Abstract
In this study, we examined the abiotic and biotic factors controlling the dynamics of soil respiration (Rs) while considering the zonal distribution of plant species in a coastal dune ecosystem in western Japan, based on periodic Rs data and continuous environmental data. We set four measurement plots with different vegetation compositions: plot 1 on bare sand; plot 2 on a cluster of young Vitex rotundifolia seedlings; plot 3 on a mixture of Artemisia capillaris and V. rotundifolia; and plot 4 on the inland boundary between the coastal vegetation zone and a Pinus thunbergii forest. Rs increased exponentially along with the seasonal rise in soil temperature, but summer drought stress markedly decreased Rs in plots 3 and 4. There was a significant positive correlation between the natural logarithm of belowground plant biomass and Rs in autumn. Our findings indicate that the seasonal dynamics of Rs in this coastal dune ecosystem are controlled by abiotic factors (soil temperature and soil moisture), but the response of Rs to drought stress in summer varied among plots that differed in dominant vegetation species. Our findings also indicated that the spatial dynamics of Rs are mainly controlled by the distribution of belowground plant biomass and autotrophic respiration.
Similar content being viewed by others
Introduction
Coastal dunes are important ecosystems that are inhabited by unique vegetation communities, but they are being threatened by the recent climate change and developmental pressures1. On the other hand, coastal dune ecosystems are gaining attention as green infrastructure that can provide many kinds of ecosystem services2, including carbon sequestration and storage3. A soil carbon analysis by Drius et al.4 suggested that Italian coastal dunes in the Natura 2000 network are carbon sinks. We have limited information, however, on the carbon cycle in coastal dune ecosystems based on CO2 flux observation data. Therefore, more CO2 flux research in coastal dunes is needed to clarify the value of coastal dune ecosystems from the viewpoint of climate change mitigation. This new information may also contribute to protecting dune ecosystems and establishing sustainable management strategies for these vulnerable ecosystems.
Soil respiration (Rs) is the second-largest carbon flux in terrestrial ecosystems and a major component of the global carbon cycle5. Rs consists of heterotrophic respiration (Rh, decomposition of plant litter and soil organic carbon (SOC) by microbiota) and autotrophic respiration (Ra) that comes from plant roots, mycorrhiza, and other rhizosphere microorganisms6. Although several studies have estimated global Rs5,7, large uncertainty in the estimation still remains8 and may be partly explained by spatial bias for Rs9. Not only spatial bias but also bias associated with ecosystem types might contribute to the uncertainty. For example, according to the recent database of Rs by Jian et al.10, Rs data in forest ecosystems (especially in temperate regions) is the most frequently recorded category from the viewpoint of the number of data in the database. More research in a wider array of ecosystems, however, would help improve Rs estimation as well as our understanding of the mechanism underlying the Rs response to environmental factors.
In most ecosystems, abiotic factors such as soil temperature and soil moisture are the major environmental factors controlling the dynamics of Rs, but the magnitude and the direction (increase or decrease) of the Rs response to those factors vary among ecosystems. Generally, Rs increases exponentially along with a rise in soil temperature11,12,13, whereas Rs has a “mountain-shaped” relationship with soil moisture, because conditions that are too dry or too wet suppress Rs14. Gao et al.15 observed an exponential relationship between soil temperature and Rs in plantation forests and a secondary forest in coastal dunes, as typically observed in other terrestrial ecosystems; they also reported that soil moisture was positively related to Rs in several stands among the study sites. Their findings suggest that soil temperature and soil moisture also exert strong control over the seasonal dynamics of Rs in coastal dunes.
In addition to abiotic factors, biotic factors are also reported to regulate the dynamics of Rs in coastal dune ecosystems. Previous studies suggested that biotic factors like root biomass and microbial population were also important indicators for the dynamics of Rs in coastal dune ecosystems15,16, however, it needs more discussion to get a consensus about which biotic factor is the most dominant one. Chapman17 estimated that 70% of Rs is caused by root respiration, based on surveys in several heathland ecosystems that included dune-heath ecosystems, suggesting a strong influence of vegetation on Rs in those ecosystems. Because coastal dune ecosystems are generally carbon-limited, interactions between vegetation and Rs might be more clearly observed than in other ecosystems.
In coastal dune ecosystems, a zonal distribution (zonation) of coastal plant species with distance from the shoreline is typically observed18. As the distance from the shoreline increases, the vegetation gradually changes from herbs to shrubs and tree species4,19, and the amount of soil organic matter is also positively correlated with the distance20. Because plant communities can be a strong driver of Rs21, the dynamics of Rs and the response to environmental factors may differ between vegetation zones. Thus, for a better understanding of Rs dynamics and the mechanism underlying the Rs response to abiotic and biotic factors in coastal dune ecosystems, it is necessary to account for the zonal distribution of plant communities. No study has yet examined, however, if the Rs dynamics and responses to abiotic and biotic factors differ among plots dominated by different coastal plant species.
In this study, we investigated the dynamics of Rs in a Japanese coastal dune ecosystem focusing on abiotic factors (soil temperature and soil moisture) and biotic factors (belowground plant biomass and microbial abundance), while considering the difference in dominant coastal vegetation. We hypothesized that the relationships between the controlling factors (abiotic and biotic) and Rs vary among plots dominated by different vegetation species. We also aimed to identify the mechanism(s) that caused the difference in the response of Rs using ecological and microbial analysis.
Results
Environmental data
The time series of the soil moisture at a depth of 30 cm and soil temperature at depths of 0–5, 5, 10, 30, and 50 cm are shown in Fig. 1a. The maximum soil temperature from June to December 2020 at 0–5 cm, based on data collected at each measurement point during each Rs measurement, was 65.0 °C measured during the daytime in late August. The maximum soil temperatures based on the average of 30-min continuous measurements at depths of 5, 10, 30, and 50 cm were 49.7, 40.1, 33.2, and 30.4 °C, respectively, in late August. The minimum soil temperature from June to December 2020 at 0–5 cm during Rs measurement was 9.3 °C during the daytime in mid-November. The minimum soil temperature at depths of 5, 10, 30, and 50 cm was 0.6, 0.9, 2.2, and 3.7 °C, respectively, in late December.
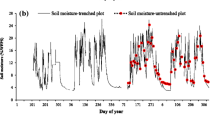
(a) Seasonal dynamics of soil moisture at a depth of 30 cm (averaged value of CS616 sensors in plots 1, 3, and 4) and soil temperature at depths of 0–5, 5, 10, 30, and 50 cm; (b) seasonal dynamics of Rs in each measurement plot. The soil temperature at the depth of 0–5 cm for all 40 measurement points in plots 1–4 is shown by pink crosses, and the average value (mean ± SD) during the daytime is shown by pink circles. Soil temperature at depths of 5, 10, and 30 cm is the average value of reference soil temperature (thermocouple) at the center of plot 3 and stand-alone soil temperature sensors in plots 1 and 4. Soil temperature at a depth of 50 cm is the reference soil temperature at the center of plot 3. Bars in (b) show the standard error of the mean (n = 10). The area of light orange represents the drought period (10 August to 4 September) when the daily averaged soil moisture was less than 3.9%, the threshold soil moisture value. This figure was created using Sigmaplot 14.5 software (Systat Software, San Jose, CA, USA, https://systatsoftware.com/sigmaplot/).
The maximum average soil moisture at the depth of 30 cm was 19.0% in the middle of June, the early summer rainy season, and the minimum value was 1.6% in early September (Fig. 1a). The remarkable decrease in soil moisture was due to limited precipitation in August. The monthly precipitation recorded at the meteorological observatory in Tottori (Japan Meteorological Agency) in August was 8.5 mm, the lowest monthly precipitation in August in the last 50 years.
Dynamics of R s in each plot
Rs values in plots 1–3 peaked at the end of July to early August, whereas Rs in plot 4 peaked in the middle of June (Fig. 1b). The maximum Rs values were 1.82 ± 0.05, 2.95 ± 0.10, 1.63 ± 0.14, and 1.99 ± 0.18 μmol CO2 m−2 s−1 in plots 1 to 4, respectively (mean ± SE, n = 10). Minimum Rs values in each plot were observed from mid-November to early December and were 0.11 ± 0.004, 0.61 ± 0.08, 0.23 ± 0.05, and 0.58 ± 0.08 μmol CO2 m−2 s−1 in plots 1–4, respectively (mean ± SE, n = 10). In plots 3 and 4, Rs was also markedly decreased at the end of August. During the measurement period from 15 June to 2 December (13 Rs measurements), the average Rs value in each plot was 1.35 ± 0.53, 2.03 ± 0.67, 1.07 ± 0.50, and 1.27 ± 0.45 μmol CO2 m−2 s−1, respectively (mean ± SD).
Soil temperature and soil moisture response of R s
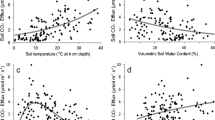
Rs increased exponentially along with the seasonal rise in soil temperature, except for late August, and the goodness of fit (R2) was best at a depth of 50, 50, 10, and 0–5 cm in plots 1–4, respectively (Fig. 2). In this study, we selected the soil temperature at the depth of 30 cm as the standard for analysis of Rs. Significant exponential relationships between 30-cm soil temperature and Rs were observed in plots 1, 2, and 3 when we used all data (Fig. 3, p < 0.001 in plots 1 and 2, p = 0.022 in plot 3), whereas there was no significant exponential relationship between soil temperature and Rs in plot 4 (p = 0.344). However, if we excluded data collected in late August (21 and 28 August), the drought period, a significant exponential relationship was also confirmed in plot 4 (Fig. 3, p = 0.006).
Change of R2 values based on the relationships between soil temperature at each depth and average Rs in each plot. For this analysis, data for late August were removed to avoid the period of drought stress. Data were measured continuously using an environmental measurement system and standalone soil temperature sensors near each plot at depths of 5, 10, and 30 cm. Soil temperature at the depth of 0–5 cm was measured simultaneously with Rs at each measurement point. Soil temperature at a depth of 50 cm was the reference soil temperature at the center of plot 3. This figure was created using Sigmaplot 14.5 software (Systat Software, San Jose, CA, USA, https://systatsoftware.com/sigmaplot/).
Relationships between soil temperature at 30 cm depth and averaged Rs in each plot. Pink squares represent data in late August 2020. Gray lines with black R2 and p values, show regression lines obtained by fitting Eq. (3) to all data (\(R_{{\text{s}}} = R_{{{\text{ref}}}} {\text{e}}^{{E_{0} \times \left( {\frac{1}{{T_{{{\text{ref}}}} - T_{0} }} - \frac{1}{{T_{{\text{s}}} - T_{0} }}} \right)}}\)). Red lines with red R2 and p values, are those obtained by excluding data from late August during the drought period. Bars show the standard error of the mean (n = 10). This figure was created using Sigmaplot 14.5 software (Systat Software, San Jose, CA, USA, https://systatsoftware.com/sigmaplot/).
Using the soil temperature response curve of Rs for which data in late August (drought period) were excluded, we analyzed the relationships between soil moisture at a depth of 30 cm and temperature-normalized Rs (RsN) in each plot. These relationships were significant in plot 3 (p = 0.025) and plot 4 (p = 0.002, Fig. 4), but they were not significant in plot 1 (p = 0.232) or plot 2 (p = 0.055). Ranges of RsN during the drought period from plots 1 to 4 were 0.76–0.83, 0.75–0.82, 0.35–0.45, and 0.33–0.44, respectively (Fig. 4).
Relationships between soil moisture at a depth of 30 cm and temperature-normalized soil respiration (RsN, observed Rs/modeled Rs) in each measurement plot. Gray lines show significant (p < 0.05) relationships between soil moisture and RsN fitted with Eq. (4) (\(R_{{{\text{sN}}}} = c_{1} {\uptheta }^{2} + c_{2} {{\uptheta + }}c_{3} (c_{1} < 0)\)). Pink squares represent RsN during the drought period. This figure was created using Sigmaplot 14.5 software (Systat Software, San Jose, CA, USA, https://systatsoftware.com/sigmaplot/).
Belowground plant biomass
We observed a large variation in the distribution of total belowground plant biomass (BPB) and rooting depth in each plot (Fig. 5). Total BPB to a depth of 220 cm was 483.8, 1604.6, 751.2, and 552.3 g m−2 for plots 1–4, respectively (n = 1). The majority of BPB was concentrated in the upper 30 cm in plot 3 (82%) and plot 4 (95%), and the BPB ratios to a depth of 50 cm were 93% (plot 3) and 98% (plot 4), respectively. In contrast, the BPB ratios in the upper 30 cm in plots 1 and 2 were both 14%, and the BPB ratios to a depth of 50 cm were both 26%.
Profile of belowground plant biomass in each measurement plot to depths of 100–220 cm from 18 May to 8 June 2021 (n = 1). The red arrow indicates that roots were distributed below 220-cm depth. This figure was created using Sigmaplot 14.5 software (Systat Software, San Jose, CA, USA, https://systatsoftware.com/sigmaplot/).
Influence of BPB on R s
There was no significant correlation between the natural logarithm of BPB in subplots and Rs on 3 November (1 day before trench treatment) when we analyzed separately in plot 1 (p = 0.327), plot 2 (p = 0.365), and plot 4 (p = 0.199), but the relationship was significant in plot 3 (Spearman’s rank correlation coefficient = 0.75, p = 0.038). However, there was a significant positive correlation (p = 0.002–0.003) when data in all subplots were analyzed together (Fig. 6).
Relationship between the natural logarithm of belowground plant biomass (BPB, g) to a depth of 50 cm in each subplot and Rs on 3 November before trench treatment. Values in the figure are Spearman’s rank correlation coefficient (rs) and p value; values in parentheses are those when we included invaded plots (gray circles) in the analysis. This figure was created using Sigmaplot 14.5 software (Systat Software, San Jose, CA, USA, https://systatsoftware.com/sigmaplot/).
Average Rs values in control and pre-trenched plots on 3 November 2020 were 0.58 ± 0.06 and 0.57 ± 0.15 μmol CO2 m−2 s−1 in plot 1, 1.69 ± 0.21 and 1.66 ± 0.30 μmol CO2 m−2 s−1 in plot 2, 1.04 ± 0.09 and 1.11 ± 0.24 μmol CO2 m−2 s−1 in plot 3, and 0.64 ± 0.23 and 0.62 ± 0.16 μmol CO2 m−2 s−1 in plot 4, respectively (mean ± SE, n = 4, 5, 3, and 4 for plots 1–4, Fig. 7a). Average soil CO2 efflux (Fc) values measured in control and trenched plots 15 days after trench treatment on 19 November were 1.23 ± 0.40 and 0.58 ± 0.03 μmol CO2 m−2 s−1 in plot 1, 1.69 ± 0.24 and 0.98 ± 0.07 μmol CO2 m−2 s−1 in plot 2, 0.90 ± 0.19 and 0.31 ± 0.03 μmol CO2 m−2 s−1 in plot 3, and 1.29 ± 0.35 and 0.49 ± 0.13 μmol CO2 m−2 s−1 in plot 4, respectively (mean ± SE, n = 4, 5, 3, and 4 for plots 1–4, Fig. 7b). Based on the Fc data collected on 19 November, Ra_50/Rs was calculated as 0.53, 0.42, 0.65, and 0.62 in plots 1–4, respectively.
Comparison of Rs values between control and trenched plots (a) on 3 November before trench treatment and (b) on 19 November, 15 days after trench treatment (n = 4, 5, 3, and 4 for plots 1–4, respectively). Rs data in pre-trenched and trenched plots are shown as squares in all plots. Circles and triangles are Rs data in control plots. This figure was created using Sigmaplot 14.5 software (Systat Software, San Jose, CA, USA, https://systatsoftware.com/sigmaplot/).
Soil organic carbon and microbial abundance
Total SOC stocks from 0 to 30 cm depth in plots 1–4 were 44.5 ± 1.5, 57.9 ± 3.6, 248.5 ± 30.5, and 328.2 ± 73.2 g C m−2 (mean ± SE, n = 3), respectively. The ratio of SOC stock in the upper 10 cm against SOC stock to a depth of 30 cm in plots 1 to 4 were 31%, 26%, 48%, and 61%, respectively. Total nitrogen stocks at 0 to 30 cm depth in plots 1–4 were 7.8 ± 0.7, 7.1 ± 0.3, 14.3 ± 1.1, and 13.5 ± 1.6 g N m−2 (mean ± SE, n = 3), respectively.
When averaged across soil depths, plots 3 and 4 had higher bacterial and fungal abundance than the other plots, whereas the bare plot (plot 1) showed the lowest bacterial and fungal abundance (Fig. 8a,b). Soil depth did not have significant effects on either bacterial or fungal abundance, but there were significant interactions between location and soil depth. Deeper soils (20–30 cm) only in plot 4 showed significantly lower microbial abundance when compared to the surface layer. Fungal abundances were from 5 to 125 times lower than bacterial abundances (Fig. 8c). There were significantly positive relationships between SOC stock (g C m−2) and log copies of genes for both bacteria (R2 = 0.39, p < 0.001) and fungi (R2 = 0.55, p < 0.001).
Gene abundances of (a) bacteria and archaea, (b) fungi, and (c) the ratio of fungi and bacteria. The level of significance was determined by two-way ANOVA. Uppercase letters indicate a statistically significant difference between vegetation plots when we averaged the values across soil depth (0–30 cm) and used the Tukey–Kramer test among vegetation plots. Lowercase letters indicate a significant difference between soil depths in each plot.
Discussion
Our study showed that seasonal dynamics of Rs in a coastal dune ecosystem were controlled mainly by soil temperature, except for the dry summer period in August 2020. Generally, the soil temperature is the primary factor controlling Rs in many natural ecosystems12,22,23. Gao et al.15 also reported a strong relationship between soil temperature and Rs in subtropical coastal dunes in China. In most cases, soil temperature at shallower layers (e.g., 5 cm) is the appropriate parameter to explain the dynamics of Rs using exponential regressions24,25,26, because the litter layer and shallow layers of soil (e.g., A horizon), which contain more SOC than the deeper layer, contributes a large portion of the total Rs27,28,29. Pavelka et al.30 showed that R2 values based on an exponential relationship between soil temperature and Rs decreased significantly at greater soil temperature depths in a Norway spruce forest and a mountain grassland in the Czech Republic. However, in plots 1 and 2 in our study, soil temperature at depths of 30 and 50 cm was a better indicator (the best fit soil temperature-depth) than that at 0–5 and 5 cm for seasonal dynamics of Rs (Fig. 2). Our result implies that the difference in best-fit soil temperature depth reflects the vertical distribution of the source of Rs along across soil depth. At least in plots 1 and 2, both the upper layer (above 30 cm) as well as the deeper layer (below 30 cm) appeared to contribute to Rs because BPB was distributed at depths of 200 cm and more (Fig. 5). In plots 3 and 4, on the other hand, 82–95% of BPB was concentrated within the top 30 cm. In addition, 48–61% of SOC was concentrated in the upper 10 cm in plots 3 and 4. Furthermore, the abundances of bacteria and fungi were both significantly higher in the upper 20 cm than at 30-cm depth in plot 4 (Fig. 8). These findings indicate that the shallower soil layer (above 30-cm depth) made a greater contribution to Rs in plots 3 and 4 as compared to that in the other plots, which may explain why soil temperature in the shallower layers (0–5 and 10 cm) fit the soil temperature response model of Rs well in plots 3 and 4. Most previous studies used surface soil temperature (0–10 cm) to examine the soil temperature response of Rs—even in desert ecosystems where extreme surface soil temperatures of > 50 °C were observed31. Therefore, when performing temperature response analysis of Rs, we recommend measuring soil temperature at multiple depths, including those at 30 cm and deeper in places where extreme soil temperature variation is expected, such as coastal dunes and deserts.
Soil moisture also had a strong impact on Rs especially in the drought period in August 2020, when a lack of precipitation contributed to drought stress and decreased soil moisture. Coastal dune ecosystems are easily influenced by drought stress32,33 because of the low water-holding capacity of sand34. Under drought stress, Rs is suppressed because of the limited microbial and plant activity, and Rh is expected to be more sensitive to drought stress than Ra35. In our study, Rs markedly decreased in plots 3 and 4 under drought stress. There are few reports regarding the effect of drought stress on Rs in the coastal dune ecosystem, but our result for the soil moisture response of Rs (a mountain-shaped relationship) is consistent with previous studies in several other types of ecosystems13,28. Compared with plots 3 and 4, however, Rs was not remarkably decreased in plots 1 and 2 (Fig. 1b). The variety of rooting depths in each plot provides a clue about the difference. BPB in plots 1 (200-cm depth) and plot 2 (> 220 cm) was distributed more deeply than in plots 3 and 4, where more than 90% of BPB was in the upper 50 cm (Fig. 5). Therefore, the deeper rooting depth may have contributed to continued plant activity despite the drought stress at the surface and prevented a decrease of Ra in plots 1 and 2. Our findings indicate that the soil moisture response of Rs differed among vegetation zones of the coastal dune ecosystem, and drought stress markedly decreased Rs in some plots. Data from multiple years will be needed to confirm whether such drought stress occurs frequently in this coastal dune ecosystem because the precipitation in August 2020 was unusually limited compared with that in other years.
The significant positive relationship between the natural logarithm of BPB to 50-cm depth and Rs in November in our study suggests that the distribution of BPB is one factor controlling the spatial dynamics of Rs in the coastal dune ecosystem (Fig. 6). Positive linear relationships between root biomass and Rs have been reported36,37,38, whereas our result showed an logarithmic relationship between BPB and Rs. This logarithmic relationship is reasonable when we consider that root respiration exponentially decreases with the increase of root diameter17,39. Lee40 reported an logarithmic relationship between root biomass and Rs using field-grown maple seedlings in central Korea. Our findings suggest that such an logarithmic relationship between root biomass and Rs is also applicable to coastal dune ecosystems.
The Ra/Rs to a depth of 50 cm was estimated as 0.42–0.65 in November (Fig. 7). Although information about the Ra/Rs is limited in coastal dune ecosystems, a study by Chapman17 in nine heathlands, including three dune-heath ecosystems, can serve as a reference. Chapman estimated that root respiration contributed up to 70% of Rs in heathland ecosystems, which is comparable to our result, suggesting that Ra is a large component of Rs in the SOC-limited dune ecosystem. Together, these results suggest that the distribution of BPB and the resulting Ra are major contributors to the spatial dynamics of Rs in coastal dune ecosystems.
In addition to plant roots, the mycorrhizal fungal network also contributes to Rs. For example, Hogberg et al.41 reported a 50% decrease of Rs as a result of large-scale forest girdling, and they suggested the decrease was due to the inhibition of carbon translocation from host trees to the ectomycorrhizal root tips and mycelia. Ashkannejhad and Horton42 showed that ectomycorrhizal symbiosis functions as a critical factor for pine establishment in the coastal dune ecosystem in Oregon. In our study, fungal abundance and the fungal/bacterial ratio were highest in the 0–10 cm layer in plot 4 (Fig. 8b,c), near a pine forest. Although it is not possible to separately evaluate the contributions of root respiration and mycorrhizal CO2 efflux to Rs in our study, this finding implies a possible contribution of ectomycorrhizal root tips and mycelial networks to Rs. The Ra/Rs to a depth of 50 cm was 0.62 in plot 4, and some fraction of the contribution would be caused by ectomycorrhizal CO2 efflux.
SOC is the source of Rh and also strongly influences Rs. For example, a study by Li et al.43 in an alpine meadow ecosystem on the Qinghai-Tibetan Plateau indicated positive relationships between SOC and Rs and their components (Rh and Ra). Morisada et al.44 estimated the average SOC stock in Japanese forests to a depth of 30 cm as 9.0 kg C m−2. Compared with that amount, the SOC stock at our study site (maximum 0.3 kg C m−2 in plot 4) was remarkably limited. Therefore, the relatively small contribution of Rh to Rs is theoretically possible under the SOC-limited condition of our study site. Our microbial analyses revealed a variety of bacterial and fungal abundance in each plot (Fig. 8), suggesting that the contribution of Rh to Rs varies among plots. Our result indicates that the zonal distribution of plant species in a coastal dune ecosystem significantly influenced the abundance of the soil microbiota. In addition, the significantly greater abundance of microbiota and SOC in the shallower layers (0–10 and 10–20 cm) compared with the deeper layer (20–30 cm) in plot 4 suggest that the shallower layer is a larger source of Rh compared with the deeper layer.
There are uncertainties regarding our findings on the influence of BPB on Rs. First, trench treatment in our study was limited to the depth of 50 cm, and we could not exclude the influence of roots in the deeper layer. The influence was likely relatively minor in plots 3 and 4 because more than 90% of BPB was concentrated in the upper 50 cm in those plots (Fig. 5). In plots 1 and 2, however, BPB was distributed at depths greater than 50 cm (Fig. 5), which certainly caused underestimation of the contribution of Ra to Rs in plots 1 and 2. We have limited information regarding the magnitude of the Ra/Rs in deeper layers as compared with that in shallower layers. Pregitzer et al.45 reported that root respiration in the surface layer (0–10 cm) was up to 40% higher than that at 20- to 30-cm and 40- to 50-cm depth in sugar maple forests in Michigan. According to the report, it is possible that Ra in the deeper layer below 50 cm less contributed to Rs compared with the Ra in the shallower layer (0–50 cm) in our study. Even though, there is no report showing the influence of deeper roots below 50 cm on Rs compared with the roots in the shallower layer. Therefore, the uncertainty of the Ra/Rs in plots 1 and 2 is larger than that in plots 3 and 4.
Second, there is uncertainty about the influence of dead root decomposition and disturbance by trench treatment on Rs in trenched plots, and this may also have caused an underestimation of the Ra/Rs. Carbon input as dead roots to trenched plots is inevitable in the trench treatment6. Some studies applied a correction for Ra/Rs by conducting root bag experiments46,47, whereas others did not23,48. In our study, we did not apply any correction for the influence of BPB on Rs because of the short experimental period (we collected root samples to a depth of 50 cm in all subplots within 2 months after trench treatment). Previous studies reported that the influence of disturbance accompanied by trench treatment ceased several months after the treatment47,49. For example, Lee et al.47 reported that Fc of trenched plots was higher compared with that of control plots until 1–2 months after trench treatment, and Fc in trenched plots significantly decreased after that period. In our study, the significant decrease of Fc in trenched plots compared with control plots was confirmed 15 days after trench treatment. It appears that the early occurrence of a Fc decrease after trench treatment was due to the relatively low soil temperature in November (Fig. 1a), such that the influence of dead root decomposition on Fc might be minor.
Third, seasonal dynamics of the Ra/Rs were not considered in this study. Previous studies reported that the Ra/Rs in the growing season was larger than that in the dormant season50,51,52. However, Lee et al.47 observed an exceptionally large contribution of Ra to Rs in November (71%) in a cool-temperate deciduous forest in central Japan. Our measurement to assess the Ra/Rs was conducted in November, the transitional period from the growing season to the dormant season. Because we did not consider seasonal trends in our estimation of the Ra/Rs in a coastal dune ecosystem, this may have led to under-or overestimation.
Conclusion
The dynamics of Rs were greatly influenced by abiotic and biotic factors in a coastal dune ecosystem. Our findings demonstrated that seasonal dynamics of Rs were controlled by soil temperature, but drought stress also strongly influenced Rs in the dry summer period, and the response of Rs to drought varied among plots dominated by different vegetation species. In addition, Ra made a large contribution to Rs, and the distribution of BPB appeared to be a factor controlling the spatial dynamics of Rs. Furthermore, the microbial analysis suggested that the zonal distribution of vegetation in the dunes also strongly influenced microbial abundance, indicating that the contribution of the microbial community to Rs likely differs among measurement plots. These findings support our hypothesis that relationships between the abiotic and biotic controlling factors and Rs vary among plots dominated by different vegetation species, reflecting the zonal vegetation distribution patterns in a coastal dune ecosystem. Our study provides important data for further examination of coastal dune ecosystems from the viewpoint of carbon cycle analysis.
Materials and methods
Site description
The study site (about 1 ha) is within a coastal dune ecosystem (35° 32′ 26.0″ N, 134° 12′ 27.5″ E) located at the Arid Land Research Center of Tottori University, Tottori, Japan. The mean annual temperature is 15.2 °C, and the mean total precipitation is 1931 mm, based on records collected from 1991 to 2020 at the Tottori observation station of the Japan Meteorological Agency. Dominant plant species around the measurement plot were Vitex rotundifolia and Artemisia capillaris. Carex kobomugi and Ischaemum anthephoroides were also scattered around the coastal side of the study site, and planted Pinus thunbergii trees cover the inland side.
Experimental design
In May 2020, we established four measurement plots at the study site (Fig. 9). Plot 1 was a gap area surrounded by V. rotundifolia seedlings. Plot 2 consisted of clusters of V. rotundifolia seedlings and was adjacent to plot 1. Within plots 1 and 2, C. kobomugi and I. anthephoroides were also scattered. Plot 3 was in a mixed area of V. rotundifolia and A. capillaris; this plot was in the center of the study site. Plot 4 was located in front of P. thunbergii trees and was in the most inland area of the study site. On 10 June 2020, we set an environmental measurement system at the center of the study site adjacent to plot 3, and we then obtained continuous data for soil temperature and soil moisture. In each plot (main plot), we set 10 plastic (polypropylene) collars (n = 10) before the start of the Rs measurement. We measured Rs every 2 weeks from 15 June to 2 December 2020 in the main plots. Vitex rotundifolia and C. kobomugi invaded a part of plot 1 in late June and early July, after the first Rs measurement on 15 June. Therefore, we set new measurement points for plot 1 in early July (Fig. 9), and flux calculations for plot 1 were conducted after removing data from the invaded area measured on June 15.
Environmental measurement system
The environmental measurement system was composed of a data logger (CR1000, Campbell Scientific Inc., Logan, UT, USA), battery (SC dry battery, Kind Techno Structure Co. Ltd, Saitama, Japan), solar panel (RNG-50D-SS, RENOGY International Inc., Ontario, CA, USA), charge controller (Solar Amp mini, CSA-MN05-8, DENRYO, Tokyo, Japan), thermocouples (E type), and soil moisture sensors (CS616, Campbell Scientific Inc.). The data logger, battery, and charge controller were kept in a plastic box to avoid exposure to rainfall and sand. Each end of the thermocouple was inserted into a copper tube (4-mm inner diameter, 5-cm length) and affixed with glue. To measure the reference soil temperature at different depths, copper tubes enclosing E-type thermocouples were buried horizontally in the sand at depths of 5, 10, 30, and 50 cm (n = 1 for each depth) at the center of plot 3 as reference soil temperature (the data was recorded every 30 min). In addition, we set stand-alone soil temperature sensors (Thermochron SL type, KN Laboratories, Inc. Osaka, Japan) at the center of plots 1 and 4 at depths of 5, 10, and 30 cm (n = 1 for each plot, each depth), and they recorded soil temperature data every 30 min. Reference soil temperature at the depth of 5, 10, and 30 cm was used for gap-filling for soil temperature measured by stand-alone sensors at each depth and plot. Soil moisture sensors were buried horizontally in the sand at a depth of 30 cm in the center of plots 1, 3, and 4 (n = 1 for each plot) and recorded data every 30 min. Raw values of soil moisture sensors were converted to volumetric soil moisture (%) using a calibration line from 0 to 15% measured in the laboratory using dune sand and three sensors (CS616) referring to the procedure of Bongiovanni et al.53. Data for precipitation at the local meteorological observatory in Tottori was downloaded from the home page of the Japan Meteorological Agency (https://www.data.jma.go.jp/gmd/risk/obsdl/index.php).
R s measurement in the main plots
Polypropylene collars (30-cm inner diameter, 5-cm depth, n = 10) were set in each measurement plot in late May 2020. The first Rs measurement was conducted on 15 June 2020. However, V. rotundifolia and C. kobomugi then invaded about half of the gap area of plot 1, so on 1 July we set 5 new polypropylene collars for plot 1 to replace the 5 invaded measurement points (Fig. 9). The second Rs measurement was conducted on 2 July, and all polypropylene collars then remained in the same position until the end of the measurement period.
Rs was measured using an automated closed dynamic chamber system54 composed of two cylindrical aluminum chambers (30 cm diameter, 30 cm height) equipped with thermistor temperature sensors (44006, Omega Engineering, Stanford, CA, USA) for measuring air temperature inside the chamber during Rs measurement. Those chambers were connected to a control box equipped with a pump, data logger (CR1000, Campbell Scientific Inc.), CO2 analyzer (Gascard NG infrared gas sensor, Edinburgh Sensors, Lancashire, UK), and thermometer (MHP, Omega Engineering). The composition of the control box is basically the same as used in previous studies54,55. The measurement period for each point was 3 min, and the CO2 concentration and air temperature inside the chamber were recorded every 5 s. During the measurement, another chamber was set on the next polypropylene collar with the lid opened, and the next measurement was started at that moment of finishing the previous measurement by automatically closing the chamber lid on the next polypropylene collar in the same plot. Soil temperature at a depth of 0–5 cm was recorded simultaneously by inserting the rod of the thermometer vertically into the soil surface near the polypropylene collar (about 1–2 m from the collar).
Rs was calculated by using the following equation:
where P is the air pressure (Pa), V is the effective chamber volume (m3), R is the ideal gas constant (8.314 Pa m3 K−1 mol−1), S is the soil surface area (m2), Tair is the air temperature inside the chamber (°C). ∂C/∂t is the rate of change of the CO2 mole fraction (μmol mol−1 s−1), which was calculated using least-squares regression of the CO2 changes inside the chamber12. For the flux calculation, we removed data for the first 35 s (dead band) of each measurement as an outlier.
Trench treatment and soil CO2 efflux (F c) measurement in subplots
In November 2020, we conducted root-cut treatment (trench treatment) in subplots using polyvinyl chloride (PVC) tubes to estimate the contribution of Ra to Rs in the soil layer above 50 cm in each plot (Ra_50/Rs). Small PVC collars (10.7 cm inner diameter, 5 cm depth, n = 10 for each plot), with the upper ends about 1–2 cm above the soil surface, were set in subplots adjacent to the main plots on 23 October 2020. Rs was measured in subplots using two cylindrical mini PVC chambers (11.8 cm inner diameter at the bottom, 30 cm height, equipped with the same thermistors as cylindrical aluminum chambers for air temperature measurement) connected to the same control box as used for Rs measurement in the main plots. The measurement period was 3 min, and the measurement procedure and the flux calculation were the same as the main plot. Rs was first measured in subplots on 3 November to examine the spatial variation of Rs before trench treatment. Using the data, we selected subplots to conduct trench treatment and control plots for comparison, while aiming to achieve a minimal difference in the average Rs between control and pre-trenched plots. On 4 November, we inserted PVC tubes (10.7 cm inner diameter, 50 cm length) into about half (n = 3–5) of the subplots (the same position as PVC collars were set on 23 October) by using a hammer and aluminum lid until the upper end of each PVC tube was 1–2 cm above the soil surface to exclude roots to a depth of about 50 cm. On 19 November, after 15 days of trench treatment, respiration was measured in the same subplots.
The Ra_50/Rs was calculated as follows:
where Fc_trenched and Fc_control (= Rs) are the Fc values in trenched and control plots on 19 November, respectively.
In late December 2020, all the belowground plant biomass (BPB) in subplots (control and trenched plots) to a depth of 50 cm was collected for biomass analysis, about 2 months after trench treatment. In the laboratory, all the collected plant materials were washed and oven-dried for 72 h at 70 °C, and then the dry weight of the BPB samples was measured.
Biomass measurement
We conducted BPB analysis from 18 May to 8 June 2021 in each plot (n = 1). At that time, 100 cm × 100 cm sampling plots near the CO2 measurement plots (100 cm × 100 cm for plots 2–4 and 50 cm × 50 cm in plot 1 because of the narrow gap area) were dug to a depth of 100–220 cm, according to the root distribution in each plot, and all plant materials were collected by passing the soil through 5- to 7-mm sieves. Once we reached a depth where no roots were visible, no more digging was conducted. In plots 2 and 3, stolons of V. rotundifolia were difficult to distinguish from roots if underground. Therefore, we defined plant material as BPB if it was underground. In the laboratory, all of the collected plant materials were washed and air-dried at room temperature for 0–6 days depending on the biomass. After that, samples were oven-dried for 15–25 h at 70–80 °C, and the dry weight of those samples was then measured.
Soil organic carbon and nitrogen
On 21 October 2020, soil pits were dug to a depth of 50 cm near each plot (n = 3), and soil core samples were collected. Cylindrical stainless core samplers (5 cm diameter, 5 cm height, 100 cc) were horizontally inserted into the soil pit at depths of 0–5, 5–10, 10–20, and 20–30 cm. In the laboratory, soil core samples were weighed and oven-dried at 105 °C for 48 h, and the dry weight was measured. Oven-dried soil samples were sieved with a 2-mm-pore stainless wire mesh screen, and visible fungal mycelia in soil samples from plot 4 were removed as well as possible. Sieved samples were ground with an agate mortar. Samples (fine powder) were oven-dried for 24 h at 105 °C and weighed before SOC and nitrogen analysis. About 1.5 g of powdered samples were used for the analysis. Organic carbon content (combustion at 400 °C) and total nitrogen in samples were analyzed using a Soli TOC cube (Elementar Analysensysteme GmbH, Langenselbold, Germany) by the combustion method.
Microbial abundance
On 21 October 2020, soil samples for microbial analysis were collected at the same time as soil core sampling for SOC and nitrogen analysis. Soil samples were collected at depths of 0–10, 10–20, and 20–30 cm using a stainless spatula and placed individually in a polyethylene bag. The bags were kept in a cooler box with ice in the field and then placed in a freezer (− 30 °C) in the laboratory soon after sampling.
DNA was extracted from 0.5 g of the fresh soils using NucleoSpin Soil (Takara Bio, Inc., Shiga, Japan) according to the manufacturer’s instructions (SL1 buffer), and the extracts were stored at − 20 °C until further analysis. Bacterial and archaeal 16S rRNA and fungal internal transcribed spacer (ITS) gene were targeted to investigate the microbial abundance. Bacterial and archaeal 16S rRNA (V4 region) and fungal ITS were determined using the universal primer sets 515F/806R and ITS1F_KYO2/ITS2_KYO2, respectively56,57.
For qPCR, samples were prepared with 10 μL of the KAPA SYBR Fast qPCR kit (Kapa Biosystems, Wilmington, MA, USA), 0.8 μL of forward primer, 0.8 μL of reverse primer, and 3 μL of 1–50 × diluted soil DNA. Nuclease-free water was added to make up to a final volume of 20 μL. Cycling conditions of 16S rRNA were 95 °C for 30 s, followed by 40 cycles at 95 °C for 30 s, 58 °C for 30 s, and 72 °C for 1 min. Cycling conditions of ITS were 95 °C for 30 s, followed by 40 cycles at 95 °C for 30 s, 55 °C for 1 min, and 72 °C for 1 min. A melting curve analysis was performed in a final cycle of 95 °C for 15 s, 60 °C for 1 min, and 95 °C for 15 s. High amplification efficiencies of 99% for bacterial and archaeal 16S rRNA genes and 101% for the fungal ITS were obtained based on the standard curves.
Data analysis
To examine the environmental response (soil temperature and soil moisture) of Rs, nonlinear and quadratic regression models were applied. We conducted F-tests by comparing the regression model to a constant model whose value is the mean of the observations (significance set at p < 0.05). For the temperature response analysis of Rs, we used the following equation58:
where Rref (µmol CO2 m−2 s−1) is the CO2 efflux at a specified reference soil temperature (Tref: 283.15 K), E0 is a fitting parameter, T0 is the soil temperature when Rs is zero (227.13 K), and Ts is the observed soil temperature (K) at different depths (0–5, 5, 10, 30, 50 cm). Based on the 1-year soil moisture data between 11 June 2020 and 10 June 2021, we defined the period when soil moisture was below the annual average − 2SD (= 3.9%) as a drought period (10 August to 4 September), and we conducted nonlinear regression for the temperature response of Rs with and without Rs data during the drought period. To avoid the confounding effects of soil temperature and soil moisture, we first divided the observed value by the simulated value of Rs based on the temperature response curve (the curve was calculated without data collected in late August 2020, during a drought period). The temperature-normalized Rs, RsN, was used to analyze the relationship between soil moisture and Rs59. The relationship was fitted with the following quadratic regression:
where θ is the volumetric soil moisture (%) and c1, c2, and c3 are fitting parameters.
To examine the relationship between BPB and Rs, we referred to the logarithmic relationship between root biomass and Rs in a previous study in a forest ecosystem40, and we calculated the natural logarithm of BPB to a depth of 50 cm (ln BPB (g)). Correlation analysis (Spearman’s rank correlation, significance set at p < 0.05) between the ln BPB in each subplot and Rs was conducted.
To examine the relationship between SOC stock (g C m−2) and microbial abundance (log copies of genes g−1 soil), linear regression analysis and F-tests were performed (significance set at p < 0.05).
We performed all the above-mentioned statistical analyses using Sigmaplot 14.5 software (Systat Software, San Jose, CA, USA, https://systatsoftware.com/sigmaplot/).
The soil microbial abundance was assessed by using two-way analysis of variance (ANOVA) in R 4.0.3., and then Tukey’s test was performed to analyze significant differences between each treatment. Differences were considered statistically significant at p < 0.05 (two-sided test).
Ethics statement
The collection of plant materials in this study complied with relevant institutional, national, and international guidelines and legislation.
Data availability
The datasets generated in this study are available from the corresponding author according to reasonable requests from readers.
References
Brown, A. C. & McLachlan, A. Sandy shore ecosystems and the threats facing them: Some predictions for the year 2025. Environ. Conserv. 29, 62–77. https://doi.org/10.1017/S037689290200005X (2002).
Barbier, E. B. et al. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 81, 169–193. https://doi.org/10.1890/10-1510.1 (2011).
Directorate-General for Environment (European Commission). Building a green infrastructure for Europe. Publications Office of the European Union. https://doi.org/10.2779/54125 (2014).
Drius, M., Carranza, M. L., Stanisci, A. & Jones, L. The role of Italian coastal dunes as carbon sinks and diversity sources. A multi-service perspective. Appl. Geogr. 75, 127–136. https://doi.org/10.1016/j.apgeog.2016.08.007 (2016).
Raich, J. W. & Schlesinger, W. H. The global carbon-dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 44, 81–99. https://doi.org/10.1034/j.1600-0889.1992.t01-1-00001.x (1992).
Epron, D. In Soil Carbon Dynamics: An Integrated Methodology (eds. Heinemeyer, A., Bahn, M., & Kutsch, W. L.) 157–168 (Cambridge University Press, 2010).
Bond-Lamberty, B. & Thomson, A. Temperature-associated increases in the global soil respiration record. Nature 464, 579–582. https://doi.org/10.1038/nature08930 (2010).
Bond-Lamberty, B. New techniques and data for understanding the global soil respiration flux. Earth’s Future 6, 1176–1180. https://doi.org/10.1029/2018ef000866 (2018).
Stell, E., Warner, D., Jian, J., Bond-Lamberty, B. & Vargas, R. Spatial biases of information influence global estimates of soil respiration: How can we improve global predictions?. Glob. Change Biol. 27, 3923–3938. https://doi.org/10.1111/gcb.15666 (2021).
Jian, J. et al. A global database of soil respiration data, version 5.0. ORNL DAAC, Oak Ridge, Tennessee, USA. https://doi.org/10.3334/ORNLDAAC/1827.
Luo, Y. Q., Wan, S. Q., Hui, D. F. & Wallace, L. L. Acclimatization of soil respiration to warming in a tall grass prairie. Nature 413, 622–625. https://doi.org/10.1038/35098065 (2001).
Teramoto, M. et al. Enhanced understory carbon flux components and robustness of net CO2 exchange after thinning in a larch forest in central Japan. Agric. For. Meteorol. 74, 106–117. https://doi.org/10.1016/j.agrformet.2019.04.008 (2019).
Zhang, X. et al. Effects of continuous drought stress on soil respiration in a tropical rainforest in southwest China. Plant Soil 394, 343–353. https://doi.org/10.1007/s11104-015-2523-4 (2015).
Harper, C. W., Blair, J. M., Fay, P. A., Knapp, A. K. & Carlisle, J. D. Increased rainfall variability and reduced rainfall amount decreases soil CO2 flux in a grassland ecosystem. Glob. Change Biol. 11, 322–334. https://doi.org/10.1111/j.1365-2486.2005.00899.x (2005).
Gao, W., Huang, Z., Ye, G., Yue, X. & Chen, Z. Effects of forest cover types and environmental factors on soil respiration dynamics in a coastal sand dune of subtropical China. J. For. Res. 29, 1645–1655. https://doi.org/10.1007/s11676-017-0565-6 (2018).
Panda, T. Significance of soil properties and microbial activity on soil CO2 emission in coastal sand dunes of Odisha, India. J. Indian Bot. Soc. 100, 148–159. https://doi.org/10.5958/2455-7218.2020.00036.4 (2020).
Chapman, S. B. Some interrelationships between soil and root respiration in lowland calluna heathland in southern England. J. Ecol. 67, 1–20. https://doi.org/10.2307/2259333 (1979).
Ishikawa, S.-I., Furukawa, A. & Oikawa, T. Zonal plant distribution and edaphic and micrometeorological conditions on a coastal sand dune. Ecol. Res. 10, 259–266. https://doi.org/10.1007/BF02347851 (1995).
Šilc, U. et al. Sand dune vegetation along the eastern Adriatic coast. Phytocoenologia 46, 339–355. https://doi.org/10.1127/phyto/2016/0079 (2016).
Hwang, J.-S. et al. Relationship between the spatial distribution of coastal sand dune plants and edaphic factors in a coastal sand dune system in Korea. J. Ecol. Environ. 39, 17–29. https://doi.org/10.5141/ecoenv.2016.003 (2016).
Metcalfe, D. B., Fisher, R. A. & Wardle, D. A. Plant communities as drivers of soil respiration: Pathways, mechanisms, and significance for global change. Biogeosciences 8, 2047–2061. https://doi.org/10.5194/bg-8-2047-2011 (2011).
Tan, Z.-H. et al. Soil respiration in an old-growth subtropical forest: Patterns, components and controls. J. Geophys. Res.-Atmos. 118, 2981–2990. https://doi.org/10.1002/jgrd.50300 (2013).
Teramoto, M., Liang, N., Ishida, S. & Zeng, J. Long-term stimulatory warming effect on soil heterotrophic respiration in a cool-temperate broad-leaved deciduous forest in northern Japan. J. Geophys. Res-Biogeochem. 123, 1161–1177. https://doi.org/10.1002/2018jg004432 (2018).
Ruehr, N. K. & Buchmann, N. Soil respiration fluxes in a temperate mixed forest: Seasonality and temperature sensitivities differ among microbial and root-rhizosphere respiration. Tree Physiol. 30, 165–176. https://doi.org/10.1093/treephys/tpp106 (2010).
Yan, T. et al. Temperature sensitivity of soil respiration across multiple time scales in a temperate plantation forest. Sci. Total Environ. 688, 479–485. https://doi.org/10.1016/j.scitotenv.2019.06.318 (2019).
Noh, N. J., Kuribayashi, M., Saitoh, T. M. & Muraoka, H. Different responses of soil, heterotrophic and autotrophic respirations to a 4-year soil warming experiment in a cool-temperate deciduous broadleaved forest in central Japan. Agric. For. Meteorol. 247, 560–570. https://doi.org/10.1016/j.agrformet.2017.09.002 (2017).
Fang, C. & Moncrieff, J. B. The variation of soil microbial respiration with depth in relation to soil carbon composition. Plant Soil 268, 243–253. https://doi.org/10.1007/s11104-004-0278-4 (2005).
Wang, D., Yu, X., Jia, G., Qin, W. & Shan, Z. Variations in soil respiration at different soil depths and its influencing factors in forest ecosystems in the mountainous area of north China. Forests 10, 1081. https://doi.org/10.3390/f10121081 (2019).
Hirano, T., Kim, H. & Tanaka, Y. Long-term half-hourly measurement of soil CO2 concentration and soil respiration in a temperate deciduous forest. J. Geophys. Res-Atmos. 108, 4631. https://doi.org/10.1029/2003JD003766 (2003).
Pavelka, M., Acosta, M., Marek, M. V., Kutsch, W. & Janous, D. Dependence of the Q10 values on the depth of the soil temperature measuring point. Plant Soil 292, 171–179. https://doi.org/10.1007/s11104-007-9213-9 (2007).
Cable, J. M. et al. The temperature responses of soil respiration in deserts: A seven desert synthesis. Biogeochemistry 103, 71–90. https://doi.org/10.1007/s10533-010-9448-z (2011).
Novo, F. G., Barradas, M. C. D., Zunzunegui, M., Mora, R. G. & Fernández, J. B. G. In Coastal Dunes: Ecology and Conservation (eds Martínez, M. L. & Psuty, N. P.) 155–169 (Springer, 2004).
Hesp, P. A. Ecological processes and plant adaptations on coastal dunes. J. Arid Environ. 21, 165–191. https://doi.org/10.1016/S0140-1963(18)30681-5 (1991).
Salisbury, E. J. Downs & Dunes; Their Plant Life and Its Environment (Bell, 1952).
Wang, Y. F. et al. Responses of soil respiration and its components to drought stress. J. Soil. Sediment. 14, 99–109. https://doi.org/10.1007/s11368-013-0799-7 (2014).
Kucera, C. L. & Kirkham, D. R. Soil respiration studies in tallgrass prairie in Missouri. Ecology 52, 912–915. https://doi.org/10.2307/1936043 (1971).
Behera, N., Joshi, S. K. & Pati, D. P. Root contribution to total soil metabolism in a tropical forest soil from Orissa, India. For. Ecol. Manag. 36, 125–134. https://doi.org/10.1016/0378-1127(90)90020-c (1990).
Tomotsune, M., Yoshitake, S., Watanabe, S. & Koizumi, H. Separation of root and heterotrophic respiration within soil respiration by trenching, root biomass regression, and root excising methods in a cool-temperate deciduous forest in Japan. Ecol. Res. 28, 259–269. https://doi.org/10.1007/s11284-012-1013-x (2013).
Jia, S. X., McLaughlin, N. B., Gu, J. C., Li, X. P. & Wang, Z. Q. Relationships between root respiration rate and root morphology, chemistry and anatomy in Larix gmelinii and Fraxinus mandshurica. Tree Physiol. 33, 579–589. https://doi.org/10.1093/treephys/tpt040 (2013).
Lee, J.-S. Relationship of root microbial biomass and soil respiration in a stand of deciduous broadleaved trees—A case study in a maple tree. J. Ecol. Environ. 42, 19. https://doi.org/10.1186/s41610-018-0078-z (2018).
Hogberg, P. et al. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411, 789–792. https://doi.org/10.1038/35081058 (2001).
Ashkannejhad, S. & Horton, T. R. Ectomycorrhizal ecology under primary succession on coastal sand dunes: Interactions involving Pinus contorta, suilloid fungi and deer. New Phytol. 169, 345–354. https://doi.org/10.1111/j.1469-8137.2005.01593.x (2006).
Li, W. et al. Nitrogen fertilizer regulates soil respiration by altering the organic carbon storage in root and topsoil in alpine meadow of the north-eastern Qinghai-Tibet Plateau. Sci. Rep. 9, 13735. https://doi.org/10.1038/s41598-019-50142-y (2019).
Morisada, K., Ono, K. & Kanomata, H. Organic carbon stock in forest soils in Japan. Geoderma 119, 21–32. https://doi.org/10.1016/S0016-7061(03)00220-9 (2004).
Pregitzer, K. S., Laskowski, M. J., Burton, A. J., Lessard, V. C. & Zak, D. R. Variation in sugar maple root respiration with root diameter and soil depth. Tree Physiol. 18, 665–670. https://doi.org/10.1093/treephys/18.10.665 (1998).
Diaz-Pines, E. et al. Root trenching: A useful tool to estimate autotrophic soil respiration? A case study in an Austrian mountain forest. Eur. J. For. Res. 129, 101–109. https://doi.org/10.1007/s10342-008-0250-6 (2010).
Lee, M. S., Nakane, K., Nakatsubo, T. & Koizumi, H. Seasonal changes in the contribution of root respiration to total soil respiration in a cool-temperate deciduous forest. Plant Soil 255, 311–318. https://doi.org/10.1023/A:1026192607512 (2003).
Noh, N.-J. et al. Responses of soil, heterotrophic, and autotrophic respiration to experimental open-field soil warming in a cool-temperate deciduous forest. Ecosystems 19, 504–520. https://doi.org/10.1007/s10021-015-9948-8 (2016).
Bond-Lamberty, B., Bronson, D., Bladyka, E. & Gower, S. T. A comparison of trenched plot techniques for partitioning soil respiration. Soil Biol. Biochem. 43, 2108–2114. https://doi.org/10.1016/j.soilbio.2011.06.011 (2011).
Li, X. et al. Contribution of root respiration to total soil respiration in a semi-arid grassland on the Loess Plateau, China. Sci. Total Environ. 627, 1209–1217. https://doi.org/10.1016/j.scitotenv.2018.01.313 (2018).
Hanson, P. J., Edwards, N. T., Garten, C. T. & Andrews, J. A. Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 48, 115–146. https://doi.org/10.1023/A:1006244819642 (2000).
Bond-Lamberty, B., Wang, C. K. & Gower, S. T. Contribution of root respiration to soil surface CO2 flux in a boreal black spruce chronosequence. Tree Physiol. 24, 1387–1395. https://doi.org/10.1093/treephys/24.12.1387 (2004).
Bongiovanni, T., Liu, P.-W., Preston, D., Feagle, S. & Judge, J. Calibrating time domain reflectometers for soil moisture measurements in sandy soils: AE519, 2/2017. EDIS 2017, https://doi.org/10.32473/edis-ae519-2017 (2017).
Abe, Y. et al. Spatial variation in soil respiration rate is controlled by the content of particulate organic materials in the volcanic ash soil under a Cryptomeria japonica plantation. Geoderma Reg. 29, e00529. https://doi.org/10.1016/j.geodrs.2022.e00529 (2022).
Sun, L., Hirano, T., Yazaki, T., Teramoto, M. & Liang, N. Fine root dynamics and partitioning of root respiration into growth and maintenance components in cool temperate deciduous and evergreen forests. Plant Soil 446, 471–486. https://doi.org/10.1007/s11104-019-04343-z (2020).
Caporaso, J. G. et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 108, 4516–4522. https://doi.org/10.1073/pnas.1000080107 (2011).
Toju, H., Tanabe, A. S., Yamamoto, S. & Sato, H. High-coverage ITS primers for the DNA-based identification of Ascomycetes and Basidiomycetes in environmental samples. PLoS One 7, e40863. https://doi.org/10.1371/journal.pone.0040863 (2012).
Lloyd, J. & Taylor, J. A. On the temperature-dependence of soil respiration. Funct. Ecol. 8, 315–323. https://doi.org/10.2307/2389824 (1994).
Wang, B. et al. Soil moisture modifies the response of soil respiration to temperature in a desert shrub ecosystem. Biogeosciences 11, 259–268. https://doi.org/10.5194/bg-11-259-2014 (2014).
Acknowledgements
We appreciate technical support for the SOC and nitrogen analysis from the Engineering Department of the Arid Land Research Center of Tottori University and advice on the statistical analysis provided by Mr. Ryuji Fukushima (ESUMI Co., Ltd.) and Dr. Shigeaki Ohtsuki (Japan Institute of Statistical Technology). We also express our gratitude to the handling editor and two anonymous reviewers for the detailed insight into our study and constructive comments. This study was funded by a grant from the Tenure-Track Program of Tottori University. This work was also partially funded by the Joint Research Program of Arid Land Research Center, Tottori University (03A2001), by the Environment Research and Technology Development Fund (JPMEERF20202006) of the Environmental Restoration and Conservation Agency of Japan, and by the Nippon Life Insurance Foundation.
Author information
Authors and Affiliations
Contributions
M.T. designed the experiment. M.T. and R.H. collected environmental data, biomass data, and soil CO2 efflux data. T.H. conducted microbial analysis, and T.T. supported the microbial analysis. N.L. developed a soil CO2 efflux measurement system and supported data collection. N.Y. introduced the study site to M.T. and discussed the experimental design with M.T. M.T. wrote the first draft of the manuscript and T.H., N.L., T.T., T.Y.I., R.H., and N.Y. provided information and feedbacks for revising the manuscript at all stages.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Teramoto, M., Hamamoto, T., Liang, N. et al. Abiotic and biotic factors controlling the dynamics of soil respiration in a coastal dune ecosystem in western Japan. Sci Rep 12, 14320 (2022). https://doi.org/10.1038/s41598-022-17787-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-17787-8
- Springer Nature Limited