. Nat. Genet. 49, 1458–1467. https://doi.org/10.1038/ng.3951 (2017).
Kichaev, G. et al. Leveraging polygenic functional enrichment to improve GWAS power. Am. J. Hum. Genet. 104, 65–75. https://doi.org/10.1016/j.ajhg.2018.11.008 (2019).
Sakaue, S. et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 53, 1415–1424. https://doi.org/10.1038/s41588-021-00931-x (2021).
Graff, M. et al. Genome-wide physical activity interactions in adiposity—A meta-analysis of 200,452 adults. PLoS Genet. 13, e1006528. https://doi.org/10.1371/journal.pgen.1006528 (2017).
Chen, J. et al. The trans-ancestral genomic architecture of glycemic traits. Nat. Genet. 53, 840–860. https://doi.org/10.1038/s41588-021-00852-9 (2021).
Hattersley, A. T. & Tooke, J. E. The fetal insulin hypothesis: An alternative explanation of the association of low birthweight with diabetes and vascular disease. Lancet 353, 1789–1792. https://doi.org/10.1016/S0140-6736(98)07546-1 (1999).
Hebbar, P. et al. Genome-wide association study identifies novel risk variants from RPS6KA1, CADPS, VARS, and DHX58 for fasting plasma glucose in Arab population. Sci. Rep. 10, 152. https://doi.org/10.1038/s41598-019-57072-9 (2020).
Richardson, T. G. et al. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLoS Med. 17, e1003062. https://doi.org/10.1371/journal.pmed.1003062 (2020).
Pott, J. et al. Genetic association study of eight steroid hormones and implications for sexual dimorphism of coronary artery disease. J. Clin. Endocrinol. Metab. 104, 5008–5023. https://doi.org/10.1210/jc.2019-00757 (2019).
Mahajan, A. et al. Refining the accuracy of validated target identification through coding variant fine-mapping in type 2 diabetes. Nat. Genet. 50, 559–571. https://doi.org/10.1038/s41588-018-0084-1 (2018).
Xu, Y. et al. Generation and phenotype identification of PAX4 gene knockout rabbit by CRISPR/Cas9 system. G3 (Bethesda) 8, 2833–2840. https://doi.org/10.1534/g3.118.300448 (2018).
Rumer, K. K., Uyenishi, J., Hoffman, M. C., Fisher, B. M. & Winn, V. D. Siglec-6 expression is increased in placentas from pregnancies complicated by preterm preeclampsia. Reprod. Sci. 20, 646–653. https://doi.org/10.1177/1933719112461185 (2013).
Huang, T. et al. FTO genotype, dietary protein, and change in appetite: The preventing overweight using novel dietary strategies trial. Am. J. Clin. Nutr. 99, 1126–1130. https://doi.org/10.3945/ajcn.113.082164 (2014).
Couto Alves, A. et al. GWAS on longitudinal growth traits reveals different genetic factors influencing infant, child, and adult BMI. Sci. Adv. 5, 3095. https://doi.org/10.1126/sciadv.aaw3095 (2019).
Guo, G., Liu, H., Wang, L., Shen, H. & Hu, W. The genome-wide influence on human BMI depends on physical activity, life course, and historical period. Demography 52, 1651–1670. https://doi.org/10.1007/s13524-015-0421-2 (2015).
Madden, A. M. & Smith, S. Body composition and morphological assessment of nutritional status in adults: A review of anthropometric variables. J. Hum. Nutr. Diet 29, 7–25. https://doi.org/10.1111/jhn.12278 (2016).
Klish, W. J. & Skelton, J. A. Definition; epidemiology; and etiology of obesity in children and adolescents, https://www.uptodate.com/contents/definition-epidemiology-and-etiology-of-obesity-in-children-and-adolescents (2018).
McCarthy, H. D. Measuring growth and obesity across childhood and adolescence. Proc. Nutr. Soc. 73, 210–217. https://doi.org/10.1017/S0029665113003868 (2014).
Liu, S., Jones, R. N. & Glymour, M. M. Implications of lifecourse epidemiology for research on determinants of adult disease. Public Health Rev. 32, 489–511. https://doi.org/10.1007/BF03391613 (2010).
Sudlow, C. et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779. https://doi.org/10.1371/journal.pmed.1001779 (2015).
Barbeira, A. N. et al. Exploring the phenotypic consequences of tissue specific gene expression variation inferred from GWAS summary statistics. Nat. Commun. 9, 1825. https://doi.org/10.1038/s41467-018-03621-1 (2018).
Watanabe, K., Taskesen, E., van Bochoven, A. & Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 8, 1826. https://doi.org/10.1038/s41467-017-01261-5 (2017).
Watanabe, K., Umicevic Mirkov, M., de Leeuw, C. A., van den Heuvel, M. P. & Posthuma, D. Genetic mapping of cell type specificity for complex traits. Nat. Commun. 10, 3222. https://doi.org/10.1038/s41467-019-11181-1 (2019).
Funding
The authors have no relevant financial or non-financial interests to disclose. Support for this research was provided by a National Institute on Minority Health and Health Disparities award for the Center of Excellence in Precision Medicine and Population Health (U54MD010722 to D.R.V.E.), a National Human Genome Research Institute award for the Vanderbilt Genomic Medicine Training Program (T32HG008341 to E.A.J.), a Eunice Kennedy Shriver National Institute of Child and Human Development award for the Building Interdisciplinary Research Careers in Women’s Health career development program (K12HD043483 to E.A.J. and J.N.H.), and a National Institute of General Medical Sciences award for the Training Program in Genetic Variation and Human Phenotypes (T32GM080178 to J.A.P.) at Vanderbilt University Medical Center.
Author information
Authors and Affiliations
Contributions
E.A.J conceptualized and designed the study, carried out analyses, interpreted the results, and prepared the initial article, and reviewed and revised the manuscript. J.N.H. contributed to the design of the study, analyses, interpretation, and preparation of the manuscript. J.A.P. and B.M. contributed to data acquisition and portions of the analysis as well as the preparation of the manuscript. S.H.J. contributed to data acquisition and the preparation of the manuscript. Both K.E.H. and D.M.A. made substantial contributions to the interpretation of data and the initial and final versions of the manuscript. T.L.E. and D.V.E. conceptualized and designed the study, coordinated, and supervised data acquisition and storage, made substantial contributions to the interpretation of data, and critically revised the manuscript. All authors approved the final article as submitted and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jasper, E.A., Hellwege, J.N., Piekos, J.A. et al. Genetically-predicted placental gene expression is associated with birthweight and adult body mass index. Sci Rep 13, 322 (2023). https://doi.org/10.1038/s41598-022-26572-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-26572-6
- Springer Nature Limited