Abstract
In this work, a novel method of solid sample pretreatment technique of bismuth fire assay (Bi-FA) combined with solid sample determination by laser ablation ICP-MS (LA-ICP-MS) was reported for the determination of ultra-trace Pt and Pd in geochemical samples. Bismuth oxide (Bi2O3) was used as fire assay collector to directly enrich Pt and Pd from solid samples, and Ag protection cupellation was employed to generate Ag granules. After cleaning, weighing and annealing, the Ag granules were compressed into thin slices and determined by LA-ICP-MS for 195Pt, 105Pd and 109Ag (109Ag was selected as the internal standard isotope). Bi2O3 provided exceptionally low blanks compared to nickel oxide and lead oxide commonly employed in fire assay procedures, and could be applied directly without purification. Different from traditional empirical coefficient method, the Chinese Certified Reference Materials (CRMs) for Pt and Pd were treated by the same procedure to obtain completely matrix matched Ag slices. And then modified empirical coefficient method and internal standard calibration strategy was used to reduce the instability of LA-ICP-MS, and random multipoint laser ablation was employed to further reduce analytical variation resulting from heterogeneity of Pt and Pd in the Ag slice. Under optimal conditions, excellent calibration curves for Pt and Pd were obtained (0.407–2958 μg g−1 and 0.407–2636 μg g−1, respectively), with correlation coefficients exceeding 0.9996. The method detection limits for Pt and Pd were 0.074 and 0.037 ng g−1, respectively. The established method was applied successfully to analysis of real geochemical samples, with determined values in good agreement with the results of traditional Pb-FA graphite furnace atomic absorption spectrometry (GF-AAS), and spiked recoveries between 87.8 and 125.0%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Laser ablation (LA) due its capability of complete ablation of any solid material, is the most frequent universal sample introduction technique for solid samples1,2. Laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) has become a powerful analytical tool for sensitive ultra-trace analysis of solid samples in recent years in different application fields, such as geological samples3,4, metallic and semiconducting materials, environmental and biological samples5,6,7 and so on.
Pt and Pd are known for their valuable properties and rare resources, which have been widely applied in petroleum, automobile, electronics, chemical industry, atomic energy, as well as environmental protection industry8,9,10. Consequently, accurate determination of Pt and Pd in geochemical samples is of great significance for geological science research and precious metal ore prospecting. However, it is difficult to accurate determination of ultra-trace Pt and Pd because of the low abundance and uneven distribution in natural Pt–Pd ore as well as the nugget effect11,12. Therefore, appropriate sample pretreatment techniques of Pt and Pd were needed prior to the determination.
Fire assay is an ancient but still used method, which plays an important role in the separation and enrichment of precious metals. The solid geochemical samples were mixed and reacted with solid fluxing agent at high temperature, then the target precious metals were concentrated by collectors to product high density alloy granule, conversely, the nonprecious metals and rock-forming elements reacted with solid flux to produce low density silicate or borate fluids13,14,15,16. Thereby, the target precious metals were successfully separated from the sample matrix. Based on the difference of collectors, fire assay methods can be divided into nickel sulfide fire assay (NiS-FA)16,17, lead fire assay (Pb-FA)18,19, antimony fire assay (Sb-FA)20, tin fire assay (Sn-FA)21,22 and so on. NiS-FA and Pb-FA are the most commonly used methods to simultaneously concentrate Pt and Pd in geochemical samples. In previous publications, also Pb fire assay buttons23,24,25 and NiS buttons26,27 were determined by LA-ICP-MS for precious metal when using external calibration against matrix-matched standards. However, due to the high and changeable reagent blank mainly from the NiO and PbO collector the accurate determination of ultra-trace Pt and Pd has become very difficult, thus the collector reagents must be purified in advance17,19. The selectivity of Sb-FA was unsatisfactory20 and Sn granules could not be removed by cupellation22. Therefore, other novel fire assay collectors were constantly searching and trying. Pt and Pd could form a series of alloys or metal inter-compounds with non-toxic Bi at high temperatures; thus Bi could quantitatively collect the precious metal elements in solid geological samples28.
In this work, a novel method of Bi-FA preconcentration combined with empirical coefficient method LA-ICP-MS for the determination of ultra-trace Pt and Pd in geochemical samples was established. Non-toxic Bi2O3 was used as the fire assay collector to enrich the precious metal elements into Bi granule, and Ag protection cupellation was employed to form Ag slice for direct laser ablation solid sample injection. Compared with Pb-FA and NiS-FA, the reagent blank of Bi2O3 was relatively low. Thus Bi2O3 could be directly employed to collect precious metal elements without purifying. Moreover, the harm of toxic collector to the analyst and environment was avoided by using the non-toxic Bi2O3. The Chinese Certified Reference Materials (CRMs) of Pt and Pd were treated by the same procedure to obtain completely matrix matched Ag slices, and the modified empirical coefficient method was employed to fit the correction curve. Laser ablation analysis of the Ag slice for direct solid sample injection was used to avoid acid digestion that was used in the traditional GF-AAS or ICP-MS determination methods, which saved the analysis time, reduced the blank value, decreased the interference of polyatomic molecular ions and the dilution effect, and eliminated acid reagents to protect the environment and the health of the analyst. The established method was successfully applied to determine Pt and Pd in real geochemical samples, and the determined values were in good agreement with the results of traditional Pb-FA GF-AAS method.
Experimental details
Instrumentation
A laser ablation system (Model GeoLas HD, Coherent, USA) coupled to the quadrupole (Q) ICP-MS (Model 7700x, Agilent, USA) was used in all experiments for the determination of Pt and Pd. The operating parameters laser ablation and mass spectrometric measurements are summarized in Tables 1 and 2. Millionth electronic balance (ME5, Sartorius, Germany) and micropipettors (100–1000 μL, Brand, Germany) were used for weighing and pipetting.
Standard solutions and reagents
The mixed standard solutions (Pd and Pt 10 μg mL−1) in 10% aqua regia bought from SPEX CertiPrep group (USA). Ag standard solution (25 g L−1) was prepared and used as cupellation protector.
The fire assay collector of Bi2O3 and other solid fluxing agent such as Na2CO3, Na2B4O7·10H2O, Na2O2, CaO, SiO2 and flour were of analytical reagent grade (AR) and purchased from Tianjin Kemiou Chemical Reagent Co., Ltd. HCl and HNO3 were of excellent reagent grade (GR, Kemiou Chemical Reagent Co., Ltd, Tianjin). Ultrapure water (18.2 MΩ cm) obtained from a Milli-Q water purification system (Millipore, Bedford, MA, USA) was used throughout the whole experiments.
Sample pretreatment and determination
Fire assay recipes, melting process, cupellation and Ag granule pretreatment
According to the mineral composition characteristics of geochemical samples (such as rock, soil, river sediment, chromite, black shale and polymetallic ore), the fire assay recipes were adjusted, as shown in Table 3. In order to have better sample decomposition and enrichment effect, some special samples should be pretreated before fire assay29. For example, sulfide rock sample should be heated in a furnace at 650 °C for 2 h; and chromite sample should be mixed well with CaO and Na2O2 and heated at about 680 °C for 1.5 h.
Raw materials according to Table 3 were mixed well in an ingredient bottle, 70% of the mixture was transferred into a 500 mL fire-clay crucible, then 250 μL of Ag standard solution (25 g L−1) was added. After drying, another 30% of the mixture was added and 20 g of covering agent (Na2CO3, Na2B4O7·10H2O, glass powder, Bi2O3 and flour were mixed well at 50 g: 20 g: 15 g: 10 g: 4 g) was uniformly added. Then the crucible was fused in a furnace heated to 1070 °C gradually and kept for 30 min. The melts in the crucible were poured into a cast iron mold. Once cooled, the Bi granule was separated from the slag.
The Bi granule was cupellated in a magnesia cupel at 940 °C until it produced a dazzling color and flashes, which was the end of cupellation process. At this stage, Bi in the granule was eliminated and the target precious metal elements were trapped in the Ag granule. The Ag granule was ultrasonic cleaned, weighed and annealed at 700 °C for 30 min, then was compressed into a thin slice (~ 0.2 mm). A blank sample was also subjected to this procedure.
Standard samples preparation
According to the fire assay recipes of Chinese Certified Reference Materials (CRMs) in Table 3, 10 g of CRMs (GBW07288, GBW07289, GBW07290, GBW07291, GBW07293, GBW07294, GBW07341 and GBW07342) and solid fluxing agent were mixed well in parallel. Then the next Ag standard solution and covering agent adding procedure and the preparation of external standard CRMs Ag slices steps were the same as “Fire assay recipes, melting process, cupellation and Ag granule pretreatment” section.
LA-ICP-MS determination
The slices of external standard CRMs series and real geochemical sample were determined by random multipoint (10 points) LA-ICP-MS for 195Pt, 105Pd and 109Ag (internal standard). The mass fractions of Pt and Pd in real geochemical samples were calculated by formula (1) as shown in Ref.29:
where \({w}_{0}\) is the mass fractions of Pt and Pd in blank Ag slice (μg g−1); \({m}_{0}\) is the mass of blank Ag slice (g); \({w}_{sam}\) is the mass fractions of Pt and Pd in real sample Ag slice (μg g−1); \({m}_{sam}\) is the mass of real sample Ag slice (g); \({m}_{s}\) is the mass of real sample (g).
Result and discussion
Comparison of different collectors
The regent blanks of Bi2O3, NiO and PbO were respectively determined by Bi-FA-ICP-MS, NiS-FA ICP-MS and Pb-FA GF-AAS, and the values were shown in Table 4. It could be seen that the reagent blank of Pt and Pd in Bi2O3 was extremely low compared to commercial PbO and NiO. Therefore, Bi2O3 could be used as collector directly without purification.
Cupellation temperature
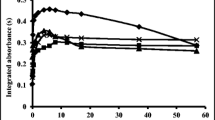
Metal Bi can be oxidized to Bi2O3 at ~ 300 °C. However, the melting point of Bi2O3 is 820 °C. Therefore, the cupellation temperature must be controlled to above 820 °C so that liquid Bi2O3 could be absorbed by the magnesia cupel. The effect of cupellation temperature was optimized with the data shown in Fig. 1. It can be seen that the cupellation speed was accelerated, and Bi content remaining in the Ag granule was also decreased with increasing of cupellation temperature. When the temperature reached to 950 °C, obvious volatilization of Bi2O3 could be observed. If the temperature was too high, the loss of Pt and Pd as well as muffle furnace would be increased. Therefore, 940 °C was selected as the cupellation temperature.
After Bi cupellation, the target Pt and Pd were trapped in the Ag granule. The Ag granule was annealed at 700 °C to further homogenize the alloy of Pt, Pd and Ag, and which was compressed into ~ 0.2 mm slices to facilitate the use of the laser ablation system.
Preparation of Ag slices
The solid samples determined by LA-ICP-MS were required to be as uniform as possible, thus the effect of annealing on the signal stability of 105Pd and 195Pt were evaluated and shown in Fig. 2. It was observed that, the signal fluctuation before annealing was obviously larger than that after annealing. Therefore, the Ag granules were annealed at 700 °C to ensure the uniformity of the target Pt and Pd inside the Ag slices.
Mass spectral interferences
Based on the principle of high abundance and free from isobaric interference, the monitored isotopes of Pt, Pd and Ag were shown in Table S1, 195Pt and 105Pd were selected as measuring isotopes. Though 107Ag and 109Ag are close in abundance, the mass charge ratio difference of 109Ag/105Pd is larger than 107Ag/105Pd, then 109Ag was selected as internal standard isotope. The possible interferences from polyatomic molecular ions on 195Pt, 105Pd and 109 Ag were shown in Table S2. After Bi-FA and cupellation, there were only trace Bi, Au, Pt, Pd, Ru, Rh and Ir reserved in Ag granule. Compared to traditional solution injection system, laser ablation solid sample injection could avoid the introduction of large amount of Cl, N, H and O into the ICP. Based on the above means, the possible interferences of polyatomic molecular ions could be effectively decreased.
Modified empirical coefficient method
The empirical coefficient method is based on the certified values and signal strength of a series of CRMs, using linear or nonlinear regression methods to obtain the coefficients for the quantification formula and allow quantitative sample analysis30, which is usually used in X-ray fluorescence analysis of solid samples. However, the traditional empirical coefficient method has a high demand on sample matrix, which requires the composition and structure of the CRMs and the real sample to be tested should be highly similar. Up to now, only a few geochemical Certified Reference Materials included soil, stream sediments, peridotite, chromite and Pt–Pd ores were developed by China. Some special samples, such as polymetallic ore and black shale, the matrix was not identical to the existing CRMs, the accuracy of the method will be affected.
In this work, a fully matrix-matched Ag slices were obtained and modified empirical coefficient method LA-ICP-MS was established for the determination of ultra-trace Pt and Pd in multiple geochemical samples. Bi-FA was employed to enrich the target precious metal elements from the CRMs and real samples (such as soil, river sediment, chromite, olivinite and Pt–Pd ore) into the Bi granule. After Ag protection cupellation, Pt and Pd were enriched in the Ag slices, fully matrix-matched was achieved and the typical Ag slices of CRMs were shown in Fig. 3. Details of the matrix-matched Pt and Pd mixed external standard CRMs series in the Ag slices are shown in Table 5. The concentrations for Pt and Pd in the media of Ag slices were 0.407–2958 μg g−1 and 0.407–2636 μg g−1, respectively. The representative time-resolved LA-ICP-MS signals of blank and certified sample were shown in Fig. S1.
Internal standard calibration strategy for LA-ICP-MS
In this work, internal standard calibration method was employed to reduce analysis error and correct biases resulting from fluctuations in laser output power as well as sample ablation amount and transport efficiency, to improve the method precision and accuracy. Due to Ag content in the Ag slices were almost identical between CRMs and real samples, then 109Ag in the Ag slices was selected as internal standard isotope for the determination of 195Pt and 105Pd. According to the basic principle and formula (Formula 2) of internal standard method29, the concentration of the target element in real sample could be calculated.
where \(w_{t}^{sam}\) and \(w_{t}^{std}\) are the concentrations of target element (Pt and Pd) in Ag slices of real and standard samples (μg g−1), respectively; \(w_{i}^{sam}\) and \(w_{i}^{std}\) are concentrations of internal standard element (Ag) in Ag slices of real and standard samples (μg g−1), respectively. In our experiment, as the concentrations of internal standard element in real and standard samples were the same by adding the same amount of Ag standard solution during Ag protection cupellation procedure, then the formula is simplified; \(I_{t}^{sam}\) and \(I_{t}^{std}\) are the signal strength of target element in Ag slices of real and standard samples (cps), respectively; \(I_{i}^{sam}\) and \(I_{i}^{std}\) are the signal strength of internal standard element in Ag slices of real and standard samples (cps), respectively.
The internal standard and non-internal standard method coefficient of variations (CVs) (n = 10) were compared in Table 6. It is observed that the CVs of 195Pt and 105Pd by non-internal standard method were between 5.71 and 6.68%. In comparison, the CVs were reduced to 2.54–4.05% when internal standard method was used.
Analytical performance
The Pt and Pd mixed external standard series were prepared with the concentrations of 0.407–2958 μg g−1 and 0.407–2636 μg g−1 in the media of Ag slices. The standard series of CRMs are shown in Table 5. At the optimum conditions, the intensities of 195Pt, 105Pd and 109Ag were detected by LA-ICP-MS, and the concentrations of target elements were calculated by formula (2).
The analytical performance of the proposed Bi-FA LA-ICP-MS method has been validated using the calibration curve equation, fit coefficient and LODs, shown in Table 7. Excellent curve fitting of Pt and Pd were obtained and shown in Fig. S2 (0.407–2958 μg g−1 and 0.407–2636 μg g−1, respectively), with the correlation coefficients exceeding 0.9996. Based on 3δblank approach as recommended by IUPAC for spectrochemical measurements, the LODs (3 * standard deviation of background/slope of calibration curve, for 10 g sample) of the proposed method for the target Pt and Pd were 0.074 and 0.037 ng g−1, respectively. The LODs for Pt and Pd obtained by this method along with other methods were compared. The results in Table 8 revealed that, due to the high enrichment factor (about 1667 fold, 10 g sample weight pre-concentrated into ~ 6 mg Ag granules) the LODs obtained in this work and our previous Pb-FA LA-ICP-MS methods29 were much lower than those low enrichment factor methods based LA-ICP-MS and NiS/Pb fire assay24,25,26,27.
Sample analysis
Under the optimal experimental and instrumental conditions, real geochemical samples were analyzed by the established Bi-FA LA-ICP-MS method and compared to Pb-FA GF-AAS method. The results are shown in Table 9. It can be seen that the determined values are in good agreement with the results of traditional Pb-FA GF-AAS, and the spiked recoveries were between 87.8 and 125.0%.
Conclusions
In this work, the method of Ag protection cupellation Bi-FA combined with LA-ICP-MS for the determination of Pt and Pd was established. Bi2O3 was used as fire assay collector, which has the advantages of lower toxicity and blank values than conventional Pb fire assay. Complete matrix match for the CRMs standards and real geochemical samples were obtained by the modified empirical coefficient method. In order to reduce analysis error and the instability of LA-ICP-MS test parameters, 109Ag was selected as internal standard isotope and internal standard calibration strategy was used. Due to the advantages of obtained by solid sample pretreatment and analysis, the sample throughput was improved and interference of polyatomic molecular ions was decreased. The established method was successfully applied to determination of Pt and Pd in real geochemical samples, and the determined values were in good agreement with the results of traditional Pb-FA GF-AAS analysis.
References
Lin, J., Liu, Y. S., Yang, Y. H. & Hu, Z. C. Calibration and correction of LA-ICP-MS and LA-MC-ICP-MS analyses for element contents and isotopic ratios. Solid Earth Sci. 1, 5–27. https://doi.org/10.1016/j.sesci.2016.04.002 (2016).
Becker, J. S., Matusch, A. & Wu, B. Bioimaging mass spectrometry of trace elements-recent advance and applications of LA-ICP-MS: A review. Anal. Chim. Acta. 835, 1–18. https://doi.org/10.1016/j.aca.2014.04.048 (2014).
Cochrane, E. E. & Neff, H. Investigating compositional diversity among Fijian ceramics with laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS): Implications for interaction studies on geologically similar islands. J. Archaeol. Sci. 33, 378–390. https://doi.org/10.1016/j.jas.2005.08.003 (2006).
Yang, W. W. et al. A simple method for the preparation of homogeneous and stable solid powder standards: Application to sulfide analysis by LA-ICP-MS. Spectrochim. Acta. B. 178, 106124. https://doi.org/10.1016/j.sab.2021.106124 (2021).
Torimoto, R. et al. Analysis of lead distribution in avian organs by LA-ICP-MS: Study of experimentally lead-exposed ducks and kites. Environ. Pollut. 283, 117086. https://doi.org/10.1016/j.envpol.2021.117086 (2021).
Papaslioti, E. M., Parviainen, A., Román Alpiste, M. J., Marchesi, C. & Garrido, C. J. Quantification of potentially toxic elements in food material by laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS) via pressed pellets. Food Chem. 274, 726–732. https://doi.org/10.1016/j.foodchem.2018.08.118 (2019).
Uryu, T., Yoshinaga, J., Yanagisawa, Y., Endo, M. & Takahashi, J. Analysis of lead in tooth enamel by laser ablation-inductively coupled plasma-mass spectrometry. Anal. Sci. 19, 1413–1416. https://doi.org/10.2116/analsci.19.1413 (2003).
Li, T. T. et al. Effect of noble metal element on microstructure and NO2 sensing properties of WO3 nanoplates prepared from a low-grade scheelite concentrate. J. Alloy. Compd. 818, 152927. https://doi.org/10.1016/j.jallcom.2019.152927 (2020).
Kaye, M. H., Lewis, B. J. & Thompson, W. T. Thermodynamic treatment of noble metal fission products in nuclear fuel. J. Nucl. Mater. 366, 8–27. https://doi.org/10.1016/j.jnucmat.2006.11.014 (2007).
Hou, Z. Q. et al. Rare earth oxides and their supported noble metals in application of environmental catalysis. J. Rare Earth. 38, 819–839. https://doi.org/10.1016/j.jre.2020.01.011 (2020).
Amossé, J. Determination of platinum-group elements and gold in geological matrices by inductively coupled plasma-mass spectrometry (ICP-MS) after separation with selenium and tellurium carriers. Geostand. Geoanal. Res. 22, 93–102. https://doi.org/10.1111/j.1751-908X.1998.tb00548.x (1998).
Pattou, L., Lorand, J. P. & Gros, M. Non-chondritic platinum group element ratios in the earth’s mantle. Nature 379, 712–715. https://doi.org/10.1038/379712a0 (1996).
Li, C. S., Chai, C. F., Li, X. L. & Mao, X. Y. Determination of platinum-group elements and gold in two Russian Candidate Reference Materials SCHS-1 and SLg-1 by ICP-MS after nickel sulfide fire assay preconcentration. Geostand. Geoanal. Res. 22, 195–197. https://doi.org/10.1111/j.1751-908X.1998.tb00692.x (1998).
Jackson, S. E., Fryer, B. J. & Gosse, W. Determination of the precious metals in geological materials by inductively coupled plasma-mass spectrometry (ICP-MS) with nickel sulphide fire-assay collection and tellurium coprecipitation. Chem. Geol. 83, 119–132. https://doi.org/10.1016/0009-2541(90)90144-V (1990).
Cerceau, C. I. et al. Recovering lead from cupel waste generated in gold analysis by Pb-Fire assay. J. Environ. Manag. 183, 771–776. https://doi.org/10.1016/j.jenvman.2016.08.052 (2016).
Sun, Y. L., Guan, X. Y. & Du, A. D. Determination of platinum group elements by inductively coupled plasma-mass spectrometry combined with nickel sulphide fire assay and tellurium coprecipitation. Spectrochim. Acta. B. 53, 1463–1467. https://doi.org/10.1016/S0584-8547(98)00167-0 (1998).
Ni, W. S., Zhang, H. L., Mao, X. J., Liu, L. & Xiao, F. Determination of ultra-trace osmium and ruthenium in geological samples by ICP-MS combined with nickel sulfide fire assay pre-concentration and microwave digestion. Microchem. J. 150, 104187. https://doi.org/10.1016/j.microc.2019.104187 (2019).
Date, A. R., Davis, A. E. & Cheung, Y. Y. The potential of fire assay and inductively coupled plasma source mass spectrometry for the determination of platinum group elements in geological materials. Analyst 112, 1217. https://doi.org/10.1039/an9871201217 (1987).
Ni, W. S. et al. Lead fire assay preconcentration and high resolution continuum source graphite furnace atomic absorption spectrometry for the determination of ultra-trace amounts of Au, Ir, Pd, Pt and Rh in rocks and minerals. Spectrochim. Acta. B. 158, 105643. https://doi.org/10.1016/j.sab.2019.105643 (2019).
Ni, W. S. et al. Simultaneous determination of ultra-trace Au, Pt, Pd, Ru, Rh, Os and Ir in geochemical samples by KED-ICP-MS combined with Sb-Cu fire assay and microwave digestion. Microchem. J. 158, 105197. https://doi.org/10.1016/j.microc.2020.105197 (2020).
Moloughney, P. E. & Faye, G. H. A rapid fire-assay atomic absorption method for the determination of platinum, pallandium and gold in ores and concentrates: A modification of the tin-collection scheme. Talanta 23, 377–381. https://doi.org/10.1016/0039-9140(76)80050-1 (1976).
Ni, W. S. et al. Simultaneous determination of ultra-trace Pt, Pd, Rh and Ir in geochemical samples by inductively coupled plasma mass spectrometry following tin fire assay preconcentration and microwave digestion. Curr. Anal. Chem. 17, 552–563. https://doi.org/10.2174/1573411016999200715160650 (2021).
Compernolle, S., Wambeke, D., Raedt, I. D. & Vanhaecke, F. Evaluation of a combination of isotope dilution and single standard addition as an alternative calibration method for the determination of precious metals in lead fire assay buttons by laser ablation-inductively coupled plasma-mass spectrometry. Spectrochim. Acta. B. 67, 50–56. https://doi.org/10.1016/j.sab.2011.12.008 (2012).
Vanhaecke, F., Resano, M., Koch, J., McIntoshd, K. & Gunther, D. Femtosecond laser ablation-ICP-mass spectrometry analysis of a heavy metallic matrix: Determination of platinum group metals and gold in lead fire-assay buttons as a case study. J. Anal. At. Spectrom. 25, 1259–1267. https://doi.org/10.1039/c002746d (2010).
Resano, M. et al. Comparison of the solid sampling techniques laser ablation-ICP-MS, glow discharge-MS and spark-OES for the determination of platinum group metals in Pb buttons obtained by fire assay of platiniferous ores. J. Anal. At. Spectrom. 21, 899–909. https://doi.org/10.1039/b603270b (2006).
Resano, M., Ruiz, E. G., McIntosh, K. S. & Vanhaecke, F. Laser ablation-inductively coupled plasma-dynamic reaction cell-mass spectrometry for the determination of platinum group metals and gold in NiS buttons obtained by fire assay of platiniferous ores. J. Anal. At. Spectrom. 237, 1599–1609. https://doi.org/10.1039/b807003b (2008).
Resano, M., McIntosh, K. S. & Vanhaecke, F. Laser ablation-inductively coupled plasma-mass spectrometry using a doublefocusing sector field mass spectrometer of Mattauch–Herzog geometry and an array detector for the determination of platinum group metals and gold in NiS buttons obtained by fire assay of platiniferous ores. J. Anal. At. Spectrom. 27, 165–173. https://doi.org/10.1039/c1ja10193e (2012).
Zhang, S. L. & Tu, H. M. Study on enrichment of precious metals in ore by bismuth assay. Mine. Res. Geol. 02, 90–102 (1981).
Ni, W. S. et al. Matrix-matched multi-external standards combined internal standard calibration strategy for the simultaneous determination of ultra-trace Au, Pt and Pd in geochemical samples by LA-ICP-MS after lead fire assay preconcentration. Microchem. J. 170, 106724. https://doi.org/10.1016/j.microc.2021.106724 (2021).
Mejía-Piña, K. G., Huerta-Diaz, M. A. & González-Yajimovich, O. Calibration of handheld X-ray fluorescence (XRF) equipment for optimum determination of elemental concentrations in sediment samples. Talanta 161, 359–367. https://doi.org/10.1016/j.talanta.2016.08.066 (2016).
Acknowledgements
This work was supported by the National Natural Science Foundation of PR China [grant numbers 22106148] and the Geological Survey Program of China Geological Survey [grant numbers DD20190573].
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ni, W., Mao, X., Yao, M. et al. Bismuth fire assay preconcentration and empirical coefficient LA-ICP-MS for the determination of ultra-trace Pt and Pd in geochemical samples. Sci Rep 12, 11555 (2022). https://doi.org/10.1038/s41598-022-15881-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15881-5
- Springer Nature Limited