Abstract
Obesity and type 2 diabetes (T2D) show an increased risk for a severe COVID-19 disease. Treatment with DPP4 inhibitor (DPP4i) results in reduced mortality and better clinical outcome. Here, we aimed to identify potential mechanisms for the observed DPP4i effect in COVID-19. Comparing T2D subjects with and without DPP4i treatment, we identified a significant increase of the anti-inflammatory adipokine sFRP5 in relation to DPP4 inhibition. sFRP5 is a specific antagonist to Wnt5a, a glycopeptide secreted by adipose tissue macrophages acting pro-inflammatory in various diseases. We therefore examined sFRP5 levels in patients hospitalised for severe COVID-19 and found significant lower levels compared to healthy controls. Since sFRP5 might consequently be a molecular link for the beneficial effects of DPP4i in COVID-19, we further aimed to identify the exact source of sFRP5 in adipose tissue on cellular level. We therefore isolated pre-adipocytes, mature adipocytes and macrophages from adipose tissue biopsies and performed western-blotting. Results showed a sFRP5 expression specifically in mature adipocytes of subcutaneous and omental adipose tissue. In summary, our data suggest that DPP4i increase serum levels of anti-inflammatory sFRP5 which might be beneficial in COVID-19, reflecting a state of sFRP5 deficiency.
Similar content being viewed by others
Introduction
The corona virus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2). Subjects with type 2 diabetes (T2D) suffering from COVID-19 exhibit an increased risk for hospitalization and higher mortality in manifest COVID-19. In addition, recent studies showed that patients with T2D are at high risk for long term complications caused by SARS-Cov-2. Since 2020, various treatments have been tested to diminish symptoms and progress of this infectious disease1,2.
Dipeptidyl peptidase IV inhibitors (DPP4i), like Sitagliptin, are efficient antidiabetics revealing a two-or threefold increase of the gastro intestinal hormone glucagon-like peptide 1 (GLP-1) serum levels. GLP-1 induces 70% of the post-prandial insulin secretion and decreases glucagon serum levels under hyperglycaemic conditions. Due to the degradation of GLP-1 by DPP4, the bioavailability of GLP-1 is short-lived. DPP4 enzymes belong to the key regulators of incretin hormones3,4. Thus, DPP4i inhibit degradation of GLP-1 by DPP4 and ameliorate glucose dependent insulin response5.
Recent studies investigated beneficial effects of DPP4i in patients with T2D and concomitant severe infection with SARS-Cov-2. A multicentre, retrospective, case–control study from northern Italy, including 338 subjects with T2D and SARS-Cov-2, was performed. All subjects were hospitalized for severe COVID-19 and exhibited pneumonia. The cohort was stratified into subjects taking sitagliptin and subjects with standard of care. It was shown that patients under DPP4i treatment showed significantly enhanced clinical outcomes, reduced mortality and an increased number of clinical discharges compared to controls6.
To examine potential mechanisms mediating the anti-COVID-19 effects of DPP4i, we investigated the association between DPP4i intake and various clinical and biochemical markers as well as co-morbidities in T2D subjects in a subset of our cross-sectional Food Chain Plus (FoCus) cohort.
Results
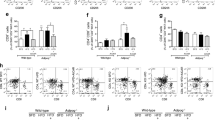
DPP4i treatment is related to increased serum concentrations of the anti-inflammatory adipokine sFRP5 in human T2D subjects
The aim of the present study was to examine, if DPP4i exert anti-inflammatory effects in treated T2D subjects which might explain the beneficial effects in Covid-19 patients. Therefore, differences in clinical and biochemical parameters between diabetic subjects with DPP4i treatment (T2D + DPP4i), without DPP4i treatment (T2D-DPP4i) and healthy controls from our FoCus cohort were investigated. While most of the tested variables were not different between the groups, a significant increase of the anti-inflammatory adipokine sFRP5 in diabetic subjects taking DPP4i compared to diabetic subjects without DPP4i treatment and healthy controls was found (p = 0.013; p = 0.0021, Fig. 1E).This is of interest, since the Wnt5a/sFRP5 system has been shown not only to be important in metabolic inflammation but also in severe bacterial sepsis7. In the present analysis, the pro-inflammatory counterpart, Wnt5a did not reveal significant differences between the groups (Fig. 1F). Besides sFRP5, parameters of glucose status: glucose and HOMA-IR (Fig. 1A,B), inflammatory markers: IL-6 and CRP (Fig. 1C,D) showed significant differences between healthy controls and diabetes subjects, but not between subjects with and without DPP4i treatment. Comorbidities, lipid profile, metabolites and anthropometry did not show significant differences between the diabetic subjects with and without DPP4i medication. Descriptive statistics and results of associations with clinical markers are shown in Tables 1 and 2.
Differences of clinical parameters between FoCus subjects with T2D taking DPP4i (T2D + DPP4i), without DPP4i treatment (T2D-DPP4i) and healthy controls were investigated: Glucose (A), HOMA-IR (B), CRP (C), IL-6 (D) were significantly higher in diabetic subjects with and without DPP4i treatment compared to healthy controls, no differences between subjects with and without DPP4i treatment. (E) Diabetic subjects with DPP4i treatment showed significant higher levels of sFRP5 compared to diabetic subjects without DPP4i treatment and healthy controls. (F) Wnt5a levels showed no differences between groups. Wilcoxon test; statistical significance p < 0.05.
Serum concentrations of sFRP5 are reduced in severe COVID-19 disease
Having found that anti-inflammatory sFRP5 is increased in DPP4i treated T2D subjects we next examined, if sFRP5 and Wnt5a are altered in COVID-19 patients. These examinations were performed in a cohort of 17 patients hospitalised on intensive care unit for severe COVID-19 and compared to healthy controls from our FoCus cohort. Table 3 displays basic characteristics of the Covid-19 subjects from the ICU-cohort and healthy controls from the FoCus cohort. Of interest, we found that subjects with COVID-19 revealed significant lower sFRP5 levels compared to healthy controls (p < 0.01, Fig. 2A). Moreover, Wnt5a levels were significantly increased in COVID-19 patients compared to healthy controls (p < 0.001, Fig. 2B).
Differences in sFRP5 and Wnt5a levels between patients from ICU cohort with severe Covid-19 infection (cases) and healthy controls from FoCus cohort. (A) Subjects with Covid-19 disease showed significant lower sFRP5 levels compared to healthy controls. (B) Levels of Wnt5a were significantly increased in cases compared to healthy controls. Wilcoxon Test; statistical significance p < 0.05.
sFRP5 is specifically expressed in mature adipocytes
So far, we found that DPP-4 inhibitor treatment is associated with increased sFRP5 serum concentrations and that COVID-19 is related to sFRP5 deficiency. sFRP5 is known to be an adipokine. In a next set of experiments, we aimed to examine, (1) which specific cell type in adipose tissue is important for sFRP5 synthesis and release in order to identify the potential DPP4i cellular target and (2) whether sFRP5 is differentially expressed in subcutaneous (SAT) versus visceral adipose tissue (VAT), since it is known that inflammatory reactions are more closely linked to visceral adipose tissue. To this end we isolated pre-adipocytes, mature adipocytes and macrophages from adipose tissue biopsies from n = 100 human subjects undergoing elective abdominal surgery (mainly bariatric surgery). The basic characteristics of the adipose tissue biopsy cohort are given in Table 4.
Protein expression of sFRP5 was investigated by western blotting. sFRP5 was not detectable in pre-adipocytes or macrophages. In contrast, mature adipocytes revealed a significant sFRP5 expression (Fig. 3) suggesting sFRP5 is an adipokine specifically secreted by fully differentiated adipocytes in humans.
Investigation of sFRP5 expression in different cell types of the adipose tissue study cohort: Western blot: sFRP5 expression was analysed in THP-1 control, visceral and subcutaneous adipocytes, pre-adipocytes and macrophages from three representative samples (patient P7, P8, P9). Only visceral and subcutaneous adipocytes showed sFRP5 expression.
Of interest, although inflammation is more linked to VAT, sFRP5 expression was found in both, mature adipocytes from subcutaneous and visceral adipose tissue biopsies with no significant difference.
Discussion
Patients with T2D and severe COVID-19 disease showed better clinical outcomes with intake of the DPP4i sitagliptin6. sDPP4 activity was described to be elevated during infections with hepatitis C virus or Epstein-Barr virus8. DPP4 cleaves the pro-inflammatory chemokine CXCL10 leading to the short NH2-terminal truncated CXCL10 form which is positively associated with hepatitis C virus infection9,10,11. Treatment with DPP4i reduced cleavage of CXCL1012 which points out that sDPP4i exhibit more therapeutic functions than increasing GLP-1 levels.
To examine further potential mechanisms of the anti-viral/anti-inflammatory DPP4i activity, we compared T2D patients treated with DPP4i (n = 23), without DPP4i (n = 46) and healthy controls (n = 46) on various clinical and biochemical levels. Significant higher levels of the Wnt5a inhibitor sFRP5 in T2D subjects with DPP4i treatment compared to T2D subjects without DPP4i treatment and healthy controls were found. Metabolic markers such as glucose and HOMA-IR and the inflammatory parameters CRP and IL-6 showed significant higher levels in diabetic subjects with and without DPP4i treatment compared to healthy controls. However, diabetic subjects with and without DPP4i medication had no differences in these clinical parameters which points out the independent elevation of SFRP5 by DPP4 inhibition. Our previous studies showed that the pro-inflammatory Wnt5a/anti-inflammatory sFRP5 system is closely related to various acute (e.g. sepsis7) as well as chronic (e.g. psoriasis13) inflammatory diseases and is also important in the context of metabolic inflammation in obesity and type 2 diabetes. Thereby, obese subjects reveal reduced concentrations of anti-inflammatory sFRP5 and increased levels of Wnt5a14,15.
In our previous clinical study in human subjects suffering from bacterial sepsis we observed stable (not reduced) sFRP5 concentrations7. This is of interest, since in the present analysis sFRP5 concentrations were reduced in severe COVID-19 disease, suggesting differences of sFRP5 in bacterial versus COVID-19 acute systemic inflammation.
Wnt5a is a glycoprotein and displays a regulator of the innate immune response by reinforcing low-grade inflammation. It is secreted by pro-inflammatory macrophages which infiltrate adipose tissue. Previous investigations of our working group showed in a FoCus subcohort of n = 896 cross-sectional subjects a positive association of Wnt5a with the clinical markers IL-6 and triglycerides. Furthermore, subjects with T2D exhibited high levels of Wnt5a which were positively correlated with fasting plasma glucose levels15. sFRP5 is a soluble Wnt5a receptor and inhibits pro-inflammatory effects of Wnt5a by sequestering it extracellularly. Accordingly, sFRP5 has been indicated as potential therapeutic target in metabolic inflammation7,15,16.
Having found DPP4i treatment to be related with increased sFRP5 serum levels, we specifically analysed differences of serum sFRP5 levels between subjects with severe COVID-19 disease and healthy Focus controls. We observed significant lower sFRP5 levels in COVID-19 patients compared to healthy controls, while Wnt5a levels were significantly increased in COVID-19 patients. Of interest, Choi et al. recently also showed significantly increased Wnt5a serum levels in severe COVID-19 infection17. Hence, results point towards the activation of wnt signalling pathway in COVID-19, that might be even more pronounced in severe inflammation resulting from SARS-CoV-2 rather than classical bacterial septic infection due to the additional reduction of anti-inflammatory sFRP5 in COVID-19 but not in bacterial sepsis. Furthermore, it might be of interest how sFRP5/wnt5a levels act in subjects with mild COVID-19 to prevent a possible more severe course of disease or post covid-19 symptoms.
To further investigate sFRP5 characteristics in metabolic inflammation, we analysed differential expression of sFRP5 on a cellular level in pre-adipocytes, adipocytes and macrophages. Results showed sFRP5 expression specifically in visceral and subcutaneous mature adipocytes while for pre-adipocytes and macrophages, no sFRP5 expression was recorded. These results confirm the findings of other working groups18,19 which indicates that compared to Wnt5a, sFRP5 reflects a specific marker of mature adipocytes. Furthermore, Wang et al. showed that sFRP5 expression showed higher levels in VAT from obese patients compared to lean controls18. These findings contradict the assumption that sFRP5 is only expressed in healthy adipocytes and reveals decreased levels in obesity20. It is also to be mentioned that sFRP5 is not only expressed in adipose tissue but also in pancreatic islets in which sFRP5 and Wnt signalling were shown to be regulators of β-cell proliferation21. Since it was observed that a SARS-Cov-2 infection can induce β-cell damage and therefore reinforce the development of T2D22, it is of importance to investigate the effects of DPP4i not only in the adipose tissue but also in pancreatic islets.
Thus, the results of our present study together with our previous results in obese subjects15 might explain obesity being a risk factor for severe disease courses of COVID-19, since sFRP5 levels are lower in both, COVID-19 and severe obesity. However, a limitation of this analysis must be mentioned that the BMI of Covid-19 subjects could not be determined because they were in intensive care. Only two subjects were diagnosed as obese.
Besides Wnt5a scavenging, the specific mechanisms of sFRP5 are still barely investigated. According to its anti-inflammatory properties, Carstensen-Kirberg et al. observed that sFRP5 decreased levels of Il-6 and NF-κB phosphorylation in TNFα treated human adipocytes23, further suggesting anti-inflammatory activities.
In summary, our data suggest that the release of the anti-inflammatory adipokine sFRP5 from mature adipocytes in humans is induced by DPP4i which might compensate the sFRP5 deficiency found in COVID-19, but not in bacterial sepsis. In addition, it might be speculated that elevated levels of sFRP5 might be an indication for a better clinical outcome of COVID-19 in obese as well as T2D patients, which could be interesting to examine in future biomarker studies.
Methods
Clinical cohorts used in the study
The Food Chain Plus (FoCus) is a cross-sectional, population-based cohort and included 511 subjects from the obesity outpatient clinic (Department of Internal Medicine I, University Medical Center Schleswig–Holstein, Campus Kiel, Germany) and 1326 people from the regional registration offices as cross-sectional control subjects. The intermediate care unit (ICU) cohort from the institute of clinical molecular biology (IKMB, University Medical Center Schleswig–Holstein, Campus Kiel, Germany) consisted of 25 Covid-19 patients including recall subjects. Adipose tissue biopsies were obtained from 100 subjects (Institute for experimental tumor research, University Medical Center Schleswig–Holstein, Campus Kiel, Germany) undergoing elective abdominal surgery, most of them bariatric therapy. All studies were approved by the local ethics committee (Christian-Albrechts University in Kiel) and all patients signed the informed consent.
We generally confirm that all experiments were performed in accordance with relevant guidelines and regulations.
Anthropometric characteristics and blood pressure measurements
Weight and height were measured by digital scales and a stadiometer. They were used to calculate BMI [weight (kg) divided by height (m)2]. Waist circumference was measured at the approximate midpoint between the lower margin of the last palpable rib and the top of the iliac crest, according to the World Health Organization24.
Biochemical analysis
After an overnight fast, blood samples from Focus cohort were obtained by venipuncture. The central laboratory (UKSH, Kiel) analysed the following parameters: C-reactive protein (CRP) by immunoturbidimetry (Hitachi Modular; Roche), fasting glucose by glucose-hexokinase-ultraviolet test (Hitachi Modular), IL-6 and fasting insulin by electrochemiluminescence immunoassay (Elecsys System 2010; Roche), and triglycerides by enzymatic test (Hitachi Modular; Roche)25. Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as fasting glucose (milligrams per deciliter) multiplied by fasting insulin (microunits per milliliter) and that product divided by 405 to determine insulin resistance. Protein concentrations of sFRP5 and Wnt5a were measured using the following ELISA kits: Wnt5a (MBS162566; sample dilution: 1:2 or 1:3 with PBS depending on available amount of serum; analytic sensitivity < 0.059 ng/mL; MyBioScource; San Diego, US), sFRP5 (SEC842Hu; analytic sensitivity < 0.58 ng/mL; Cloud-Clone Corp; Hölzel Diagnostika, Cologne, Germany). Investigations were performed according to the manufacturer’s instructions. Variables of comorbidities and intake of DPP4i were evaluated by a questionnaire.
Isolation of adipocytes, pre-adipocytes and macrophages from human adipose tissue biopsies
Subcutaneous and visceral adipose tissue (SAT, VAT) biopsies from bariatric surgeries or other surgeries (lean controls) were mechanically cut to a homogeneous mixture and digested with collagenase type II (Sigma Aldrich; Darmstadt, Germany) for 1 h at 37 °C in a shaking incubator. After digestion the samples were filtered with a sieve (355 µm; 280 µm) to separate cells from undigested tissue. Afterwards, the samples were centrifuged (1000 rpm; 5 min). Adipocytes from the upper phase were pipetted in a reaction vessel and frozen at − 80 °C. Remaining supernatants were removed and the cell pellets were resuspended in 5 mL red cell lysis buffer and incubated for 5 min at room temperature. After centrifugation, cell pellets were resuspended in 10 mL isotonic NaCl solution (Berlin Chemie AG, Germany). Up to 1 × 107 cells were resuspended in 80 µl MACS-buffer and 20 µl CD14 magnetic micro-beads (Miltenyi Biotec; Bergisch-Gladbach, Germany) and incubated for 15 min at 4 °C. Afterwards, cells were washed with 1 mL MACS-buffer and centrifuged (300xg for 10 min). Cells were resuspended in 500 µL MACS-buffer. For magnetic separation of macrophages and pre-adipocytes, MACS MS columns with pre-separation filters and a magnetic MiniMACS Separator (Miltenyi Biotec; Bergisch-Gladbach, Germany) was used. Macrophages and pre-adipocytes samples were centrifuged (200xg; 12 min), resuspended in 200 µl APL lysis buffer and frozen at − 80 °C.
Preparation of total protein from mature adipocytes, pre-adipocytes and macrophages
Protein was isolated from primary human pre-adipocytes and macrophages using the AllPrep Protein Kit (Qiagen; Hilden, Germany) following the manufacturer’s instructions. Total protein of human adipocytes was isolated using the Minute Total Protein Extraction Kit for Adipose Tissue/Cultured Adipocytes (Invent Biotechnologies, MN, USA). Protein concentrations were measured with the spectrophotometer Nanodrop one (Thermo Fisher Scientific, Inc., MA, USA). Samples, containing low protein were desalted with Zeba Spin Columns 0.5 mL 7K MWCO (Thermo Fisher Scientific, Inc., MA, USA) following the manufacturer`s instructions. Samples were dried overnight at room temperature using the Speed-Vac machine concentrator 5301 (Eppendorf; Hamburg, Germany) or at − 80 °C using the lyophilisation machine Alpha 2-4 LSC (Christ; Osterode am Harz, Germany). A complete set of proteins (from visceral and subcutaneous adipocytes, pre-adipocytes and macrophages) could be isolated from n = 70 patients which were used for further analysis. Since general loading control markers such as β-actin, α-tubulin and GAPDH were not suitable, the membranes were stained with Ponceau-S solution.
Western-blotting
A Bradford assay was conducted and the protein assay dye reagent (BIO-RAD; Ca, USA) was used. Protein (30 µg per sample) was added to 4 × loading buffer (bromophenol blue and Laemmli buffer), heated to 95 °C for 5 min, and separated on a 12.5% SDS-PAGE gel. Afterwards, proteins were transferred to polyvinylidene fluoride membranes (Carl Roth, Karlsruhe, Germany). The following antibodies were used according to the instructions of the manufacturer: sFRP5 (Santa Cruz Biotechnology; Dallas, Texas; USA) and secondary antibody anti-rabbit (Cell signalling; Danvers, Massachusetts, USA). Membranes were incubated with SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific; Waltham, Massachusetts, USA) for 5 min at room temperature and the signals were detected with the Molecular Imager ChemiDoc XRS+ (Bio Rad; Hercules, California, USA).
Statistical analysis
Statistical analysis was performed using open source R software and SPSS Statistics Software Version 24. Statistical significance was set at p < 0.05.
From FoCus cohort, a subcohort of patients was divided into subjects with type 2 diabetes taking DPP4i (n = 23; “T2D + DPP4i”), without DPP4i treatment (n = 46; “T2D-DPP4i”) and healthy subjects (n = 46; healthy controls). Groups were matched by age and gender. Continuous variables were tested for normal distribution using the Shapiro–Wilk test. To determine between-group differences, Wilcoxon rank sum test was performed. Categorical variables are shown as absolute numbers and percentages. x2 test and Fisher test were used for between-group analyses.
The ICU cohort contained 25 subjects. Since recalls were excluded, 17 Covid-19 subjects of the ICU cohort were selected and matched by gender and age with healthy controls of FoCus cohort (n = 34). Since Covid-19 subjects were in intensive care, the BMI could not be determined. However, 2 ICU subjects were diagnosed as obese. Healthy controls had a BMI lower than 25. sFRP5 and Wnt5a differences between groups were determined using Wilcoxon rank sum test.
For descriptive statistics, variables of adipose tissue cohort were tested for normal distribution using the Shapiro–Wilk test and are expressed as median (IQR) according to non-normal distribution. Mann–Whitney U test were used to determine group-differences.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Pujari, R., Thommana, M. V., Ruiz Mercedes, B. & Serwat, A. Therapeutic options for COVID-19: A review. Cureus 12(9), e10480 (2020).
Corrao, S., Pinelli, K., Vacca, M., Raspanti, M. & Argano, C. Type 2 diabetes mellitus and COVID-19: A narrative review. Front. Endocrinol. (Lausanne) 12, 609470 (2021).
Gallwitz, B. Clinical use of DPP-4 inhibitors. Front. Endocrinol. (Lausanne) 10, 389 (2019).
Schlicht, K. et al. Circulating levels of soluble dipeptidylpeptidase-4 are reduced in human subjects hospitalized for severe COVID-19 infections. Int. J. Obes. (Lond) 44(11), 2335–2338 (2020).
Scott, L. J. Sitagliptin: A review in type 2 diabetes. Drugs 77(2), 209–224 (2017).
Solerte, S. B. et al. Sitagliptin treatment at the time of hospitalization was associated with reduced mortality in patients with type 2 diabetes and COVID-19: A multicenter, case-control, retrospective observational study. Diabetes Care 43(12), 2999–3006 (2020).
Schulte, D. M. et al. The wingless-related integration site-5a/secreted frizzled-related protein-5 system is dysregulated in human sepsis. Clin. Exp. Immunol. 180(1), 90–97 (2015).
Shao, S., Xu, Q., Yu, X., Pan, R. & Chen, Y. Dipeptidyl peptidase 4 inhibitors and their potential immune modulatory functions. Pharmacol. Ther. 209, 107503 (2020).
Barreira da Silva, R. et al. Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat. Immunol. 16(8), 850–858 (2015).
Meissner, E. G. et al. Dynamic changes of post-translationally modified forms of cxcl10 and soluble dpp4 in hcv subjects receiving interferon-free therapy. PLoS ONE 10(7), e0133236 (2015).
Riva, A. et al. Truncated CXCL10 is associated with failure to achieve spontaneous clearance of acute hepatitis C infection. Hepatology 60(2), 487–496 (2014).
Decalf, J. et al. Inhibition of DPP4 activity in humans establishes its in vivo role in CXCL10 post-translational modification: prospective placebo-controlled clinical studies. EMBO Mol. Med. 8(6), 679–683 (2016).
Gerdes, S., Laudes, M., Neumann, K., Baurecht, H. & Mrowietz, U. Wnt5a - a potential factor linking psoriasis to metabolic complications. Exp. Dermatol. 23(6), 439–440 (2014).
Tong, S. et al. Sfrp5/Wnt pathway: A Protective regulatory system in atherosclerotic cardiovascular disease. J. Interferon Cytokine Res. 39(8), 472–482 (2019).
Relling, I. et al. Role of wnt5a in Metabolic Inflammation in Humans. J. Clin. Endocrinol. Metab. 103(11), 4253–4264 (2018).
Ouchi, N. et al. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science 329(5990), 454–457 (2010).
Choi, E. Y. et al. Wnt5a and Wnt11 as acute respiratory distress syndrome biomarkers for severe acute respiratory syndrome coronavirus 2 patients. Eur. Respir. J 56(5), 2001531 (2020).
Wang, R. et al. SFRP5 acts as a mature adipocyte marker but not as a regulator in adipogenesis. J. Mol. Endocrinol. 53(3), 405–415 (2014).
Lv, C., Jiang, Y., Wang, H. & Chen, B. Sfrp5 expression and secretion in adipocytes are up-regulated during differentiation and are negatively correlated with insulin resistance. Cell Biol. Int. 36(9), 851–855 (2012).
Schulte, D. M. et al. Pro-inflammatory wnt5a and anti-inflammatory sFRP5 are differentially regulated by nutritional factors in obese human subjects. PLoS ONE 7(2), e32437 (2012).
Rebuffat, S. A. et al. Downregulation of Sfrp5 promotes beta cell proliferation during obesity in the rat. Diabetologia 56(11), 2446–2455 (2013).
Keiichiro, M., Seiho, N., Hitoe, M., Hirokazu, T. & Keizo, A. SARS-CoV-2 Infection and Pancreatic β Cell Failure. Biology 11(1), 22 (2022).
Carstensen, M. et al. Effect of Sfrp5 on cytokine release and insulin action in primary human adipocytes and skeletal muscle cells. PLoS ONE 9(1), e85906 (2014).
Nishida, C., Ko, G. T. & Kumanyika, S. Body fat distribution and noncommunicable diseases in populations: Overview of the 2008 WHO expert consultation on waist circumference and waist-hip ratio. Eur. J. Clin. Nutr. 64(1), 2–5 (2010).
Heinsen, F.-A. et al. Beneficial effects of a dietary weight loss intervention on human gut microbiome diversity and metabolism are not sustained during weight maintenance. Obes Facts 9(6), 379–391 (2016).
Acknowledgements
We especially want to thank all participants of all study cohorts as well as all who contributed to the studies.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
M.L. designed the study. J.Brandes, I.Z., N.R., K.H., K.T., W.S., J.Beckmann, F.T. conducted the research. J.Brandes, I.Z., N.R., K.S. analysed the data. J.Brandes and M.L. wrote the paper. M.L. had primary responsibility for the final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brandes, J., Zobel, I., Rohmann, N. et al. Dipeptidylpeptidase (DPP)-4 inhibitor therapy increases circulating levels of anti-inflammatory soluble frizzle receptor protein (sFRP)-5 which is decreased in severe COVID-19 disease. Sci Rep 12, 14935 (2022). https://doi.org/10.1038/s41598-022-18354-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18354-x
- Springer Nature Limited
This article is cited by
-
Insulin may increase disease severity and mortality of COVID-19 through Na+/H+ exchanger in patients with type 1 and type 2 diabetes mellitus
Journal of Endocrinological Investigation (2022)