Abstract
This systematic review and meta-analysis aim to provide the best evidence on the association between metabolic syndrome (MetS) and uric acid (UA) by determining the size of the effect of this biomarker on MetS. The review protocol is registered with PROSPERO (CRD42021231124). The search covered the PubMed and Scopus databases. Methodological quality was assessed with the STROBE tool, overall risk of bias with RevMan (Cochrane Collaboration) and quality of evidence with Grade Pro. Initially, 1582 articles were identified. Then, after excluding duplicates and reviewing titles and abstracts, 1529 articles were excluded from applying the eligibility criteria. We included 43 papers (56 groups) comparing UA concentrations between subjects 91,845 with MetS and 259,931 controls. Subjects with MetS had a higher mean UA of 0.57 mg/dl (95% CI 0.54–0.61) (p < 0.00001). Given the heterogeneity of the included studies, the researchers decided to perform subgroups analysis. Men with MetS have a higher UA concentration mg/dl 0.53 (95% CI 0.45–0.62, p < 0.00001) and women with MetS 0.57 (95% CI 0.48–0.66, p < 0.00001) compared to subjects without MetS. Assessment of UA concentration could provide a new avenue for early diagnosis of MetS, as a new biomarker and the possibility of new therapeutic targets.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS) is defined as a set of metabolic abnormalities, including dysglycaemia, central obesity, dyslipidaemia (elevated triglycerides and decreased HDL-cholesterol) and hypertension. These alterations increase the risk of type 2 diabetes mellitus and cardiovascular disease1. The pathogenesis of Mets is not well understood but involves complex interactions between genetic background, hormones, and environmental factors such as air pollution, toxins and nutrients2. Previous evidence supports that insulin resistance (IR), oxidative stress and low-grade inflammation play a central role3.
Chronic low-grade systemic inflammation appears to be a central mechanism underlying the pathophysiology of MetS3,4. This inflammation is characterised by an increase in pro-inflammatory mediators and the activation of several inflammatory pathways that are significantly associated with cardiovascular events5. In addition, the increased concentration of pro-inflammatory substances is primarily related to obesity, especially central obesity, resulting in altered endocrine function of visceral adipose tissue6.
Due to the increasing prevalence of obesity, the prevalence of MetS has grown worldwide, and it is expected to continue increasing in the coming years7. In this respect, the adult population with MetS is estimated between 20 and 30% in most countries8.Due to the complexity of MetS, with diverse influences and implications for other diseases, it is not easy to make a clear-cut distinction of the diagnostic ability of the various biomarker groups. Moreover, the subdivision has limitations: the complexity of the syndrome, interactions of various biochemical pathways and the overlap of markers9.
Nevertheless, some studies have shown an association between MetS and the following variables indicative of inflammatory processes: uric acid (UA), C-reactive protein (CRP), liver transaminases (ALT), erythrocyte sedimentation rate (ESR), leukocytes, among others10,11,12. Likewise, through magnetic resonance spectroscopy, different metabolites have been identified in urine, highlighting glucose, lipids, aromatic amino acids, salicylic acid, maltitol, trimethylamine N-oxide and p-cresol sulphate, which have been associated with the progression of MetS13.
UA is an enzymatic end product of purine metabolism in humans14. Hyperuricaemia is a metabolic disease caused by increased formation or reduced serum uric acid (SUA) excretion. Alterations in SUA homeostasis have been correlated with several diseases such as gout, MetS, cardiovascular disease, diabetes, hypertension and kidney disease15.
Although SUA levels are often associated with MetS16,17, hyperuricaemia is not included among the diagnostic criteria that have been proposed internationally for the definition of this pathology. However, the pro-oxidant action of hyperuricaemia may induce inflammation and endothelial dysfunction by decreasing the availability of nitric oxide, thus promoting the development of the pathologies discussed above18,19,20,21.
Given that the prevalence of MetS increases worldwide and raises the risk of morbidity and mortality, identifying biomarkers for the early detection of this pathology is of great importance22. Therefore, the main Aim is to provide the best evidence on the association between MetS and UA by determining the effect size of this biomarker.
Methods
Literature search and selection
A systematic review and meta-analysis were carried out, following the criteria established by the PRISMA statement23. The search covered the PubMed and Scopus databases. The search strategy was developed by combining the following Medical Subject Headings (MeSH) descriptors: "metabolic syndrome", "uric acid", using the Boolean operator AND. The review was carried out from 2015 to May 2021. In addition, hand searching the reference lists of included studies supplemented the tracking of the available literature. The systematic review was registered in PROSPERO with ID CRD42021231124.
Eligibility criteria
We included longitudinal, cross-sectional, case–control and cohort studies, which investigated the association between MetS and UA. In addition, their results had to include the mean and standard deviation of the study parameters. Furthermore, only papers in English and Spanish and those articles collected data in subjects older than 18 years were considered. Finally, abstracts and unpublished studies comparing subjects with and without MetS were excluded.
Data collection
Two authors (E.R.C. and M.R.S.) separately screened all articles obtained in the search to eliminate duplicates. Then, two other authors (D.P.J. and R.M.L.) independently read the title and abstract and applied the eligibility criteria to select the articles that were finally included in the review. Finally, a fifth authors (M.V.A.) acted as a judge in case of discrepancy. One researcher (E.R.C.) oversaw extracting the data, verified by a second researcher (G.M.R.). A third researcher (M.R.S.) resolved the disagreement in case of a tie.
The extracted articles were drawn up with a table with the main characteristics (author, year, country, study design, reporting guidelines, age of participants, MetS, Aims, conclusions).
The following data were extracted from each study: citation, details of the study population (including age and sex), study design, sample size, study, aims, the mean and standard deviation of UA in those subjects with and without MetS.
Evaluation of the qualitative synthesis
Four authors (R.M.L., D.P.J., G.M.R. and E.R.C.) were responsible for the evaluation of the qualitative synthesis through a triple analysis:
-
1.
Assessment of methodological quality. The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement24 was used for observational studies.
-
2.
Risk of bias assessment. Researchers were using the Cochrane Collaboration25 tool included in the REVMAN 5.4.2. software, the risks of selection, conduct, detection, attrition, and reporting were analysed.
-
3.
Assessment of the quality of evidence. With the help of the Grade Protool, the evidence profile table was developed, establishing the following levels26:
-
High: high confidence in the match between the actual and estimated effect.
-
Moderate: moderate confidence in the effect estimate. There is a possibility that the actual effect is far from the estimated effect.
-
Low: limited confidence in the estimate of the effect. The actual effect may be far from the estimated effect.
-
Very low: low confidence in the estimated effect. The actual effect is very likely to be different from the estimated effect.
-
Statistical analysis (evaluation of the quantitative synthesis or meta-analysis)
For the meta-analysis, the Cochrane Review Manager software (RevMan 5.4.2) was used to perform the statistical calculations and create the forest plots and funnel plots. Due to the difference in effect size of the included studies, a meta-analysis was performed using the Mantel–Haenszel random-effects method according to the DerSimonian and Laird model. The difference between arithmetic means with a 95% confidence interval was used to measure effect size. The risk of publication bias was assessed using the funnel plot. Heterogeneity was analysed using the Chi-square test and the inconsistency index (I2). According to the Cochrane Collaboration tool, heterogeneity was classified as: unimportant (0–40%), moderate (30–60%), substantial (50–90%) and considerable (75–100%).
Results
Characteristics of the studies
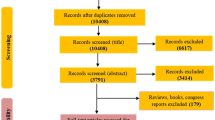
Initially, 1582 articles were identified. Then, after excluding duplicates and reviewing titles and abstracts, 1529 articles were excluded from applying the eligibility criteria. Finally, a total of 43 articles were selected for systematic review and meta-analysis (Fig. 1).Given the large number of articles found in the search, it was divided into three subgroups: (i) articles providing UA data globally without distinction of sex (n = 24); (ii) articles with disaggregated data for men (n = 17) and (iii) women (n = 15). The detailed characteristics of the selected studies are shown in Table 1. Regarding research design, all studies were observational. Twenty-seven studies27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53 defined MetS according to the third report of the National Cholesterol Education Program (NCEP-Adult Treatment Panel (ATP III)54. Seven studies55,56,57,58,59,60,61 assessed metabolic syndrome using the International Diabetes Federation (IDF) criteria62. Four studies63,64,65,66 used the harmonised criteria67. Three studies68,69,70 used Chinese Medical Association criteria71; Sumiyoshi et al.72 used the Japanese criteria73 and, finally, Osadnik74 used the criteria defined in the study by Buscemi et al.75.
Concerning the articles' origin, twelve (27.9%) were conducted in China34,38,39,42,48,50,61,64,66,68,69,70. In total, the 43 selected papers compared UA concentrations between 91,845 subjects with MetS and 259931controls. The age of study participants ranged from 18 to 90 years.
Methodological quality assessment
All papers scored 16 points or more out of the 22 items included (highest tercile). No article was excluded for insufficient methodological quality. Table 1 shows a column with the score for each of the reports.
Bias risk analysis
Overall (Fig. 2), the main biases were: random sequential generation, allocation and participant and staff concealment, and blinding of outcome assessment, affecting 72% of the reports. Figure 3 represents the individual assessment of the included studies.
Quantitative analysis. Meta-analysis
Meta-analysis 1
This analysis comprises 43 papers, including men and women, together or separately, resulting in 56 groups (Fig. 4). Subjects with MetS had a mean UA 8.2% higher than those without this syndrome (5.89 mg/dl vs. 5.44 mg/dl; p < 0.00001). The funnel plot (Fig. 5) shows a low risk of publication bias. The sensitivity analysis performed to assess the pooled estimate's stability concerning each meta-analysis study did not show that any study significantly affected the heterogeneity of the meta-analysis; therefore, none was excluded. Given the heterogeneity of the included studies, it was decided to perform subgroup analysis.
Meta-analysis 2
Figure 6, which includes 17 studies, represents the results obtained when analysing the presence of UA in men with and without MetS. In this case, men with MetS showed a higher mean UA, (0.53 mg/dl; 95% CI 0.45 − 0.62; p < 0.00001; I2 = 97%). Figure 7 shows that there is a low risk of publication bias.
Meta-analysis 3
Figure 8 compiles the results of 15 studies that examined the association between UA in women and the presence of MetS. The results show that UA level was associated with the diagnosis of METS (0.57 mg/dl; 95% CI 0.48–0.66; p < 0.00001; I2 = 97%). This meta-analysis also observed a low risk of publication bias (Fig. 9).
Quality of evidence
Table 2 shows the evidence profile of the three meta-analyses, providing specific information regarding the overall certainty of the evidence of the studies included in the comparison, the magnitude of the studies examined and the sum of the data available for the outcomes assessed.
Discussion
A systematic review and meta-analysis were conducted to analyse the most recent evidence on the relationship between MetS and UA. Forty-three studies were selected, the effect size and the limitations that have conditioned the results of the different studies were quantified.
Of the included papers, 26 directly associated UA with MetS28,29,30,33,35,36,38,40,41,42,43,44,45,46,48,49,50,56,57,59,60,61,63,65,66,72, and 17 reports collected data indirectly27,31,32,34,37,39,47,51,52,53,57,58,64,68,69,70,74, i.e. they study parameters related to MetS and collect data associated with UA. These studies had limitations, but overall, all demonstrated a sufficient degree of methodological reliability and quality in terms of the association of UA and MetS.
This meta-analysis provides evidence of a relationship between UA level and MetS. The concentration of UA in subjects with MetS was significantly higher than in the control group. The meta-analysis is notable for its large sample size, with 91,845 subjects in the MetS group and 259,931 in the control group. Given the heterogeneity of the included studies, it was decided to perform subgroup analysis. The results obtained show that men with MetS have a higher UA concentration than those without MetS (mean difference (MD): mg/dl 0.53; 95% CI 0.45–0.62; p < 0.00001). This was also observed in women (MD 0.57 mg/dl; 95% CI 0.48–0.66, p < 0.00001).
Changes in the UA concentrations in human fluids can reflect the metabolic state, immunity, and other human body functions. If the concentration of UA in the blood exceeds normal, the human body fluid becomes acidic, which affects the normal function of human cells, leading to long-term metabolic disease76. UA correlates with obesity, diabetes mellitus76, hypertension77, cardiovascular disease78 and chronic kidney disease79, where UA acts as an oxidant, inducing oxidative stress and endothelial dysfunction80.
Previous studies have reported significant associations between hyperuricaemia and individual elements of the metabolic syndrome81,82. The study by Norvik et al.83 showed that elevated UA levels are associated with components of the MetS, such as hypertriglyceridaemia, insulin resistance, elevated blood pressure and low high-density lipoprotein cholesterol. Xu et al.84 concluded that the relationship between SUA and elevated body mass index, hypertension and hyperglycaemia varies by sex. Reducing SUA levels by adopting a healthier lifestyle may be a valuable strategy to reduce the burden of MetS84.
Overall, the results have shown that people with MetS have 8.2% more UA, so reducing UA could positively impact the development of this syndrome. The results found by several authors85,86,87 support this. Yuan et al.85, in a meta-analysis based on prospective studies of various populations, suggest that for every 1 mg/dl increase in SUA level, the risk of MetS increases by 30% with a linear dose–response relationship. Liu et al.86 observed a consistent and linear causality of increased UA on the incidence of MetS, concluding that SUA could be an individualised predictor in detecting systemic/hepatic metabolic abnormalities. It is estimated that people with high UA are 1.6 times more likely to develop MetS87. Therefore, reducing SUA levels could be a potential treatment to prevent comprehensive metabolic disorders.
At the methodological level, the assessment of risks of bias in studies is a major issue in this type of research, in line with PRISMA recommendations. Studies with similar methodologies but with discrepancies in quality may have biased results. Among all the papers included in this review, only ten studies29,35,38,41,42,50,56,63,65,68 had performed this step correctly. The quality of the evidence obtained is "very low" since observational studies have been analysed where there is a high risk of bias and, in addition, they present a very high inconsistency (heterogeneity).
One of the main strengths of this review is the comprehensive search that covered a wide geographical area. In addition, a large sample size of subjects with and without MetS was included, which strengthened the study's statistical power.
The interpretation of the findings in this systematic review and meta-analysis must be made considering some limitations. First, most of the studies are from China, making it difficult to generalise the results to other countries. Author bias should also be a limitation since the same research team wrote several studies. Finally, it should be noted that there is still a lack of uniformly accepted diagnostic criteria for the diagnosis of MetS.
Conclusions
Current diagnostic criteria for MetS vary, although there is a consensus on the main components of the syndrome. None of these criteria includes UA levels in the definition of MetS.
The results have shown that UA levels are associated with the presence of MetS. In particular, subjects with MetS have been found to have higher plasma UA. The assessment of UA concentration could provide a new avenue for early diagnosis, identifying new biomarkers, and discovering new therapeutic targets.
A detailed understanding of the components of MetS is essential for the development of effective prevention strategies and appropriate intervention tools, which could curb its increasing prevalence and limit its comorbidity.
However, well-designed, high-quality randomised controlled trials are needed to confirm these findings.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Meng, G. et al. Comparing the diagnostic ability of inflammatory markers in metabolic syndrome. Clin. Chim. Acta 475, 1–6. https://doi.org/10.1016/j.cca.2017.09.023 (2017).
Zapulla, D. Environmental stress, erythrocyte dysfunctions, inflammation and the metabolic syndrome: Adaptations to CO2 increases. J. Cardiometab. Syndr. 3(1), 30–34. https://doi.org/10.1111/j.1559-4572.2008.07263.x (2008).
Ranasinghe, P., Mathangasinghe, Y., Jayawardena, R., Hills, A. P. & Misra, A. Prevalence and trends of metabolic syndrome among adults in the Asia-Pacific region: A systematic review. BMC Public Health 17(1), 101. https://doi.org/10.1186/s12889-017-4041-1 (2017).
Welty, F. K., Alfaddagh, A. & Elajami, T. K. Targeting inflammation in metabolic syndrome. Transl. Res. 167(1), 257–280. https://doi.org/10.1016/j.trsl.2015.06.017 (2016).
Akboga, M. K. et al. Platelet to lymphocyte ratio as a novel indicator of inflammation is correlated with the severity of metabolic syndrome: A single center large-scale study. Platelets 27(2), 178–183. https://doi.org/10.3109/09537104.2015.1064518 (2016).
Liang, Y. et al. Childhood obesity affects adult metabolic syndrome and diabetes. Endocrine 50(1), 87–92. https://doi.org/10.1007/s12020-015-0560-7 (2015).
Gao, H. G. et al. High-sensitivity C-reactive protein leads to increased incident metabolic syndrome in women but not in men: A five-year follow-up study in a Chinese population. Diabetes Metab. Syndr. Obes. 13, 581–590. https://doi.org/10.2147/dmso.s241774 (2020).
Grundy, S. M. Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol. 28(4), 629–636. https://doi.org/10.1161/ATVBAHA.107.151092 (2008).
Robberecht, H. & Hermans, N. Biomarkers of metabolic syndrome: Biochemical background and clinical significance. Metab. Syndr. Relat. Disord. 14(2), 47–93 (2016).
Sun, H. et al. The longitudinal increments of serum alanine aminotransferase increased the incidence risk of metabolic syndrome: A large cohort population in China. Clin. Chim. Acta 488, 241–247. https://doi.org/10.1016/j.cca.2018.10.033 (2019).
Zaha, C. D. et al. Influence of inflammation and adipocyte biochemical markers on the components of metabolic syndrome. Exp. Ther. Med. 20(1), 121–128. https://doi.org/10.3892/etm.2020.8663 (2020).
Vahit, D., Mehmet, K., Samet, Y. & Hüseyin, E. Assessment of monocyte to high density lipoprotein cholesterol ratio and lymphocyte-to-monocyte ratio in patients with metabolic syndrome. Biomark. Med. 11(7), 535–540. https://doi.org/10.2217/bmm-2016-0380 (2017).
Bruzzone, C. et al. A molecular signature for the metabolic syndrome by urine metabolomics. Cardiovasc. Diabetol. 20(1), 155. https://doi.org/10.1186/s12933-021-01349-9 (2021).
Chiou, W. K. et al. The relationship between serum uric acid level and metabolic syndrome: Differences by sex and age in Taiwanese. J. Epidemiol. 20, 219–224 (2010).
Liu, N. et al. The role of oxidative stress in hyperuricemia and xanthine oxidoreductase (XOR) inhibitors. Oxid. Med. Cell Longev. https://doi.org/10.1155/2021/1470380 (2021).
Nejatinamini, S. et al. Association between serum uric acid level and metabolic syndrome components. J. Diabetes Metab. Disord. 14(1), 70. https://doi.org/10.1186/s40200-015-0200-z (2015).
Gonçalves, J. P., Oliveira, A., Severo, M., Santos, A. C. & Lopes, C. Cross-sectional and longitudinal associations between serum uric acid and metabolic syndrome. Endocrine 41, 450–457. https://doi.org/10.1007/s12020-012-9629-8 (2012).
Grassi, D., Desideri, G. & Ferri, C. New insight into urate-related mechanism of cardiovascular damage. Curr. Pharm. Des. 20, 6089–6095. https://doi.org/10.2174/1381612820666140417095730 (2014).
Battelli, M. G., Bortolotti, M., Polito, L. & Bolognesi, A. The role of xanthine oxidoreductase and uric acid in metabolic syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 1864(8), 2557–2565. https://doi.org/10.1016/j.bbadis.2018.05.003 (2018).
Arrigo Francesco, G. C. et al. Serum uric acid predicts incident metabolic syndrome in the elderly in an analysis of the Brisighella Heart Study. Sci. Rep. 8, 11529. https://doi.org/10.1038/s41598-018-29955- (2018).
Foster, C., Smith, L. & Alemzadeh, R. Excess serum uric acid is associated with metabolic syndrome in obese adolescent patients. J. Diabetes Metab. Disord. 19(1), 535–543. https://doi.org/10.1007/s40200-020-00507-2 (2020).
Abdel-Moneim, A., Mahmoud, B., Sultan, E. A. & Mahmoud, R. Relationship of leukocytes, platelet indices and adipocytokines in metabolic syndrome patients. Diabetes Metab. Syndr. 13(1), 874–880. https://doi.org/10.1016/j.dsx.2018.12.016 (2019).
Urrutia, G. & Bonfill, X. Declaración PRISMA: Una propuesta para mejorar la publicación de revisiones sistemáticas y metaanálisis. Med. Clin. 135(11), 507–511. https://doi.org/10.1016/j.medcli.2010.01.015 (2010).
Von, E. E. et al. Declaración de la Iniciativa STROBE (Strengthening the Reporting of Observational studies in Epidemiology): Directrices para la comunicación de estudios observacionales. Gac. Sanit. 22, 144–150 (2008).
Higgins, J. P. T. et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928 (2011).
Guyatt, G. et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 64, 383–394 (2011).
Akboğa, M. K. et al. Increased serum YKL-40 level is associated with the presence and severity of metabolic syndrome. Anatol. J. Cardiol. 16(12), 953–958. https://doi.org/10.14744/AnatolJCardiol.2016.6933 (2016).
Ali, N. et al. Association between serum uric acid and metabolic syndrome: A cross-sectional study in Bangladeshi adults. Sci. Rep. 10(1), 7841. https://doi.org/10.1038/s41598-020-64884-7 (2020).
Chang, H. C., Chen, S. P. & Yang, H. J. Including selective metabolic components in current diagnostic criteria does not improve discriminative validity for metabolic syndrome: A risk score approach. J. Int. Med. Res. 47(3), 1298–1311. https://doi.org/10.1177/0300060518822919 (2019).
Chen, Y. et al. The association of uric acid with the risk of metabolic syndrome, arterial hypertension or diabetes in Young subjects: An observational study. Clin. Chim. Acta 478, 68–73. https://doi.org/10.1016/j.cca.2017.12.038 (2018).
Cheng, Y. Y. et al. Examining the gender difference in the association between metabolic syndrome and the mean leukocyte telomere length. PLoS One 12(7), e0180687. https://doi.org/10.1371/journal.pone.0180687 (2017).
Ding, C., Yang, Z., Wang, S., Sun, F. & Zhan, S. The associations of metabolic syndrome with incident hypertension, type 2 diabetes mellitus and chronic kidney disease: A cohort study. Endocrine 60(2), 282–291. https://doi.org/10.1007/s12020-018-1552-1 (2018).
Fawzy, M. & Alsel, B. Association of serum uric acid levels components of metabolic syndrome: A cross-sectional analysis in a Saudi adult population. Int. J. Biomed. 10, 457–466. https://doi.org/10.21103/Article10(4)_OA25 (2020).
He, S. et al. Hemoglobin concentration is associated with the incidence of metabolic syndrome. BMC Endocr. Disord. 21, 53. https://doi.org/10.1186/s12902-021-00719-4 (2021).
Jeong, J. & Suh, Y. J. Association between serum uric acid and metabolic syndrome in Koreans. J Korean Med. Sci. 34(48), e307. https://doi.org/10.3346/jkms.2019.34.e307 (2019).
Kawada, T., Otsuka, T., Inagaki, H., Wakayama, Y. & Katsumata, M. Biological markers, lifestyles and metabolic syndrome in workers. Diabetes Metab. Syndr. Clin. Res. Rev. 9(2), 71–73. https://doi.org/10.1016/j.dsx.2015.02.009 (2015).
Lee, Y.-B. et al. Change in serum bilirubin level as a predictor of incident metabolic syndrome. PLoS One 11(12), e0168253. https://doi.org/10.1371/journal.pone.0168253 (2016).
Li, Q. et al. An increase in normal SUA level within the normal range predicts risk of metabolic syndrome, especially in women: A cross-sectional study. Endocr. Care. https://doi.org/10.1055/s-0042-117010 (2016).
Liang, Q. et al. Association of changes in metabolic syndrome status with the incidence of thyroid nodules: A prospective study in Chinese adults. Front. Endocrinol. 11, 582. https://doi.org/10.3389/fendo.2020.00582 (2020).
Mukhopadhyay, P. et al. Uric acid and its correlation with various metabolic parameters: A population-based study. Indian J. Endocrinol. Metab. 23(1), 134–139. https://doi.org/10.4103/ijem.IJEM_18_19 (2019).
Nejatinamini, S. et al. Association between serum uric acid level and metabolic syndrome components. J. Diabetes Metab. Disord. 14(1), 70. https://doi.org/10.1186/s40200-015-0200-z (2020).
Ni, W. et al. Association of serum uric acid with metabolic syndrome and its components: A cross-sectional study in Chinese coastal population. Metab. Syndr. Relat. Disord. 18(2), 103–109. https://doi.org/10.1089/met.2019.0043 (2020).
Onat, A. et al. Sex-specific predictors of metabolic syndrome independent of its components. J. Investig. Med. 63(6), 796–801. https://doi.org/10.1097/JIM.0000000000000203 (2015).
Pugliese, N. R. et al. The importance of including uric acid in the definition of metabolic syndrome when assessing the mortality risk. Clin. Res. Cardiol. https://doi.org/10.1007/s00392-021-01815-0 (2021).
Rhee, C. et al. Clinical markers associated with metabolic syndrome among military aviators. Aerosp. Med. Hum. Perform. 86(11), 970–975. https://doi.org/10.3357/amhp.4362.2015 (2015).
Sreckovic, B. et al. The correlation between metabolic syndrome quantification scores and numerous laboratory parameters related to this syndrome. Vojnosanit. Pregl. 77(8), 189–795. https://doi.org/10.2298/vsp180626132S (2020).
Tabak, O. et al. The relationship between circulating irisin, retinol binding protein-4, adiponectin and inflammatory mediators in patients with metabolic syndrome. Arch. Endocrinol. Metab. 61(6), 515–523. https://doi.org/10.1590/2359-3997000000289 (2017).
Tao, J. et al. Serum uric acid to creatinine ratio and metabolic syndrome in postmenopausal Chinese women. Medicine 99(17), e19959. https://doi.org/10.1097/MD.0000000000019959 (2020).
Vigna, L. et al. Gender-related association between uric acid, homocysteine, γ-glutamyltransferase, inflammatory biomarkers and metabolic syndrome in subjects affected by obesity. Biomark. Med. 11(10), 857–865. https://doi.org/10.2217/bmm-2017-0072 (2017).
Wang, J., Wang, Y., Chen, F., Ma, G. & Wang, D. Measurement of the combined levels of serum uric acid and alanine aminotransferase and the risk of metabolic syndrome in a population aged 60 years or more in Northeastern China. Med. Sci. Monit. 20(26), e916459. https://doi.org/10.12659/MSM.916459 (2020).
Wu, C. et al. Predictability of cardiorespiratory fitness on the risk of developing metabolic syndrome and diabetes mellitus in Taiwan adults: Preliminary analysis of a cohort study. Obes. Res. Clin. Pract. 12, 541–546. https://doi.org/10.1016/j.orcp.2018.07.006 (2018).
Yen, Y. et al. Associations of metabolic syndrome and its components with mortality in the elderly. Medicine 94(23), e956. https://doi.org/10.1097/MD.0000000000000956 (2015).
Zomorrodian, D. et al. Metabolic syndrome components as markers to prognosticate the risk of developing chronic kidney disease: Evidence-based study with 6492 individuals. J. Epidemiol. Community Health 69(6), 594–598. https://doi.org/10.1136/jech-2014-205160 (2015).
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 285(19), 2486–2497 (2001).
Ahmadnezhad, M. et al. Association between serum uric acid, high sensitive C-reactive protein and pro-oxidant-antioxidant balance in patients with metabolic syndrome. BioFactors 44(3), 263–271. https://doi.org/10.1002/biof.1424 (2018).
Martins, C. C. et al. Increased oxidative stress and inflammatory markers contrasting with the activation of the cholinergic anti-inflammatory pathway in patients with metabolic syndrome. Clin. Biochem. 89, 63–69. https://doi.org/10.1016/j.clinbiochem.2020.12.007 (2021).
Nardin, M., Verdoia, M., Barbieri, L. & De Luca, G. Impact of metabolic syndrome on mean platelet volume and its relationship with coronary artery disease. Platelets 30, 615–623. https://doi.org/10.1080/09537104.2018.1499885 (2019).
Tayefi, M. et al. Evaluating of associated risk factors of metabolic syndrome by using decision tree. Comp. Clin. Pathol. https://doi.org/10.1007/s00580-017-2580-6 (2017).
Yu, T. Y. et al. Serum uric acid: A strong and independent predictor of metabolic syndrome after adjusting for body composition. Metabolism https://doi.org/10.1016/j.metabol.2015.11.003 (2015).
Yu, T.-Y. et al. The protective effects of increasing serum uric acid level on development of metabolic syndrome. Diabetes Metab. 43(4), 504. https://doi.org/10.4093/dmj.2018.0079 (2018).
Zhang, H. et al. Sex-specific associations of serum uric acid with metabolic syndrome in Chinese rural population: The RuralDiab study. Clin. Chim. Acta 480, 119–125. https://doi.org/10.1016/j.cca.2018.02.003 (2018).
Alberti, G., Zimmet, P. & Shaw, J. The metabolic syndrome a new worldwide definition. IDF epidemiology task force consensus group. Lancet 366, 1059e62 (2005).
Klongthalay, K.-S. Increased uric acid and life style factors associated with metabolic syndrome in Thais. Ethiop. J. Health Sci. 30(2), 199. https://doi.org/10.4314/ejhs.v30i2.7 (2020).
Liu, C.-F., Zhou, W.-N., Lu, Z., Wang, X.-T. & Qiu, Z. The associations between liver enzymes and the risk of metabolic syndrome in the elderly. Exp. Gerontol. 106, 132–136. https://doi.org/10.1016/j.exger.2018.02.026 (2018).
Porchia, L. M., Gonzalez, M., Torres, E., Ruiz-Vivanco, G. & Pérez, R. Low serum uric acid concentration augments insulin effects on the prevalence of metabolic syndrome. Diabetes Metab. Syndr. 12(3), 325–331. https://doi.org/10.1016/j.dsx.2017.12.012 (2018).
Wang, H., Shi, L., Liu, C., Liu, S. & Shi, S. Association between uric acid and metabolic syndrome in elderly women. Open Med. 13(1), 172–177. https://doi.org/10.1515/med-2018-0027 (2018).
Alberti, K. G. M. M. et al. Harmonizing the metabolic syndrome: A joint Interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120(16), 1640–1645. https://doi.org/10.1161/circulationaha.109.192644 (2009).
Wang, C. et al. Metabolic syndrome and risks of carotid atherosclerosis and cardiovascular events in community-based older adults in China. Asia Pac. J. Clin. Nutr. 28(4), 870–878. https://doi.org/10.6133/apjcn.201912_28(4).0024 (2019).
Wang, J. Y., Zhang, L., Liu, J., Yang, W. & Ma, L. N. Metabolic syndrome, ApoE genotype, and cognitive dysfunction in an elderly population: A single-center, case-control study. World J. Clin. Cases 9(5), 1005–1015. https://doi.org/10.12998/wjcc.v9.i5.1005 (2021).
Yang, T. & Pei, D. Association of cystatin C levels with metabolic syndrome incidence: A nested case-control study with propensity score matching. J. Int. Med. Res. 49(1), 300060520986311. https://doi.org/10.1177/0300060520986311 (2021).
Chinese Medical Association. The Suggestion on Chinese Metabolic Syndrome (Chinese Medical Association, 2004).
Sumiyoshi, H. et al. Association of uric acid with incident metabolic syndrome in a Japanese general population. Int. Heart J. 60, 830–835. https://doi.org/10.1536/ihj.18-444 (2019).
Matsuzawa, Y. Definition and the diagnostic standard for metabolic syndrome. Committee to Evaluate Diagnostic Standards for Metabolic Syndrome. J. Jpn. Soc. Intern. Med. 94, 794–809 (2005).
Osadnik, K. et al. Calcium and phosphate levels are among other factors associated with metabolic syndrome in patients with normal weight. Diabetes Metab. Syndr. Obes. 13, 1281–1288. https://doi.org/10.2147/DMSO.S232497 (2020).
Buscemi, S. et al. Characterization of metabolically healthy obese people and metabolically unhealthy normal-weight people in a general population cohort of the ABCD study. J. Diabetes Res. 2017, 9294038. https://doi.org/10.1155/2017/9294038 (2017).
Xiong, Q., Liu, J. & Xu, Y. Effects of uric acid on diabetes mellitus and its chronic complications. Int. J. Endocrinol. 2019, 9691345. https://doi.org/10.1155/2019/9691345 (2019).
Kuwabara, M. et al. Relationship between serum uric acid levels and hypertension among Japanese individuals not treated for hyperuricemia and hypertension. Hypertens. Res. 37(8), 785–789. https://doi.org/10.1038/hr.2014.75 (2014).
Shahin, L., Patel, K. M., Heydari, M. K. & Kesselman, M. M. Hyperuricemia and cardiovascular risk. Cureus 13(5), e14855. https://doi.org/10.7759/cureus.14855 (2021).
Viazzi, F. et al. Metabolic syndrome, serum uric acid and renal risk in patients with T2D. PLoS One 12(4), e0176058. https://doi.org/10.1371/journal.pone.0176058 (2017).
Yan, D. et al. Uric acid is independently associated with diabetic kidney disease: A cross sectional study in a Chinese population. PLoS One 10(6), e0129797. https://doi.org/10.1371/journal.pone.0129797 (2015).
Thottam, G. E., Krasnokutsky, S. & Pillinger, M. H. Gout and metabolic syndrome: A tangled web. Curr. Rheumatol. Rep. 19(10), 60. https://doi.org/10.1007/s11926-017-0688-y (2017).
Billiet, L., Doaty, S., Katz, J. D. & Velasquez, M. T. Review of hyperuricemia as new marker for metabolic syndrome. ISRN Rheumatol. 2014, 852954. https://doi.org/10.1155/2014/852954 (2014).
Norvik, J. V. et al. Overweight modifies the longitudinal association between uric acid and some components of the metabolic syndrome: The Tromsø Study. BMC Cardiovasc. Disord. 16, 85. https://doi.org/10.1186/s12872-016-0265-8 (2016).
Xu, J., Liu, C., Fu, L., Li, L. & Wang, T. The association of serum uric acid with metabolic syndrome and its components—From a single-clinical centre in China. Int. J. Clin. Pract. 75(4), e13845. https://doi.org/10.1111/ijcp.13845 (2021).
Yuan, H. et al. Serum uric acid levels and risk of metabolic syndrome: A dose–response meta-analysis of prospective studies. J. Clin. Endocrinol. Metab. 100, 4198–4207. https://doi.org/10.1210/jc.2015-2527 (2015).
Liu, Z., Que, S., Zhou, L. & Zheng, S. Dose–response relationship of serum uric acid with metabolic syndrome and non-alcoholic fatty liver disease incidence: A meta-analysis of prospective studies. Sci. Rep. 5, 14325. https://doi.org/10.1038/srep14325 (2015).
Chen, L. Y. et al. Relationship between hyperuricemia and metabolic syndrome. J. Zhejiang Univ. Sci. B 8(8), 593–598. https://doi.org/10.1631/jzus.2007.B0593 (2007).
Author information
Authors and Affiliations
Contributions
Two authors (E.R.C. and M.R.S.) separately screened all articles obtained in the search to eliminate duplicates. Then, two other authors (D.P.J. and R.M.L.) independently read the title and abstract and applied the eligibility criteria to select the articles that were finally included in the review. Finally, a fifth authors (M.V.A.) acted as a judge in case of discrepancy. One researcher (E.R.C.) oversaw extracting the data, verified by a second researcher (G.M.R.). A third researcher (M.R.S.) resolved the disagreement in case of a tie. All authors have participated in search of the literature, analysis and evaluation of quality, results and writing. Finally, the authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Raya-Cano, E., Vaquero-Abellán, M., Molina-Luque, R. et al. Association between metabolic syndrome and uric acid: a systematic review and meta-analysis. Sci Rep 12, 18412 (2022). https://doi.org/10.1038/s41598-022-22025-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22025-2
- Springer Nature Limited