Abstract
Persistent abnormalities in microcirculatory function are associated with poor clinical outcomes in patients with circulatory shock. We sought to identify patients with acutely reversible microcirculatory dysfunction using a low-dose topical nitroglycerin solution and handheld videomicroscopy during circulatory shock after cardiac surgery. Forty subjects were enrolled for the study, including 20 preoperative control and 20 post-operative patients with shock. To test whether microcirculatory dysfunction is acutely reversible during shock, the sublingual microcirculation was imaged with incident dark field microscopy before and after the application of 0.1 mL of a 1% nitroglycerin solution (1 mg/mL). Compared to the control group, patients with shock had a higher microcirculation heterogeneity index (MHI 0.33 vs. 0.12, p < 0.001) and a lower microvascular flow index (MFI 2.57 vs. 2.91, p < 0.001), total vessel density (TVD 22.47 vs. 25.90 mm/mm2, p = 0.005), proportion of perfused vessels (PPV 90.76 vs. 95.89%, p < 0.001) and perfused vessel density (PVD 20.44 vs. 24.81 mm/mm2, p < 0.001). After the nitroglycerin challenge, patients with shock had an increase in MFI (2.57 vs. 2.97, p < 0.001), TVD (22.47 vs. 27.51 mm/mm2, p < 0.009), PPV (90.76 vs. 95.91%, p < 0.001), PVD (20.44 vs. 26.41 mm/mm2, p < 0.001), venular RBC velocity (402.2 vs. 693.9 µm/s, p < 0.0004), and a decrease in MHI (0.33 vs. 0.04, p < 0.001. Thirteen of 20 patients showed a pharmacodynamic response, defined as an increase in PVD > 1.8 SD from shock baseline. Hemodynamics and vasoactive doses did not change during the 30-min study period. Our findings suggest a topical nitroglycerin challenge with handheld videomicroscopy can safely assess for localized recruitment of the microcirculatory blood flow in patients with circulatory shock and may be a useful test to identify nitroglycerin responsiveness.
Similar content being viewed by others
Introduction
Circulatory shock is a life-threatening, generalized form of acute circulatory failure where there is an imbalance between oxygen demand, supply, or utilization1,2. Traditionally shock is categorized by macrocirculatory etiologies including hypovolemic, distributive, obstructive, and cardiogenic shock, but downstream effects on functional capillary density and blood flow are heterogeneous3. A number of mechanisms have been identified for reduced microcirculatory blood flow during shock states, including inflammatory-mediated vascular endothelial injury, microthrombosis, and an inadequate balance between vasoconstrictive and vasodilating molecules which can lead to a global or heterogeneous reduction in capillary blood flow4,5. Unfortunately, many of the often used therapies to reverse hemodynamic derangements do not reverse microcirculatory impairment6,7,8. Interventions during the optimization and stabilization phases of shock resuscitation are limited, and often guided by surrogate markers of tissue perfusion2.
Nitric oxide (NO) impacts many of the key pathologic changes observed in shock, including regulation of vascular endothelial function, microvascular permeability, leukocyte adherence, and platelet activation at the capillary level9. Vascular endothelial injury and impaired endothelial NO production have been implicated as a mechanism of shock severity. Nitroglycerin (NTG) is an endothelium-independent nitric oxide donor and vasodilator that has been shown to increase capillary density and blood flow in patients with vasopressor-dependent septic shock and acute heart failure10,11. Initiation of vasodilatory therapy in patients with vasoactive-dependent shock may be counterintuitive and might only benefit a select group of patients. In this case, a strategy that identifies nitroglycerin-responsive patients prior to systemic administration would be ideal.
Microdosing novel therapeutics in conjunction with local tissue imaging has gained popularity in fields outside of critical care to test for important physiologic responses to treatment12. A microdose is defined as being below the dose calculated to yield a pharmacological effect and less than 100 µg of the active drug13. In healthy adults, low-dose topical nitroglycerin has been used to measure microcirculatory reserve using sublingual incident dark-field (IDF) microscopy, but this approach could also be used in patients with shock to test for a physiologic response without exposing patients to undesired effects that may be experienced with systemic administration14. Our primary aim was to determine if a microdose of topical nitroglycerin could improve microcirculatory density and capillary blood flow in critically ill patients with vasoactive-dependent circulatory shock. A secondary aim was to identify nitroglycerin responders using a patient-specific pharmacodynamic response threshold15.
Results
Forty-four patients were consented for enrollment in the study. Twenty-four patients with post-operative shock received the topical nitroglycerin challenge and 20 preoperative patients served as a control group. Control patients had common chronic medical conditions for patients with cardiovascular disease but did not have any pre-operative end-organ injury such as chronic kidney disease, reduced left ventricular ejection fraction, chronic liver disease. Two patients were excluded from the experimental arm prior to receiving the study drug (one did not require post-operative vasopressors, and one did not have a properly functioning pulmonary artery catheter). Twenty-two total subjects received the study drug to assess for microcirculation responsiveness. Two additional subjects were excluded after enrollment for having an inadequate number of quality videos to meet consensus standards. The CONSORT flow diagram is included in Supplemental Data. Patient information and demographics are in Table 1. Perioperative and clinical data are in Table 2. There was no difference in clinical status between NTG responders and non-responders at any given time point.
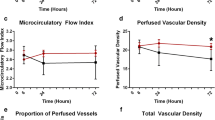
Compared to the control group, patients with postoperative shock had reduced microvascular flow index (MFI), heterogeneity (MHI), total vessel density (TVD), proportion of perfused vessels (PPV), and perfused vessel density (PVD) (Table 3).
Video quality analysis
Three hundred and twenty-six video clips of the sublingual microcirculation were obtained, stabilized, and graded for quality prior to inclusion for final analysis. Eighty-six videos were excluded from the NTG challenge group for having a Massey score > 10, leaving 240 acceptable videos for final analysis. The analyzed video clips were of good quality with a mean Massey score of 0.3 ± 0.4 (illumination 0.0 ± 0.1, duration 0.0 ± 0.2, focus 0.1 ± 0.2, content 0.1 ± 0.2, stability 0.0 ± 0.1, pressure 0.1 ± 0.3). Bland–Altman bias was 0.021 (95% limit of agreement − 0.11 to 0.15).
Effect of topical nitroglycerin on the sublingual microcirculation
Nitroglycerin administration reduced flow heterogeneity, increased microcirculatory density, and increased venular RBC velocity (RBCv) within 3 min of nitroglycerin microdosing (Table 4). There was no difference in total vessel density (TVD; 25.90 ± 3.85 vs. 27.51 ± 3.77 mm/mm2, p = 0.19), proportion of perfused vessels (PPV; 95.89 ± 2.99 vs. 95.91 ± 3.04%, p = 0.98), perfused vessel density (PVD; 24.81 ± 3.51 vs. 26.41 ± 3.50 mm/mm2, p = 0.16), microvascular flow index (MFI; 2.91 ± 0.12 vs. 2.97 ± 0.06, p = 0.26), and heterogeneity (MHI; 0.12 ± 0.15 vs. 0.04 ± 0.08, p = 0.15) between control subjects and patients with shock after the NTG challenge. All microcirculation parameters returned to baseline values 30 min after the NTG challenge.
Pharmacodynamic (PD) response to nitroglycerin was defined as an increase in PVD > 5.46 mm/mm2 compared to baseline using a pooled baseline SD of 3.03. Baseline microcirculation characteristics of NTG responders and non-responders are listed in Table 5. Of the 20 subjects included in the final analysis, 13 had a significant PD response to the topical challenge (Fig. 1).
Hemodynamics during the topical nitroglycerin challenge
There was no significant change in mean arterial blood pressure, central venous pressure, cardiac index, or systemic vascular resistance index during the 30-min challenge (Fig. 2). Patient norepinephrine equivalents at baseline, NTG administration, and at 30 min were mixed venous oxygen saturation and capillary refill time also did not change during the challenge.
Discussion
Based on the results of this study, we were able to show that (i) sublingual capillary density and blood flow is reduced during postoperative circulatory shock compared to a preoperative control; (ii) reduced microcirculation density and flow could be increased using a topical endothelium-independent nitric oxide donor without any effect on systemic hemodynamics; and (iii) we could identify nitroglycerin responsive patients using a pharmacodynamic statistical method used in early phase 0 trials.
Circulatory shock is defined as an imbalance between oxygen demand, supply, or utilization2. Compared to our control group, patients with shock had lower capillary density and blood flow indicating reduced tissue perfusion. Current resuscitation strategies to reverse shock include augmenting intravascular volume and vasoactive therapies to normalize systemic hemodynamics, but unfortunately, these interventions do not always lead to improvements in capillary blood flow7,16,17. In this study, we found that topical nitroglycerin increased regional PVD and reduced heterogeneity in patients with shock which have been associated with poor clinical outcomes7. The MFI and RBCv of our shock cohort also increased after the NTG challenge, but both of these values appear to be close to values that may be considered within the normal range based on consensus definitions—specifically MFI18. Large studies to define human reference ranges have yet to be completed. It is unclear if targeting higher values would have any clinical or physiologic impact. The small vessel abnormalities identified with IDF are likely the result of a number of common post-cardiopulmonary bypass pathologies including a SIRS-related vasoplegia, low cardiac output, vascular endothelial injury, and microvascular shunting5,19. Nitric oxide donors are of particular interest as they have been found to be a mediator of endothelial ischemia–reperfusion injury and can provide a number of other tissue-protective effects including reduction of small vessel resistance, leukocyte adherence, platelet aggregation, and vascular endothelial cell permeability20,21.
The rapid change in perfused vessel density and flow heterogeneity suggests that an endothelium-independent nitric oxide donor can reverse early post-operative derangements in capillary blood flow. The time-course of the observed change in microcirculatory flow was consistent with the known pharmacokinetics of nitroglycerin. Although nitroglycerin does provide a vasodilatory effect on both arteries and veins, evidence suggest the most significant effect is on the venous system22. A significant reduction in venular pressure would increase the pressure differential across the capillary network to improve PVD and flow homogeneity identified with IDF. It is not possible to determine if the topical solution primarily acted on the pre- or post-capillary resistance vessels within the microvascular unit using IDF microscopy. As expected, the duration of the pharmacodynamic response was transient, lasting less than 30 min, which is consistent with well-studied nitroglycerin pharmacokinetics23.
Administration of a vasodilatory medication to patients with vasopressor-dependent hypotension is a significant departure from conventional treatment strategies and could result in significant hypotension. Topical nitroglycerin has been shown to improve local microcirculation without disturbing hemodynamics in hemorrhagic shock and in healthy volunteers14,24. We chose our dose of NTG based on the previous findings of Hilty et al., who also identified an increase in microcirculatory blood flow without a change in hemodynamics in healthy volunteers14. By incorporating handheld video microscopy (HVM) imaging, we were able to identify a pharmacodynamic effect of nitroglycerin using a solution that delivered less than 2% of the standard dose of sublingual nitroglycerin without causing a change in hemodynamics. Our method appears to be a low-risk test for identifying nitroglycerin responsiveness in patients with shock.
When evaluating our NTG response groups for potential confounders, both NTG responder and non-responder groups were similar. There was no difference in vasoactive medications, vasoactive dose, mean arterial pressure, central venous pressure, or other hemodynamic measures between NTG responders and non-responders. Nitroglycerin responders had a longer cardiopulmonary bypass and cross clamp time, which has been associated with more pronounced vascular endothelial injury and decreased NO production5,25. Our measurements of postoperative microcirculation parameters are similar to other reports in the literature when using consensus standard analysis methods5,26.
In our study, 65% of patients increased their PVD > 1.8 SD from baseline, indicating that NO donor responsiveness is likely individualized. In fact, NTG responders had lower baseline perfused vessel density, largely due to a reduced TVD. There was no difference in baseline MHI between groups, yet 15/20 subjects who received the NTG challenge resulted in an MHI that approached 0 indicating minimal flow heterogeneity and essentially uniform blood flow through most capillaries.
Previous research in vasoactive-dependent septic shock utilized a fixed-dose nitroglycerin infusion (30 mcg/min) which improved peripheral perfusion but did not improve sublingual microcirculatory function or survival compared to placebo27. It is possible that previous studies included patients that were either not responsive to nitroglycerin therapy or the dose administered was inadequate to stimulate regional blood flow in the vascular beds being imaged. The hesitancy to administer vasodilators in patients requiring vasopressor therapy is understandable, because current small molecule therapeutics cannot limit their effects to sites of microvascular injury. The development of novel NO-donor or NO-preserving therapeutics using nanocarrier technology, designed to target injured vascular endothelial cells, may be able to improve capillary blood flow in disturbed microvascular beds while minimizing undesirable side effects that can occur with current small molecule therapeutics28,29,30.
There are a few limitations to our study that should be identified. We did not perform a stepwise increase in nitroglycerin dosing, therefore it is possible that patients labeled as non-responders did not receive a high enough dose of nitroglycerin to improve capillary blood flow. While incorporating HVM to identify a real-time response to nitroglycerin can identify a physiologic response, significant time-constraints for the analysis of video sequences are a barrier to use in routine clinical practice. Development of software capable of providing real-time automated analysis would reduce the time required to interpret changes in PVD, allowing for more timely, individualized therapeutic interventions. Finally, there may be natural variation in sublingual microcirculation over time, which could account for a portion of the change we observed after the nitroglycerin challenge. While we did not perform an analysis to evaluate for regression to the mean, our findings overall reflected the anticipated change in microcirculatory function based on established nitroglycerin pharmacology. Future research that examines natural variation in microcirculatory flow over time on a larger dataset would be a valuable addition to the literature.
In conclusion, we were able to demonstrate that abnormalities in functional capillary density, heterogeneity, and flow during circulatory shock can be reversed using an endothelium-independent nitric oxide donor. The effect appears to be most pronounced in patients with abnormal perfused vessel density. Individual responsiveness can be safely tested with the use of a low-dose topical nitroglycerin solution in conjunction with HVM.
Methods
Patient selection
Adult patients (age > 18 years) receiving elective cardiac surgery requiring cardiopulmonary bypass were screened for eligibility from August to October 2021. Written informed consent was obtained from eligible patients prior to surgery. Patients were excluded if they had a nitroglycerin allergy, were taking oral phosphodiesterase inhibitors, were unable to tolerate microcirculatory flow image acquisition or did not have a pulmonary artery catheter for continuous cardiac output monitoring after surgery. Patients only received the study drug if they met the clinical criteria for circulatory shock, defined as having postoperative vasopressor-dependent hypotension or low cardiac output requiring inotropic support, with signs of end-organ injury (capillary refill time > 3 sec, lactate > 2 mmol/dL, SvO2 < 60%). A second cohort of patients were enrolled to serve as a preoperative control, which were matched to the experimental group for age, co-morbidities, and planned operation. Study data were collected and recorded using a well-established clinical database tool (REDCap, Vanderbilt University, Nashville, TN) hosted at the University of Pennsylvania31,32.
Study design and drug administration
This investigator-initiated, open-label study was conducted at the Hospital of the University of Pennsylvania, an urban quaternary academic medical center. The study was approved by the University of Pennsylvania’s institutional review board, registered with ClinicalTrials.gov (NCT05102734), and conducted in accordance with the principles of the Declaration of Helsinki. Sample size was determined based on an anticipated 30% increase in TVD, and setting a one-sided α of 0.1, and ß of 0.8. A 1% topical nitroglycerin solution was prepared immediately prior to enrollment by reconstituting 400 mcg of nitroglycerin (Pfizer Pharmaceuticals, New York, NY, USA) in 4 mL of sterile water within a dropper bottle that would administer the solution in 0.05 mL aliquots. Two drops (0.1 mL) of the 1% nitroglycerin solution (5 mcg or 2.27 × 10−2 μmol per drop) were applied to the sublingual space after obtaining baseline post-operative microcirculation images. A microdose (approximately 1/50th of the lowest therapeutic sublingual dose) was administered to avoid any systemic effects.
Microcirculatory imaging and analysis
Sublingual microcirculation imaging was performed using HVM (CytoCam, Braedius Medical BV, the Netherlands) in the preoperative staging area (for controls) or within 2 hours of arriving to the intensive care unit after surgery by a trained member of the investigative team (JCG or FMT)33. The sublingual microcirculation was measured at 3 time points: baseline, 3 min post-nitroglycerin, and then 30 min later. Images were obtained by gently placing the videomicroscope under the subject’s tongue until an adequate view of the microcirculation was obtained. A series of six second video clips (120 frames) duration were taken at each time point with attention to quality factors, especially the absence of pressure artifact, excess saliva, and proper location in accordance with the accepted consensus for microcirculation analysis. Image quality was assessed using the 6-factor Massey quality score, which scores each video for appropriate illumination, duration, focus, content, stability, and pressure. Images were only included in the final analysis if the Massey quality score was < 1034. Acceptable clips were deidentified and coded for analysis after enrollment was complete.
Microcirculation videos were exported using commercial software (CCTools 2, Braedius Medical BV, the Netherlands) and manually analyzed using the validated Automated Vascular Analysis software (AVA 3.2; Microvision Medical B.V., the Netherlands). Video analysis was performed by trained investigators (JCG and FMT) who were blinded to the conditions of the subject. Only microvessels ≤ 20 μm in diameter were included in the calculation of TVD and PVD.
Red blood cell flow velocity was calculated within appropriate venules 20–30 μm in diameter by manually measuring the slope of individual RBC movement within software generated space–time diagrams18. Microcirculatory function was quantified according to the current best practice guidelines for microcirculation imaging18. Blood flow within each microvessel was graded using a semiquantitative scale, based on the vascular flow pattern ranging from 0 to 3 (0 = no flow, 1 = intermittent, 2 = sluggish, or 3 = continuous flow). Vessel perfusion was dichotomized as nonperfused (no flow or intermittent flow) or perfused (continuous or sluggish) for the calculation of PPV and PVD.
Individual pharmacodynamic response to the nitroglycerin challenge
An exploratory analysis was planned to identify the incidence of a patient specific pharmacodynamic response to the nitroglycerin challenge. A PD response threshold was set as a post-administration PVD > 1.8 standard deviations from the baseline, which was calculated by averaging the intra-patient variance between the three baseline PVD video sequences, which was then pooled across the entire cohort. This 1.8SD threshold was set to achieve 90 percent confidence interval (assuming a one-sided alpha, since there was only an anticipated increase in PVD). This method has been used in previous Phase 0 trials to provide a statistically rigorous threshold for individualized PD responses to novel therapeutic interventions15.
Patient data and safety monitoring
Subject demographics, pre-operative risk score (euroSCORE II) and medical history were collected during initial screening. Cardiac index (CI), central venous pressure (CVP), pulmonary artery pressure (PAP), and mixed venous oxygen saturation (SvO2) were continuously monitored by pulmonary artery catheter (Edwards Lifesciences LLC, Irvine, CA, USA). Arterial blood pressure was measured continuously using a standard invasive arterial line. Systemic hemodynamic data, vasopressor infusion doses, and mixed venous oxygen saturation were recorded at baseline then every 3 min for 30 min after application of the topical nitroglycerin solution35. The vasopressor-inotrope score (VIS) was calculated to summarize the degree of post-operative vasoactive support was required at the time of the study36. Norepinephrine equivalents were calculated for each patient at each time point37.
Statistical analysis
Data normality was assessed using the D’Agostino-Pearson omnibus normality test. Global hemodynamic and microcirculation variables are reported as mean ± SD. Variables that were not normally distributed are reported as median with interquartile range [25th–75th percentiles]. Student t-test was used to test for differences between normally distributed variables. Mann–Whitney U test was used to analyze non-normal data. Repeated measure one-way ANOVA with Tukey’s post-hoc testing was used to assess for changes in microcirculatory function during the nitroglycerin challenge. Inter-rater reliability between coders was assessed in 10% of the videos using a Bland–Altman plot. Statistical analysis was conducted using Prism v 8.0 (Graph-Pad Software, San Diego, CA). Statistical significance was assumed at p < 0.05.
Ethics approval and consent to participate
This study was approved by the University of Pennsylvania’s institutional review board (IRB # 829765) and informed consent was obtained prior to enrollment. All consent forms were copied in triplicate, one given to the subject, the second placed in the official medical record, the third kept in a secured location within the PI’s office.
Data availability
All original data and materials are kept in a locally managed REDCap database at the University of Pennsylvania. The dataset supporting the results of this report is available via the Zenodo research data repository38. The deidentified microcirculation dataset can be found here: https://zenodo.org/record/5768720.
Abbreviations
- AU:
-
Arbitrary units
- CABG:
-
Coronary artery bypass grafting
- CI:
-
Cardiac index
- CVP:
-
Central venous pressure
- MAP:
-
Mean arterial pressure
- MFI:
-
Microcirculatory flow index
- MHI:
-
Microcirculatory heterogeneity index
- NO:
-
Nitric oxide
- NTG:
-
Nitroglycerin
- PAP:
-
Pulmonary artery pressure
- PPV:
-
Proportion of perfused vessels
- PVD:
-
Perfused vessel density
- RBCv:
-
Red blood cell velocity
- SvO2:
-
Mixed venous oxygen saturation
- TVD:
-
Total vessel density
- VIS:
-
Vasopressor-inotrope score
References
Cecconi, M. et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intens. Care Med. 40(12), 1795–1815 (2014).
Vincent, J. L., De Backer, D., Finfer, S. R. & Vincent, J. L. Circulatory shock. N. Engl. J. Med. 369(18), 1726–1734 (2013).
Klijn, E., Den Uil, C. A., Bakker, J. & Ince, C. The heterogeneity of the microcirculation in critical illness. Clin. Chest Med. 29(4), 643–654 (2008).
Koning, N. J., Simon, L. E., Asfar, P., Baufreton, C. & Boer, C. Systemic microvascular shunting through hyperdynamic capillaries after acute physiological disturbances following cardiopulmonary bypass. Am. J. Physiol.-Heart Circ. Physiol. 307(7), H967–H975 (2014).
Dekker, N. A. M. et al. Postoperative microcirculatory perfusion and endothelial glycocalyx shedding following cardiac surgery with cardiopulmonary bypass. Anaesthesia 74(5), 609–618 (2019).
De Backer, D. et al. Challenges in the management of septic shock: A narrative review. Intens. Care Med. 45(4), 420–433 (2019).
Greenwood, J. C. et al. Severe impairment of microcirculatory perfused vessel density is associated with postoperative lactate and acute organ injury after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 35(1), 106–115 (2021).
Tachon, G. et al. Microcirculatory alterations in traumatic hemorrhagic shock. Crit. Care Med. 42(6), 1433–1441 (2014).
Vincent, J. L., Zhang, H., Szabo, C. & Preiser, J. C. Effects of nitric oxide in septic shock. Am. J. Respir. Crit. Care Med. 161(6), 1781–1785 (2000).
Spronk, P. E. et al. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet 360(9343), 1395–1396 (2002).
den Uil, C. A. et al. Dose-dependent benefit of nitroglycerin on microcirculation of patients with severe heart failure. Intens. Care Med. 35(11), 1893–1899 (2009).
Burt, T. et al. Phase 0/microdosing approaches: Time for mainstream application in drug development? Nat. Rev. Drug Discov. 19(11), 801–818 (2020).
Bertino, J. S., Greenberg, H. E. & Reed, M. D. American College of Clinical Pharmacology Position Statement on the use of microdosing in the drug development process. J. Clin. Pharmacol. 47(4), 418–422 (2007).
Hilty, M. P. et al. Assessment of endothelial cell function and physiological microcirculatory reserve by video microscopy using a topical acetylcholine and nitroglycerin challenge. ICMx 5(1), 26 (2017).
Rubinstein, L. V. et al. The statistics of phase 0 trials. Stat. Med. 29(10), 1072–1076 (2010).
Ince, C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit. Care 19, S8 (2015).
Trzeciak, S. et al. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: Relationship to hemodynamics, oxygen transport, and survival. Ann. Emerg. Med. 49(1), 88–98 (2007).
Ince, C. et al. Second consensus on the assessment of sublingual microcirculation in critically ill patients: Results from a task force of the European Society of Intensive Care Medicine. Intens. Care Med. 44(3), 281–299 (2018).
De Backer, D. et al. Microcirculatory alterations in cardiac surgery: Effects of cardiopulmonary bypass and anesthesia. Ann. Thorac. Surg. 88(5), 1396–1403 (2009).
Massoudy, P. et al. Sodium nitroprusside in patients with compromised left ventricular function undergoing coronary bypass: Reduction of cardiac proinflammatory substances. J. Thorac. Cardiovasc. Surg. 119(3), 566–574 (2000).
Anaya-Prado, R., Toledo-Pereyra, L. H., Lentsch, A. B. & Ward, P. A. Ischemia/reperfusion injury. J. Surg. Res. 105(2), 248–258 (2002).
Arnold, W. P., Mittal, C. K., Katsuki, S. & Murad, F. Nitric oxide activates guanylate cyclase and increases guanosine 3’:5’-cyclic monophosphate levels in various tissue preparations. Proc. Natl. Acad. Sci. U.S.A. 74(8), 3203–3207 (1977).
Hashimoto, S. & Kobayashi, A. Clinical pharmacokinetics and pharmacodynamics of glyceryl trinitrate and its metabolites. Clin. Pharmacokinet. 42(3), 205–221 (2003).
Truse, R. et al. Effect of topical iloprost and nitroglycerin on gastric microcirculation and barrier function during hemorrhagic shock in dogs. J. Vasc. Res. 54(2), 109–121 (2017).
Sodha, N. R., Clements, R. T. & Sellke, F. W. Vascular changes after cardiac surgery: Role of NOS, COX, kinases, and growth factors. Front. Biosci. (Landmark Ed.) 14(2), 689–698 (2009).
Koning, N. J., Vonk, A. B. A., Vink, H. & Boer, C. Side-by-side alterations in glycocalyx thickness and perfused microvascular density during acute microcirculatory alterations in cardiac surgery. Microcirculation 23(1), 69–74 (2016).
Boerma, E. C. et al. Effects of nitroglycerin on sublingual microcirculatory blood flow in patients with severe sepsis/septic shock after a strict resuscitation protocol: A double-blind randomized placebo controlled trial. Crit. Care Med. 38(1), 93–100 (2010).
Lambden, S. Bench to bedside review: Therapeutic modulation of nitric oxide in sepsis-an update. Intens. Care Med. Exp. 7(1), 64 (2019).
Shuvaev, V. V., Tliba, S., Nakada, M., Albelda, S. M. & Muzykantov, V. R. Platelet-endothelial cell adhesion molecule-1-directed endothelial targeting of superoxide dismutase alleviates oxidative stress caused by either extracellular or intracellular superoxide. J. Pharmacol. Exp. Ther. 323(2), 450–457 (2007).
Shuvaev, V. V. & Muzykantov, V. R. Targeted modulation of reactive oxygen species in the vascular endothelium. J. Control. Release 153(1), 56–63 (2011).
Harris, P. A. et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42(2), 377–381 (2009).
Harris, P. A. et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 95, 103208 (2019).
Hutchings, S., Watts, S. & Kirkman, E. The Cytocam video microscope. A new method for visualising the microcirculation using incident dark field technology. Clin. Hemorheol. Microcirc. 62(3), 261–271 (2016).
Massey, M. J. et al. The microcirculation image quality score: Development and preliminary evaluation of a proposed approach to grading quality of image acquisition for bedside videomicroscopy. J. Crit. Care 28(6), 913–917 (2013).
Boden, W. E., Padala, S. K., Cabral, K. P., Buschmann, I. R. & Sidhu, M. S. Role of short-acting nitroglycerin in the management of ischemic heart disease. Drug Des. Dev. Ther. 9, 4793–4805 (2015).
Nguyen, H. V. et al. Temporary biventricular pacing decreases the vasoactive-inotropic score after cardiac surgery: A substudy of a randomized clinical trial. J. Thorac. Cardiovasc. Surg. 146(2), 296–301 (2013).
Goradia, S., Sardaneh, A. A., Narayan, S. W., Penm, J. & Patanwala, A. E. Vasopressor dose equivalence: A scoping review and suggested formula. J. Crit. Care 61, 233–240 (2021).
Greenwood, J. C., Talebi, F. M., Jang, D., Abella, B. S. Using a Topical Nitroglycerin Challenge to Detect Reversible Microcirculatory Dysfunction in Patients with Circulatory Shock. https://zenodo.org/record/5768720 (Accessed 8 December 2021) (2021).
Acknowledgements
The authors extremely grateful to the subjects who took part in the study, as well as for the support from the Abramson Emergency Medicine & Critical Care Research Fund and the University of Pennsylvania’s Center for Resuscitation Science for its continued research support and guidance.
Funding
JCG is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, award number KL2TR001879. DHJ is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health, Award Number K08HL136858. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
J.C.G., F.M.T., D.H.J., and A.E.S. made substantial contributions to the conception or design of the work; the acquisition, analysis, or interpretation of data; drafted the work or substantively revised the manuscript. J.E.T., J.T.G., J.G.T.A., J.H., M.A.A., J.S.B., V.R.M., and T.J.K. made substantial contributions to the conception or design of the work; drafted the work or substantively revised the manuscript. F.S.S. made substantial contributions to the conception or design of the work; the analysis, or interpretation of data; drafted the work or substantively revised the manuscript. J.B. made substantial contributions to the conception or design of the work; the analysis, or interpretation of data; drafted the work or substantively revised the manuscript. B.S.A. made substantial contributions to the conception or design of the work; the analysis, or interpretation of data; drafted the work or substantively revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Greenwood, J.C., Talebi, F.M., Jang, D.H. et al. Topical nitroglycerin to detect reversible microcirculatory dysfunction in patients with circulatory shock after cardiovascular surgery: an observational study. Sci Rep 12, 15257 (2022). https://doi.org/10.1038/s41598-022-19741-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-19741-0
- Springer Nature Limited
This article is cited by
-
Investigation of Cerebral Mitochondrial Injury in a Porcine Survivor Model of Carbon Monoxide Poisoning
Journal of Medical Toxicology (2024)
-
Angiopoietin-2 is associated with capillary leak and predicts complications after cardiac surgery
Annals of Intensive Care (2023)