Abstract
Although speech declines rapidly in some individuals with amyotrophic lateral sclerosis (ALS), longitudinal changes in speech have rarely been characterized. The study objectives were to model the rate of decline in speaking rate and speech intelligibility as a function of disease onset site, sex, and age at onset in 166 individuals with ALS; and estimate time to speech loss from symptom onset. We also examined the association between clinical (speaking rate/intelligibility) measures and patient-reported measures of ALS progression (ALSFRS-R). Speech measures declined faster in the bulbar-onset group than in the spinal-onset group. The rate of decline was not significantly affected by sex and age. Functional speech was still maintained at 60 months since disease onset for most patients with spinal onset. However, the time to speech loss was 23 months based on speaking rate < 120 (w/m) and 32 months based on speech intelligibility < 85% in individuals with ALS-bulbar onset. Speech measures were more responsive to functional decline than were the patient-reported measures. The findings of this study will inform future work directed toward improving speech prognosis in ALS, which is critical for determining the appropriate timing of interventions, providing appropriate counseling for patients, and evaluating functional changes during clinical trials.
Similar content being viewed by others
Introduction
Most individuals with amyotrophic lateral sclerosis (ALS) progressively lose the ability to speak and, therefore, eventually require assistive devices to fulfill communication needs1,2,3,4. The onset and progression of dysarthria, the speech disorder associated with ALS, is a major concern of patients who invariably want to know “when” and “how fast” their loss of speech will occur. Despite the critical importance of accurate speech prognosis to clinical care, the longitudinal decline of speech has rarely been characterized, leading clinicians to speculate based on their selective and subjective experiences. Improving predictive models of speech decline is not only needed to better advise patients regarding the expected course of their disease, but also critical for optimizing ALS clinical trials and treatment strategies for maintaining communication abilities throughout the disease.
Although the course of overall functional decline due to ALS has been modeled extensively using clinical scales such as the Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-R)5,6,7,8,9, the focus on speech decline has been limited. Studies of speech are needed because the rate of speech decline may be decoupled from that occurring in other parts of the motor system5 and the disease progression and expression are known to be very heterogeneous5,6,7,8,9,10,11,12,13. The few existing studies of longitudinal speech decline in ALS suggest that speech remains functional for an average of 18 months from the first bulbar symptom14. For individuals who experience their first symptom in bulbar muscle (i.e., bulbar-onset), speech tends to decline faster than for those with spinal-onset ALS14 and can decline as fast as 20% a year15. While these estimates are useful, they are derived from only a few studies, some of which had design issues that limit interpretation, including a relatively small number of participants. For instance, the estimates of speech decline in Makkonen et al.14 were based on only 9 participants (four bulbar and five spinal) who completed their follow-up session. In addition, the model of speech intelligibility decline provided by Rong et al.15 did not account for site of onset and was calculated in months from ALS diagnosis, which may be problematic as participants could be at different stages of their disease at the time of diagnosis. Ideally, predictive models of ALS speech decline will account for the influence of potential risk factors such as site of disease onset, age at onset, and sex on group and individual trends. Additional research is also needed to identify the most responsive markers of speech decline.
In the current study, we used measures of speaking rate and speech intelligibility as indices of speech function because of their widespread use in clinical evaluation of bulbar function, disease severity, and motor speech impairment in ALS. Bulbar involvement is often characterized by slowed speaking rate and reduced speech intelligibility1,16. During the course of speech decline, it has been reported that (1) changes in speaking rate precedes the onset of intelligibility decline in individuals with ALS, and (2) intelligibility declines in a faster rate when speaking rate passes the threshed of 120 w/m1,17. Further, speaking rate is significantly associated with clinical measures of overall ALS and bulbar disease severity, and has necessary psychometric properties (high reliability, construct validity, and sensitivity (AUC = 0.81)) to detect bulbar signs prior to the onset of overt bulbar symptoms (i.e., the presymptomatic stage)18. The aims of the study were to (1) identify the status of speaking rate and speech intelligibility in ALS subgroups at baseline relative to normative data accounting for sex and age; (2) determine the rate of decline in functional speech measures, while controlling for ALS site of onset, age at onset, and sex; (3) estimate the time to speech loss in ALS subgroups; (4) compare the slope of change in speech measures to the slope of change in self-reported ratings (the ALSFRS-R total score and bulbar subscore); and (5) determine if the decline in speech measures are associated with the decline in the ALSFRS-R total score and bulbar subscore. The comparative analysis between speech measures and ALSFRS-R scores is important because ALSFRS-R scores are commonly used in routine clinical evaluations, clinical trials, and patient-oriented research to estimate the overall progression of ALS. Results from this work may help clinicians improve estimates of the rate of speech decline and, as such, optimize patient care and the design of individualized treatment plans.
Methods
Participants
One hundred sixty-six individuals with ALS (71 females) aged from 33 to 86 years old (M = 58 years, SD = 10) were included in this study. All participants in the ALS group were diagnosed with ALS by a neurologist using the revised El Escorial criteria19 and had no history of other neurological disorders. The ALS group was subdivided by site of symptom onset. Thirty participants (18 females) exhibited bulbar onset, while 136 participants (53 females) exhibited spinal onset. Unequal sample sizes in the ALS-Spinal and ALS-bulbar groups are expectable given the higher prevalence of ALS-spinal compared to ALS-bulbar onset. It’s been reported that the prevalence of ALS patients being characterized as spinal onset predominates that of bulbar onset by almost 58–82%20. In addition, 167 healthy controls (HC) (76 males, 91 females) aged from 29 to 77 years old (M = 58 years, SD = 10 years) were included as controls. Control group participants had no history of any known neurological, cognitive, speech, or swallowing impairment. Healthy controls volunteered to participate in the study and were recruited through flyers and Ads.
The data obtained from individuals with ALS and healthy controls were collected from 2009 to 2018 at multiple sites in the United States and Canada to be a good representative of the population in North America. The study sites located in the United States included the MGH Institute of Health Professions (Boston), University of Nebraska, and University of Dallas. The study sites located in Canada included the Sunnybrook Research Institute in Toronto and the sites involved in the Canadian ALS Neuroimaging Consortium (CALSNIC)21. The number of participants recruited from the United States and Canada are provided in Table 1. All participants were native speakers of English and none had any reported hearing or vision impairment or literacy disability that could have impacted reading of speech stimuli. All participants with ALS as well as normal controls had no cognitive impairment as determined by the MoCA test. In addition, none of the subjects were on medications known to affect speech production. Written informed consent was obtained from all participants and the study was approved by the Institutional Review Board of all institutions involved in the data collection for this study. The study was conducted in accordance with the relevant guidelines and regulations outlined in the Declaration of Helsinki. Table 1 represents demographic and clinical information about the participants.
Procedure
Participants were seen across multiple sessions during which they recorded a variety of speech and non-speech tasks. There was a large variation across participants in terms of the number of data collection sessions (M = 3.5 sessions, SD = 3.82, min = 1 session, max = 25 sessions). In this heterogeneous dataset, a substantial portion of the participants had only two data points. Because creating linear models that have many data points for some individuals and only two data points for a large portion of individuals introduces model complexities and leads to overfitting, we analyzed changes between the first and last recordings of the Sentence Intelligibility Test (SIT)22 by each participant to obtain more accurate estimations of the rate of decline in speech measures. In fact, the change score is often used in clinical trial settings. For example, the combined assessment of function and survival (CAFS) score is a well-accepted method of analyzing clinical trial data that looks at the change between the first and last measurements23. Therefore, all the 166 participants with ALS had both the baseline and follow-up data and, there were no dropouts due to death or loss to follow-up. The time interval between the two sessions ranged from 1 to 37 months (M = 9.63, SD = 7.25).
Functional speech measures
Measures of speaking rate and speech intelligibility were extracted from recordings of each participant’s reading of 11 randomly generated sentences from the SIT22. The number of words in each sentence ranged from five to 15. Participants were asked to read the sentences using their habitual speaking rate and loudness. Audio recordings of the participants were collected using the procedure reported in previous studies11,15. Subsequently, a trained research assistant who was unfamiliar with the SIT sentences and blind to the participants' diagnoses, listened to the audio recordings and transcribed the sentences produced by the participants. Speaking rate was calculated for each sentence as the number of words per minute (w/m) averaged across the 11 sentences. A percent intelligibility score for each sentence was calculated as the percent of intelligible (correctly transcribed) words out of the total number of words contained in the sentence. The percent intelligibility scores of all 11 sentences were averaged to derive the total percent intelligibility score. The inter and intrarater reliability of SIT transcriptions were reported in a recent study by our lab, which used a subset of data used in this study to create an empirical classification system for speech severity in patients with dysarthria secondary to ALS. Overall, the intraclass correlation (ICC) for intrarater transcription reliability was reported as 0.91 (p < 0.001) and the ICC for interrater transcription reliability ranged from 0.92 to 0.96 (p < 0.001)24, demonstrating excellent reliability in SIT transcription.
Patient-reported measure of function
The ALSFRS-R is a well-established and reliable measure of global motor (i.e., bulbar, fine motor, gross motor, and respiratory) disability in patients with ALS25. It is commonly used in clinics to assess ALS disease progression and as an endpoint in ALS clinical trials and survival outcomes7,26,27,28. A measure of patient-reported bulbar function was derived from the ALSFRS-R bulbar subscore, which includes three items: speech, swallowing, and saliva management, with possible scores ranging from 0 (maximum disability) to 12 (normal function). Please refer to Table 1 for information about the ALSFRS-R total score and ALSFRS-R bulbar subscore of individuals with ALS at baseline and follow-up sessions.
Statistical analyses
Examining the distribution of sex and age across groups
ANOVA test was used to examine whether there were any differences between the groups (HC, ALS-spinal, and ALS-bulabr) in terms of the age of participants. In addition, the proportion of male and female participants in each group was examined using chi-squared tests.
Statistical analyses related to the aims of the study
Aim 1 examined the ALS subgroups’ speaking rate and speech intelligibility at baseline relative to normative data accounting for sex and age. This aim facilitates interpretation of the longitudinal results by showing, where the individuals with ALS stand in terms of speech functions at baseline relative to normative data and how their speech change at various stages of the disease. Separate multiple regression analyses were used to examine the effect of group, sex, and age on speaking rate and speech intelligibility. In the regression models, the variable group had three levels [HC, ALS-spinal, and ALS-bulbar], and the variables sex, and age were binary and defined in two levels of analysis as follows: sex [males vs. females]; age at onset [younger than 60 years vs. older than 61 years]. The variable “age” was dichotomized because the cut-off point of 60 years yielded relatively equal sample sizes across the groups (Fig. 1). Subsequently, t-tests were performed to identify between-group differences. Bonferroni correction was used to control the family-wise error rate in multiple comparisons.
Aim 2 determined the rate of decline of functional speech measures while accounting for clinical and demographic factors. To address this aim, the lme() function from the nlme packages in R statistical software (i386 3.6.1), was used to fit linear mixed effects (LMEs) models on longitudinal speaking rate and intelligibility data. LMEs models were used to mitigate the effect of unequal time-interval between the baseline and follow-up sessions, as well as missing and unbalanced data, on the reliability of slope of change in the outcome measures29,30. In the first step, separate LMEs models were created for speaking rate and intelligibility with variables “time”, “site of onset”, “sex”, “age”, and (site of onset + sex + age) × time as fixed effects and “subject” as the random effect. In the next step, fixed effect variables that were not statistically significant were removed from the LMEs models using a backward selection approach. Subsequently, a simplified LMEs model was produced for the effect of site of onset on the rate of decline in speaking rate and intelligibility irrespective of sex and age (non-significant variables). In LMEs models, the time scale was based on months relative to baseline.
Because the baseline and follow-up sessions occurred at different time points relevant to the onset of ALS symptoms across all participants, the time (in months) of baseline and follow-up sessions were subtracted from the baseline time (in month) to align the baseline data for all subjects to 0 month from symptom onset. For example, if the baseline and follow-up sessions occurred at 5 and 12 months after symptom onset, the time points were shifted to 0 and 7 months, respectively. This technique allowed us to estimate the net rate of speech decline per month in each ALS subgroup (spinal-onset vs. bulbar onset). In the LMEs models, the variables of onset site, sex, and age were dichotomous and defined in two levels of analysis as follows: site of onset [spinal vs. bulbar]; sex [males vs. females]; age at onset [younger than 60 years at symptom onset (34–60 years, M = 52.63, SD = 5.88) vs. older than 61 years at symptom onset (61–88 years, M = 69, SD = 6.27)].
Aim 3 sought to estimate the time to speech loss. Speech loss was defined as either speaking rate of below 120 (w/m) or speech intelligibility below 85%1,15,31. Kaplan–Meier test was used to estimate the likelihood of maintaining speech functions based on the time to the occurrence of speaking rate below 120 (w/m), and intelligibility below 85%. These thresholds were selected based on the literature suggesting that speaking rate is a significant predictor of the intelligibility decline1,17 such that the slowness of speaking rate to approximately 120 words per minute (w/m), marks the initiation of accelerated decline in speech intelligibility (below 85%). Kaplan–Meier tests were fitted on the follow-up data (not the baseline) to estimate the fraction of individuals with ALS who maintain speech function until the follow-up session. Because all participants used in the study completed their follow-up session, there were no missing values due to mortality in our Kaplan–Meier analyses. In survival analysis models, the time scale was based on months since symptom onset which has been shown to be the best timescale in longitudinal studies of ALS, rendering models with increased precision and the lowest AIC14,32.
Aim 4 examined the difference in slope of change between speech measures and self-reported measures of ALSFRS-R (total score and bulbar subscore). To address this goal, separate pairwise t-tests were used for individuals in ALS-bulbar and ALS-spinal subgroups to compare the slope of change in speaking rate and intelligibility to the slope of change in the ALSFRS-R total score and ALSFRS-R bulbar subscore. The slope of change in speech and ALSFRS-R measures were calculated by subtracting the measures obtained at follow-up from measures obtained at baseline divided by the time interval. The p-value was adjusted (Bonferroni correction in multiple comparisons.
Aim 5 evaluated associations between changes in instrumental measures of speech function (intelligibility, speaking rate) and self-report ratings (the ALSFRS-R total score and bulbar subscore) across disease progression. The associations between the slope of change in functional speech measures and the slope of change in the ALSFRS-R total and bulbar subscores were assessed using the Pearson correlation tests. Bonferroni correction was performed to adjust the p-value in multiple tests.
Results
Distribution of sex and age across groups
The variable age demonstrated normal distribution in all groups as displayed in Fig. 1. The average age was almost similar in the ALS subgroups As well as healthy controls (between 58 and 60 years). The ANOVA tests showed no statistically significant differences among the three groups with regard to age. In addition, results of the chi-squared tests demonstrated that the proportion of male and female participants was only statistically significantly different in the ALS-spinal group (p = 0.01) with 83 individuals being male and 53 individuals being female in this group.
Results related to study aims
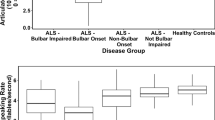
Multiple regression analyses revealed significant effect of group on both speaking rate and intelligibility (Table 2). Neither sex nor age appeared to be significant factors in speaking rate differences observed between healthy controls and ALS subgroups. However, sex was observed to have a statistically significant effect on speech intelligibility such that females demonstrated a better speech intelligibility by only 0.78% (less than 1%) compared to men, which is negligible and not clinically meaningful. Mean (SD) values of baseline speaking rate (w/m) in healthy controls, ALS-spinal, and ALS-bulbar groups were 187.56 (28.08), 168.54 (33.32), and 118.67 (34.62), respectively. Mean (SD) values of baseline intelligibility scores in healthy controls, ALS-spinal, and ALS-bulbar groups were 98.42% (1.95), 97.76% (2.77), and 92.93% (6.12), respectively. T-tests indicated that speaking rate was significantly lower for the ALS-spinal (p < 0.001) and ALS-bulbar (p < 0.001) groups than for the HC group and for the ALS-bulbar group relative to the ALS-spinal group (p < 0.001). In addition, the baseline measure of intelligibility in the ALS-bulbar group was significantly lower than intelligibility in both the ALS-spinal and the HC groups (p < 0.001). The observed difference between the HC and ALS-spinal groups in speech intelligibility (p = 0.02) did not reach the adjusted p-value for multiple comparisons (p = 0.017) and was considered not significant (Fig. 2).
Baseline speaking rate and % intelligibility score in ALS-spinal, and ALS-bulbar groups relative to normative data (HC = healthy controls). ****Represents comparisons that remained significant after Bonferroni adjustment of p-values (p-values less than 0.017 was considered statistically significant).
Results of LMEs models for speaking rate and intelligibility demonstrated a significant effect of site of onset and a significant time × site of onset interaction (Table 3). Results of simplified LMEs models demonstrated that speaking rate is significantly lower in the ALS-bulbar group compared to ALS-spinal group by approximately 51 (w/m) at time 0 (baseline) and declines at a significantly faster rate (Table 4). While the slope of speaking rate declines by 1.74 (w/m) per month in individuals with spinal onset ALS, it declines by 4.16 (w/m) per month in individuals with bulbar onset ALS (Fig. 3). Similarly, speech intelligibility was significantly lower and showed faster rate of decline in the ALS-bulbar than ALS-spinal group. Individuals in the ALS-bulbar group demonstrated about 14% lower intelligibility at time 0 compared to those in the ALS-spinal group. The slope of decline in intelligibility of the ALS-bulbar group was steeper by about 1.3% per month compared to that of ALS-spinal group (1.61% vs. 0.28% decline per month in the intelligibility scores (words understood) of ALS-bulbar and ALS-spinal groups respectively) (Fig. 4).
Kaplan–Meier tests demonstrated significantly reduced likelihood of maintaining speech functions at 12, 24, 36, 48, and 60 months since symptom onset for individuals with bulbar-onset compared to those with spinal onset (Fig. 5). While the median time to exhibiting a speaking rate lower than 120 (w/m) was 23 months for those with bulbar-onset ALS, 60% of participants with spinal-onset ALS maintained a speaking rate greater than 120 (w/m) over the 60-month follow-up.
Individuals in the ALS-spinal group were more resilient to intelligibility decline than speaking rate decline over the 60-month follow-up. About 80% of individuals in the ALS-spinal group maintained intelligibility greater than 85% during the 60-month follow-up period. However, the median time to exhibiting intelligibility lower than 85% was 32 months from symptom onset for the ALS-bulbar group.
In individuals with bulbar-onset ALS, the slope of change in both speaking rate and speech intelligibility were observed to be significantly faster than the slope of change in their ALSFRS total score and bulbar subscore. In individuals with spinal-onset ALS, however, the only significant difference in slope of change was between speaking rate and ALSFRS-R bulbar subscore, such that speaking rate declined faster than the ALSFRS-bulbar score in that group (Table 5).
Finally, Pearson correlation tests indicated that only in the ALS-spinal group, there were statistically significant associations between the slope of decline in speech intelligibility and ALSFRS-R total score and ALSFRS-R bulbar subscore as well as between speaking rate and ALSFRS-R bulbar subscore. However, the observed correlations were mild and below r = 0.50 (Table 6).
Discussion
Our findings indicate that individuals in the ALS-spinal and ALS-bulbar groups had slower rates of speech compared to normal controls at baseline. Baseline speech intelligibility of individuals with bulbar-onset ALS was also lower than normal controls. The findings also suggested that the rate of decline in speech measures were driven primarily by site of symptom onset rather than sex or age at onset. Group trends showed that on average, speaking rate and intelligibility scores were lower (by approximately 51 w/m and 14% words understood) in the bulbar-onset ALS group compared to the spinal-onset ALS group at baseline. In addition, the rates of decline in speaking rate and intelligibility were faster in the bulbar-onset ALS group (4.16 w/m per month, 1.61% words understood per month) than in the spinal-onset ALS group (1.74 w/m per month, 0.28% words understood per month). Although the rate of decline varied significantly across individuals, the median time to speech loss defined as speaking rate slower than 120 (w/m) or speech intelligibility below 85% was relatively short for those with bulbar-onset ALS. While the median time to exhibit a speaking rate slower than 120 (w/m) and ineligibility below 85% was respectively 23 and 32 for the bulbar-onset ALS group, functional speech was still maintained for the majority of individuals in the spinal-onset group over 60 months since symptom onset. In fact, at 60 months (5 years) after symptom onset, 60% of participants with spinal-onset ALS exhibited a speaking rate greater than 120 (w/m) and 80% of them exhibited speech intelligibility greater than 85%. Finally, clinical speech measures (i.e., speaking rate and speech intelligibility) were more responsive to functional decline than were the patient-reported measures (i.e., ALSFRS-R total score and bulbar subscore). The findings of this study have important implications for future work directed toward improving speech prognosis in ALS, which is critical for determining the appropriate timing of interventions, providing appropriate counseling for patients, and evaluating functional changes during clinical trials.
ALS site of onset: the major influential factor for the rate of decline in speech functions
Literature on the effect of onset site on ALS progression has consistently reported that patients with bulbar-onset ALS demonstrate deterioration of functional measures (e.g., clinical scales) at a markedly increased rate compared to individuals with spinal-onset ALS. The majority of these studies used the ALSFRS-R25 as a prognostic index to assess global function in patients with ALS and its rate of decline7,26,27,28,33. Consistent with these self-reported measures, our models demonstrated significantly faster decline of speaking rate and intelligibility in individuals with bulbar-onset ALS. Additionally, our findings are consistent with previous findings of significant differences in speaking rate and/or intelligibility decline for those with bulbar vs. spinal-onset ALS17,34.
Our models also confirm prior work suggesting that speaking rate declines with a steeper slope than speech intelligibility over the course of the disease regardless of site of onset1,17,35,36. Speaking rate has been found to be a significant predictor of intelligibility decline, with the accelerated decline in speech intelligibility (below 85%) not occurring until speaking rate has slowed to approximately 120 w/m1,17.
Although several studies on models of ALS disease progression have attributed a faster rate of functional decline in older patients and females than in younger patients and males32,37,38, findings of this study suggested that rates of speech decline are driven primarily by site of symptom onset and are not influenced by sex or age. Our findings converge with Makkonen et al.14, who reported that among onset site, sex, and age, only the onset site significantly predicted the rate of decline in speech measures of individuals with ALS.
Within-group variability in rate of speech decline was large for individuals with spinal and bulbar-onset ALS
The rate of speech decline for some individuals in both the spinal and bulbar groups diverged significantly from that of the overall group trend (e.g., faster rate of decline in some individuals in the ALS-spinal group and slower rate of decline in the ALS-bulbar group). Although the divergence only occurred in a small number of cases in each group, these findings provide further evidence in support of motor phenotype heterogeneity in ALS10 and exemplify the need for improved patient stratification. Therefore, objective criteria such as imaging-based biomarkers39, brain/plasma proteomics40, or assessment of bulbar motor involvement13 are necessary.
Results of this work suggest that speech measures can be used to uncover covert patient subgroups within the ALS-spinal and ALS-bulbar groups. Stratifying individuals with ALS into clinically relevant subgroups contributes to a better understanding of disease mechanisms and prognosis. In addition, subgroup analyses may enhance the quality of care for ALS patients by informing decision making, optimizing clinical trial planning/interpretation, and contributing to the development of effective therapeutic options.
Time to speech loss in individuals with bulbar ALS is relatively short
Because ALS progresses rapidly in some affected individuals, an important goal of clinical management is to predict functional changes in patients’ speech performance in order to facilitate intervention planning, such as the introduction of assistive technology before the patient’s ability to learn these skills is hindered by the severity of their condition2,41,42. An important finding of this study was that the median time to speech loss based on speaking rate slower than 120 (w/m), was approximately 23 months for individuals with bulbar-onset ALS. In contrast, 60% of individuals with spinal-onset ALS were observed to maintain functional speech at 60 months since symptom onset. If we use speech intelligibility below 85% to index speech loss, the median time to speech loss was 32 months since symptom onset for individuals with bulbar-onset ALS. At 60 months since symptom onset, 80% of participants with spinal-onset ALS maintained speech intelligibility greater than 85%, whereas only 10% of individuals with bulbar-onset ALS demonstrated speech intelligibility greater than 85%. Rong et al.15 modeled time to speech loss in individuals with ALS and found that at 2.5 months after diagnosis, 71% of participants with ALS maintained sentence intelligibility scores higher than 85% and 41% of them demonstrated a speaking rate faster than 120 (w/m). At three years after diagnosis, however, only about 20% of their participants with ALS exhibited a speaking rate greater than 120 (w/m) and 30% of them had sentence intelligibility greater than 85%. Differences in study findings can likely be attributed to the fact that their model was based on time from diagnosis (years) rather than time since symptom onset. Considering time from diagnosis may be problematic because diagnostic criteria can vary across clinics and it may, therefore, capture different stages of disease across individuals with ALS. In addition, Rong et al.15 included individuals with ALS irrespective of the site of onset while clinical observations and empirical evidence have consistently indicated that the rate of disease progression and functional decline is significantly more rapid in individuals with bulbar onset than spinal onset26,43,44.
Objective speech measures may be better markers of functional decline than patient-reported measures
Research has documented increased sensitivity and responsiveness of instrumental bulbar measures over patient-reported measures of bulbar dysfunction45. Our findings further support this assertion, as the slope of change in speech measures was statistically greater than the slope of change in ALS functional measures (i.e., ALSFRS-R total score and ALSFRS-R bulbar subscore). The slower rate of decline in clinical measures of ALS function may be expected as a result of the multidimensionality of the ALSFRS-R total score and the ALSFRS-R bulbar subscore, which is different from speech measures that objectively represent performance of participants in a single domain (i.e., speech). In addition, the change over time in speech measures was mildly associated with changes in ALS functional measures only for the spinal onset group. This finding is comparable with a study conducted by Barnett et al.18, who reported only mild-to-moderate associations between speaking rate and clinical measures of overall ALS and bulbar disease severity. These findings suggest that ALSFRS-R scores—including the bulbar subscore—do not effectively capture functional decline in speech motor performance over the course of bulbar decline, particularly in individuals with bulbar-onset ALS with greater speech impairment severity and a faster rate of decline in functional speech measures.
Conclusions and study limitations
In sum, this study quantified the rate of decline in functional speech measures controlling for important clinical and demographic factors and provided estimates of time to speech loss in individuals with spinal and bulbar-onset ALS. These findings inform our understanding of speech decline over the course of ALS disease progression and will contribute to the literature supporting best practices for timely intervention. A number of limitations should, however, be acknowledged. First, because of the clinical heterogeneity of ALS, each patient may demonstrate a unique trajectory of speech decline, making prediction within an individual difficult. Although we accounted for factors such as onset site, age at onset, and sex, the models presented in this study only provided simplistic estimates of the rate of decline in functional speech measures of individuals with ALS. Multiple pathophysiological, clinical, epidemiological, and genetic factors singly or in combination, influence the rate of ALS progression. However, producing models of ALS progression that simultaneously account for these variables is almost impractical due to high inter-individual variability in the underlying pathophysiological and clinical manifestations of ALS, heterogeneity in the expression of the disease symptoms, collinearity among predictors, and complex interactions of multivariate factors. Second, the distribution of sex was unbalanced between the ALS-spinal and ALS-bulbar groups, which despite the robustness of LMEs models to unequal sample sizes, might have influenced the findings related to the effect of sex on speech measures of ALS subgroups. Third, we did not track the “time of the day” when the ALSFRS was scored and if that can influence the association between the ALSFRS scores and speech measures. Although we were not able to examine this, it has been reported that “time of day” does not significantly influence speech measures46. In addition, the data used in this study were collected during visits that took place during a relatively confined time window (between 10:00 am and 4:00 pm rather than early morning and late at night) which could minimize the potential confounding effect of “time of day” on speech measures.
Data availability
The datasets used and/or analysed during the current study will be made available from the corresponding author on reasonable request.
References
Yorkston, K., Strand, E., Miller, R., Hillel, A. & Smith, K. Speech deterioration in amyotrophic lateral sclerosis: Implications for the timing of intervention. J. Med. Speech. Lang. Pathol. 1, 35–46 (1993).
Beukelman, D., Fager, S. & Nordness, A. Communication support for people with ALS. Neurol. Res. Int. 2011, 1–6 (2011).
Creer, S., Enderby, P., Judge, S. & John, A. Prevalence of people who could benefit from augmentative and alternative communication (AAC) in the UK: determining the need. Int. J. Lang. Commun. Disord. 51, 639–653 (2016).
Brownlee, A. & Bruening, L. M. Methods of communication at end of life for the person with amyotrophic lateral sclerosis. Top. Lang. Disord. 32, 168–185 (2012).
Abdulla, S. et al. Validation of the German version of the extended ALS functional rating scale as a patient-reported outcome measure. J. Neurol. 260, 2242–2255 (2013).
Westeneng, H.-J. et al. Prognosis for patients with amyotrophic lateral sclerosis: development and validation of a personalised prediction model. Lancet Neurol. 17, 423–433 (2018).
Kollewe, K. et al. ALSFRS-R score and its ratio: A useful predictor for ALS-progression. J. Neurol. Sci. 275, 69–73 (2008).
Grad, L. I., Rouleau, G. A., Ravits, J. & Cashman, N. R. Clinical Spectrum of Amyotrophic Lateral Sclerosis (ALS). Cold Spring Harb. Perspect. Med. 7, a024117 (2017).
Ticozzi, N. & Silani, V. Genotypic and phenotypic heterogeneity in amyotrophic lateral sclerosis. In Neurodegenerative Diseases 279–295 (Springer International Publishing, 2018).
Ravits, J. M. & La Spada, A. R. ALS motor phenotype heterogeneity, focality, and spread: Deconstructing motor neuron degeneration. Neurology 73, 805–811 (2009).
Yunusova, Y., Green, J. R., Wang, J., Pattee, G. & Zinman, L. A protocol for comprehensive assessment of bulbar dysfunction in amyotrophic lateral sclerosis (ALS). J. Vis. Exp. https://doi.org/10.3791/2422 (2011).
Yorkston, K., Beukelman, D. & Hakel, M. Speech Intelligibility Test for Windows (1996).
Stipancic, K. L. et al. Two distinct clinical phenotypes of bulbar motor impairment in amyotrophic lateral sclerosis. Front. Neurol. 12, 715 (2021).
Makkonen, T., Ruottinen, H., Puhto, R., Helminen, M. & Palmio, J. Speech deterioration in amyotrophic lateral sclerosis (ALS) after manifestation of bulbar symptoms. Int. J. Lang. Commun. Disord. 53, 385–392 (2018).
Rong, P., Yunusova, Y., Wang, J. & Green, J. R. Predicting early bulbar decline in amyotrophic lateral sclerosis: A speech subsystem approach. Behav. Neurol. 2015, 1–11 (2015).
Ball, L. J., Willis, A., Beukelman, D. R. & Pattee, G. L. A protocol for identification of early bulbar signs in amyotrophic lateral sclerosis. J. Neurol. Sci. 191, 43–53 (2001).
Ball, L. J., Beukelman, D. R. & Pattee, G. L. Timing of speech deterioration in people with amyotrophic lateral sclerosis. J. Med. Speech. Lang. Pathol. 10, 231–235 (2002).
Barnett, C. et al. Reliability and validity of speech & pause measures during passage reading in ALS. Amyotroph. Lateral Scler. Front. Degener. 21, 42–50 (2020).
Brooks, B. R., Miller, R. G., Swash, M. & Munsat, T. L. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 1, 293–299 (2000).
Longinetti, E. & Fang, F. Epidemiology of amyotrophic lateral sclerosis: An update of recent literature. Curr. Opin. Neurol. 32, 771–776 (2019).
Kalra, S. et al. Authors Dr . Sanjay Kalra , MD , FRCPC Professor , Department of Medicine (Neurology ) Neurosciences and Mental Health Institute University of Alberta 7–132F Clinical Sciences Building 11350–83 Ave. (2020).
Yorkston, K., Beukelman, D. & Hakel, M. Speech Intelligibility Test (SIT) for Windows. (2007).
Berry, J. D. et al. The Combined Assessment of Function and Survival (CAFS): A new endpoint for ALS clinical trials. Amyotroph. Lateral Scler. Front. Degener. 14, 162–168 (2013).
Stipancic, K. L. et al. “You Say Severe, I Say Mild”: Toward an empirical classification of dysarthria severity. J. Speech Lang. Hear. Res. 64, 4718–4735 (2021).
Cedarbaum, J. M. et al. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. J. Neurol. Sci. 169, 13–21 (1999).
Chio, A. et al. Early symptom progression rate is related to ALS outcome: A prospective population-based study. Neurology 59, 99–103 (2002).
Kaufmann, P. et al. The ALSFRSr predicts survival time in an ALS clinic population. Neurology 64, 38–43 (2005).
Kimura, F. et al. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology 66, 265–267 (2006).
Garcia, T. P. & Marder, K. Statistical approaches to longitudinal data analysis in neurodegenerative diseases: huntington’s disease as a model. Curr. Neurol. Neurosci. Rep. 17, 14 (2017).
Molenberghs, G. & Verbeke, G. A review on linear mixed models for longitudinal data, possibly subject to dropout. Stat. Model. 1, 235–269 (2001).
Niimi, M. N. S. Changes over time in dysarthric patients with amyotrophic lateral sclerosis (ALS): A study of changes in speaking rate and maximum repetition rate (MRR). Clin. Linguist. Phon. 14, 485–497 (2000).
Daghlas, I., Lever, T. E. & Leary, E. A retrospective investigation of the relationship between baseline covariates and rate of ALSFRS-R decline in ALS clinical trials. Amyotroph. Lateral Scler. Front. Degener. 19, 206–211 (2018).
Rooney, J., Burke, T., Vajda, A., Heverin, M. & Hardiman, O. What does the ALSFRS-R really measure? A longitudinal and survival analysis of functional dimension subscores in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 88, 381–385 (2017).
Connaghan, K. P. et al. Use of Beiwe Smartphone App to Identify and Track Speech Decline in Amyotrophic Lateral Sclerosis (ALS). In Interspeech 2019 4504–4508 (ISCA, 2019). https://doi.org/10.21437/Interspeech.2019-3126.
Kent, R. D. et al. Impairment of speech intelligibility in men with amyotrophic lateral sclerosis. J. Speech Hear. Disord. 55, 721–728 (1990).
Nishio, M. & Niimi, S. Changes over time in dysarthric patients with amyotrophic lateral sclerosis(ALS). II. A study of changes in speaking rate and maximum repetition Rate(MRR). Jpn. J. Logop. Phoniatr. 40, 8–16 (1999).
del Aguila, M. A., Longstreth, W. T., McGuire, V., Koepsell, T. D. & van Belle, G. Prognosis in amyotrophic lateral sclerosis: A population-based study. Neurology 60, 813–819 (2003).
Rooney, J. et al. Survival analysis of irish amyotrophic lateral sclerosis patients diagnosed from 1995–2010. PLoS ONE 8, e74733 (2013).
Bede, P. et al. Grey matter correlates of clinical variables in amyotrophic lateral sclerosis (ALS): a neuroimaging study of ALS motor phenotype heterogeneity and cortical focality. J. Neurol. Neurosurg. Psychiatry 84, 766–773 (2013).
Leoni, E. et al. Combined tissue-fluid proteomics to unravel phenotypic variability in amyotrophic lateral sclerosis. Sci. Rep. 9, 4478 (2019).
Ball, L. J., Beukelman, D. R. & Pattee, G. AAC clinical decision making for persons with ALS. Perspect. Augment. altern. commun. 11, 7–13 (2002).
Londral, A. R., Encarnacao, P. & Azevedo, L. Selecting AAC devices for persons with ALS taking into consideration the disease progression. Assist. Technol. Res. Ser. 25, 12–17 (2009).
Magnus, T. et al. Disease progression in amyotrophic lateral sclerosis: Predictors of survival. Muscle Nerve 25, 709–714 (2002).
Muddasir Qureshi, M. et al. Analysis of factors that modify susceptibility and rate of progression in amyotrophic lateral sclerosis (ALS). Amyotroph. Lateral Scler. 7, 173–182 (2006).
Allison, K. M. et al. The diagnostic utility of patient-report and speech-language pathologists’ ratings for detecting the early onset of bulbar symptoms due to ALS. Amyotroph. Lateral Scler. Front. Degener. 18, 358–366 (2017).
Stegmann, G. M. et al. Early detection and tracking of bulbar changes in ALS via frequent and remote speech analysis. npj Digit. Med. 3, 132 (2020).
Acknowledgements
This research was funded by the NIH-NIDCD grants R01DC009890 (Co-PIs: Jordan R. Green and Yana Yunusova), R01DC0135470 (PI: Jordan R. Green), R01DC017291 (Co-PIs: Yana Yunusova and Jordan R. Green), K24DC016312 (PI: Jordan R. Green), K23DC019179 (PI: Marziye Eshghi), and R15DC018944 (PI: Kathryn P. Connaghan). The Canadian ALS Neuroimaging Consortium (CALSNIC) is funded by the Canadian Institutes of Health Research (CIHR), ALS Canada, Brain Canada, and the Shelly Mrkonjic ALS Research Fund. We would like to thank Perman Gochyyev (a biostatistician at MGH Institute of Health Professions) for reviewing the statistical analyses used in this study.
Author information
Authors and Affiliations
Contributions
M.E. had a major role in study conceptualization and design, data analyses, interpretation of the findings, and writing the manuscript. Y.Y. contributed to study conceptualization, data collection, interpretation of findings, and reviewed the manuscript and provided feedback. K.P.C. reviewed the manuscript and provided feedback. B.J.P. reviewed the manuscript and provided feedback. M.F.M. reviewed the manuscript and provided feedback. J.D.B. reviewed the manuscript and provided feedback. L.Z. contributed to data collection, reviewed the manuscript and provided feedback. S.K. contributed to data collection. L.K. contributed to data collection. A.G. contributed to data collection. A.D. contributed to data collection. J.R.G. had a major role in the study conceptualization and design, interpretation of the findings, and manuscript preparation. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eshghi, M., Yunusova, Y., Connaghan, K.P. et al. Rate of speech decline in individuals with amyotrophic lateral sclerosis. Sci Rep 12, 15713 (2022). https://doi.org/10.1038/s41598-022-19651-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-19651-1
- Springer Nature Limited
This article is cited by
-
Detecting bulbar amyotrophic lateral sclerosis (ALS) using automatic acoustic analysis
BioMedical Engineering OnLine (2024)
-
At-home wearables and machine learning sensitively capture disease progression in amyotrophic lateral sclerosis
Nature Communications (2023)