Abstract
The aim of our study was (I) To compare back muscle oxygenation and perfusion as well as Biering–Sorensen muscle endurance (BSME) test holding times between chronic non-specific low back pain (CNSLBP) patients and asymptomatic controls matched for age, body mass index (BMI), sex and physical activity, and (II) to investigate factors associated with BSME holding times. Muscle perfusion (tHb) and oxygenation (SmO2) were measured by near-infrared spectroscopy (NIRS) based oximetry in three back muscles during the BSME. Reliability of tHb and SmO2 was assessed in a separate sample. BSME holding time and SmO2 were compared between patients (n = 45) and controls (n = 45) and factors associated with BSME holding time were assessed using multiple linear regression. Reliability for SmO2 was excellent (ICC = 0.87–0.99). THb showed poor to moderate reliability and was not further used. Groups differed for BSME holding time (P = 0.03), pain intensity (P ≤ 0.0005) and subcutaneous tissue thickness (P = 0.01) but not for NIRS measures. Physical activity and BMI were associated with BSME holding times. Insufficient muscle oxygenation does not seem to be a major factor contributing to CNSLBP. Future investigation should evaluate other determinants of BSME holding times, such as motivation and recruitment of auxiliary muscles.
Similar content being viewed by others
Introduction
Low back pain (LBP) is a common condition, globally the number one cause for years lived with disability1. In most instances, no specific cause can be identified1,2, resulting in the unsatisfactory diagnostic category ‘non-specific LBP’ (NSLBP). Despite the absence of a clear cause, it has been reported that patients with NSLBP have altered muscle perfusion, resulting in decreased endurance capacity of paraspinal muscles3,4 and impaired muscle oxygenation5,6. In fact, it has been observed that changes in oxygen levels in the erector spinae muscle during back flexion and extension were only altered in patients with NSLBP but not in patients with specific causes for LBP, such as structural abnormalities4. This might indicate that altered muscle perfusion and/or oxygen levels are patho-physiologically relevant in NSLBP. In rodents, it has been shown that already a slight increase in muscle metabolites (i.e. protons, lactate and adenosintriphosphate), as would be expected with reduced perfusion, can activate nociceptors7. The synergistic effect of these three metabolites in eliciting muscle pain has been confirmed in healthy human subjects8.
However, the majority of studies that investigated muscle perfusion in patients with (chronic) NSLBP (CNSLBP) used dynamic muscle tests3,5,6, although the assessment of isometric endurance capacity has been suggested to be the most appropriate method to examine muscle fatiguability in CNSLBP9. Indeed, CNSLBP is associated with a decrease in isometric endurance capacity10,11. The gold standard to assess the isometric endurance capacity of back muscles is the Biering–Sorensen Muscular Endurance test (BSME)10,12. Two studies that used the BSME in conjunction with near infrared spectroscopy (NIRS) based oximetry to measure changes in muscle perfusion and muscle oxygenation provided mixed results: one study did not report differences in BSME holding times between CNSLBP patients and healthy controls, but found an increased recovery time of oxygenation in the patients13. The other study found significantly decreased holding times of CNSLBP patients in the BSME, but the muscle perfusion and oxygenation parameters did not differ between patients and controls (recovery time was not determined)11. The isometric endurance capacity as measured by the BSME is known to be influenced by age, sex, body mass index (BMI) and the subject's activity level14,15,16,17,18. However, the latter was not considered in these two studies. Therefore, the first aim of the present study was to compare BSME holding times as well as muscle oxygenation and perfusion during the BSME between CNSLBP patients and asymptomatic controls matched for physical activity, age, sex and BMI and controlling for subcutaneous tissue thickness.
Furthermore, changes in muscle oxygenation parameters during the BSME (the change from the minimum value during the BSME to the maximum value during recovery as well as the change during rest to the maximum or minimum value during the BSME) best predicted BSME holding times in a multiple regression model in healthy men (20). Similarly, the same muscle oxygenation parameters correlated with BSME holding times in physically active CNSLBP patients (13). In addition, it is conceivable that the cardiovascular response during the BSME19 influences BSME holding times. Therefore, the second aim of this study was to investigate the influence of muscle oxygenation on BSME holding times in addition to age, sex, physical activity, BMI, mean arterial pressure (MAP), pulse pressure (PP) and heart rate (HR) to better understand which factors determine the BSME holding times across CNSLBP patients and asymptomatic controls.
Methods
This is an explorative observational study using an individually matched sample design. CNSLBP patients were compared to asymptomatic controls, individually matched for age, BMI, sex and physical activity as assessed by the Baecke Sportscore20. The study was approved by the ethics committee of the canton of Zurich, Switzerland (2016-00647) and registered at clinicaltrials.gov (NCT02955407). Each participant signed an informed consent prior to be included in the study. All experiments were performed in accordance with national and international guidelines and regulations, and the Declaration of Helsinki. Patients and asymptomatic controls were recruited via the Policlinic for Chiropractic Medicine at the University Hospital Balgrist, Zurich, Switzerland, through personal communication and digital distribution of study information among hospital staff as well as through advertisements at Zurich University, Zurich, Switzerland. Each subject signed an informed written consent before inclusion in the study.
Participants
Eligibility criteria were age between 18 and 65 years and Caucasian origin (due to requirements of NIRS oximetry measurements). Caucasian origin was rated by the clinician conducting the entrance examination, asking about the European descendance of the test subjects’ parents. Exclusion criteria were prior spinal surgery, fractures, inflammation or tumor, any neurological pathologies, radiologically confirmed spinal instability, severe chronic diseases that impact physical activity, osteoporosis or cardiovascular diseases as well as pregnancy. Additionally, patients had to have LBP for more than three months, either continuously or recurrent episodes. Asymptomatic controls were excluded if they had LBP at the time of measurement or more than one episode of LBP lasting for more than one week previously in their life.
Back muscle perfusion and oxygenation
Blood perfusion of three back muscles (M. longissimus, M. multifidus and M. iliocostalis) was assessed using NIRS oximetry [Muscle Oxygen Monitor (Moxy) (Fortiori Design LLC, Minnesota, USA)] during the BSME. In a previous study the Moxy device was found to be more sensitive to changes in muscle oxygenation and perfusion compared to an alternative, therefore it was chosen as the measurement device in this study21. The probes were placed on the right side of the back as recommended by the SENIAM (Surface ElectroMyoGraphy for the NON-Invasive Assessment of Muscles) group (Fig. 1)22. For the M. longissimus, the probe was placed at two finger width laterally from the spinous process of the first lumbar vertebra (L1). For the M. multifidus, the electrode was placed on a line from the caudal tip of the posterior spina iliaca superior to the space between L1 and L2, at the level of the L5 spinous process (i.e. about 2–3 cm from the midline). For the iliocostalis muscle, the probe was placed one finger width medial from the line from the posterior spina iliaca superior to the lowest point of the lower rib, at the level of L2. The positions for the probes were palpated and marked in standing upright position. Because subcutaneous tissue is known to influence NIRS oximetry measurements23, its thickness was measured before the NIRS measurements. The experimenter held the subcutaneous tissue at the NIRS oximetry probe location for the M. longissimus and M. multifidus between two fingers and measured it using a caliper (GPM, Caliper, DKSH Switzerland Ltd, Zurich, Switzerland). Each location was measured three times and the average of the three measurements was used. Measurements above 13 mm resulted in exclusion of the subject from the study, as recommended in the manual provided by the manufacturer24.
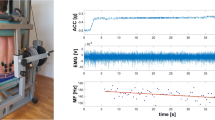
Positioning of the test subject and NIRS oximetry devices during the Biering–Sorensen muscle endurance (BSME) test. (b) Schematic representation of the location of M. longissimus, M. iliocostalis and M. multifidus, (c) positioning of the NIRS oximetry devices in the back, (d) close-up of the NIRS oximeter. (d) Visualization of a test subject performing the BSME test.
Biering–Sorensen muscle endurance test
For the BSME, the subject was lying prone on a treatment table with the pelvic bone on the edge of the table, the arms folded across the chest and the upper part of the body extending the treatment table. A 2D inclinometer (Noraxon U.S.A. INC, Arizona, USA) was placed at the level of the vertebra T7 in order to control the subject’s position during the test. The subject was instructed to hold the upper part of the body horizontally as long as possible. Deviations of more than five degrees from the horizontal testing position were signaled by a tone from the inclinometer. Required adjustments were instructed by the investigator. If the subject deviated from the horizontal position more than twice, the BSME test was stopped. After 240 s, the test was terminated by the investigator25. If subjects terminated early, the time and the reason for early cessation were noted.
Rating of pain intensity
Participants were asked to rate their back pain intensity right before the measurement started on a numerical rating scale (NRS). Ranges from 0 to 10, 0 representing no pain at all and 10 the worst imaginable pain26. It is a valid and reliable tool to assess a patient`s perceived pain in various medical settings26.
Baecke physical activity questionnaire
The Baecke physical activity questionnaire is a self-administered questionnaire about habitual physical activity20. It consists of sixteen items covering three components; work, sport and leisure-time. The items for work and leisure time are scored on a five-point Likert-scale ranging from 1 to 5. One indicating least activity and five indicating most activity. A total score can be calculated by summing up, work index, sport index and leisure index. The items for sport are divided into three sub-levels: time, intensity and proportion of the two most frequently played sports. A simple sport score can be calculated by the sum of intensity, time and proportion and is then converted to a five-point Likert-scale20,27. For subjects who do not perform any sport a rating of 0 is given20. The sport score of the questionnaire was used to assess subjects' physical activity levels and to match LBP subjects and asymptomatic controls.
Systemic physiological measures
Blood pressure and heart rate were measured throughout the experiment non-invasively. The measurement device (SOMNOtouch NIBP SOMNOMedics GmbH, Randersacker, Germany) was attached at the fingertip and wrist. Based on these data the following parameters were calculated: mean arterial pressure (MAP), pulse pressure (PP) and heart rate (HR). MAP is defined as the average of arterial blood pressure during a cardiac cycle28. PP is defined as the difference between the systolic and diastolic blood pressures29.
Experimental protocol
Before subjects were included in the study, they were examined by a chiropractor at the University Hospital Balgrist, Zurich, Switzerland, to check in- and exclusion criteria. Afterwards, the participant laid down on the treatment table prone, with the arms next to the body. Three straps were fixed around the pelvis, the knees and the ankles to avoid rotation of the legs (Fig. 1). The NIRS oximetry devices were attached on the corresponding marked position on the skin after wiping off the marker to avoid light absorption. The BSME was started after a baseline NIRS measurement of 2–3 min. After termination of the BSME, the subject stayed in prone position for another three minutes, during which the NIRS measurements were continued. To maintain a stable position during baseline and post-test measurements, the upper body of the participant was placed on two boxes which had the same height as the treatment table. After the baseline measurements, the treatment table was raised to lift the participants upper body off the boxes. At the end of the BSME test the treatment table was lowered to carry the upper body of the participant again.
Reliability testing
To assess reliability of the NIRS measurements with this study’s set-up, 15 asymptomatic controls performed the BSME twice, following the same experimental protocol as described above, with a time interval of 7 days between the two measurements (maximal difference in time of day: 1 h).
Data analysis
Muscle oxygenation was measured as the absolute tissue oxygen saturation (SmO2, measured in %); muscle perfusion was inferred from changes in total hemoglobin (tHb). SmO2 reflects the ratio between oxygen supply and consumption on the muscle level and is considered an accurate quantitation of oxygen changes in the muscle30, while tHb reflects the sum of the absolute concentrations of oxygenated hemoglobin (O2Hb) and deoxygenated hemoglobin (HHb) (tHb = O2Hb + HHb)30, which indicates changes in tissue blood volume and thus is influenced at short term only by perfusion. NIRS oximetry measurement data were processed by first interpolating missing values, resampling to 2 Hz and low-pass filtering by locally weighted regression fitting using a 2nd order polynomial (LOESS, window length (w) = 10 s). The following parameters were calculated for SmO2 and well as for tHb, based on changes relative to baseline, for SmO2 and well as tHb: area under the curve (AUC) for the whole trial (i.e. start until end of BSME), Median 1 (i.e. first minute after trial start to the second minute after trial start [duration 1 min]), Median 2 (i.e. time from the second minute after trial start until the end of the trial [variable duration across subjects]) and Median 3 (i.e. 10 s before the end of task to end of task [10 s]). The recovery time of SmO2 and tHb was defined as the time elapsed to reach 63.2% (corresponding to the exponential constant 1/e) of the post BSME maximum value. It was calculated by a non-linear regression analysis and a fitted exponential function. The calculated regression coefficient was used to define the time constant in seconds.
The NIRS data of the healthy controls who did the procedure twice were statistically analysed for test–retest reliability using the intraclass correlation coefficient (ICC)31,32,33 based on a 2-way mixed effects, single measurement, absolute agreement model34. Values of less than 0.5 were considered poor, between 0.5 and 0.75 moderate, and greater 0.75 excellent reliability34. Only measures with excellent reliability were kept for further analyses. Before subjecting the measures with excellent reliability to further analyses, they were corrected for subcutaneous tissue thickness, which differed significantly between groups and was significantly related to the NIRS measures. This was achieved by linear regression with NIRS measures as dependent variable and subcutaneous tissue thickness as independent variable and keeping the unstandardized residuals (termed in the following ‘corrected’). Clinical and demographic characteristics as well as BSME holding times and corrected NIRS measures of the CNSLBP patients and their matched controls were descriptively analyzed and compared using unpaired t-tests35. In addition, two-way ANCOVAs using corrected NIRS measures as dependent variables, gender and group as independent variables and BMI and age as covariates were performed to test whether there was an effect of group when gender, BMI and age were controlled for. Because a pain rating above 2 was used as an inclusion criteria in another study on BSME and NIRS in CNSLBP13, the group comparisons were repeated with a reduced sample of patients with pain intensity ratings above 2 at the day of examination and their matched asymptomatic controls. To assess the importance of possible influencing factors on BSME holding times as dependent variable, the following parameters were included as independent variables into a multiple regression model: muscle oxygenation (corrected SmO2), physical activity scores, BMI, pain intensity, MAP, PP and HR (continuous variables) as well as sex and group (LBP/asymptomatic controls) (categorical variables).
Data were collected and stored in the research electronic data capture (REDCap Version 9.6.1). The statistical analysis was performed using IBM SPSS Statistics (Version 26) statistical software package (SPSS, Inc., Chicago, IL, USA). The significance level was set for all analyses to α = 0.05.
Results
Reliability
15 asymptomatic controls (8 females, 7 males), mean age 35 years (SD 15 years), were included in the reliability study. Excellent reliability was found for SmO2 in the M. longissimus and M. multifidus (ICC = 0.87–0.99) for AUC, Median 1, Median 2 and Median 3 (Table 1). In contrast, poor reliability was found for SmO2 (AUC, Median 1, Median 2, Median 3) in the M. iliocostalis (ICC = 0.00–0.38). Data for M. iliocostalis were lacking in four subjects due to restricted space on the subjects' back or poor quality of the data. THb was found to have poor to moderate reliability in all examined muscles for AUC, Median 1, Median 2 and Median 3 (ICC = 0.11–0.72) and was therefore excluded from further analysis. The ICC for recovery time could only be calculated for SmO2 of the M. longissimus and M. multifidus because data for M. iliocostalis and tHb in general was often missing due to poor quality of the data. The reliability for these measures was poor (ICC = 0.25 and − 0.22).
Main study
Demographic and clinical characteristics
142 participants were recruited for the main study. 26 of the CNSLBP patients were excluded because they were not able to perform the BSME test (e.g. due to high blood pressure) or because the subcutaneous tissue thickness exceeded 13 mm (n = 3). The asymptomatic subjects matched to these patients were also excluded to keep the matching pattern. Thus, the final study sample consisted of 90 participants; 45 CNSLBP patients and their individually-matched 45 asymptomatic controls. The groups demonstrated no differences in demographic and clinical characteristics, except for the BSME holding time, pain intensity and subcutaneous tissue thickness (Table 2). 17 of the asymptomatic subjects were able to maintain the BSME position for the maximum of 240 s, whereas only 7 of the CNSLBP patients completed the maximum.
Group comparison
Qualitatively, the following time course of muscle oxygenation was observed for the M. longissimus in asymptomatic controls and CNSLBP patients: during the resting baseline period muscle oxygenation was stable. At the start of the BSME test, there was a rapid decrease of oxygenation reaching a plateau with only small variations in oxygenation. After cessation of the BSME test, muscle oxygenation increased rapidly and baseline levels were reached approximately 2 min after the end of the BSME test (Fig. 2a,b). Oxygenation of the M. multifidus in many asymptomatic subjects changed only very little during baseline, BSME test and recovery (Fig. 2a). Oxygenation in the M. multifidus in many CNSLBP patients seemed to decrease somewhat during the BSME test and to slowly increase after cessation of the test (Fig. 2b).
Muscle oxygenation of M. longissimus and M. multifidus of a representative asymptomatic subject (a) and a representative CNSLBP patient (b) before (0–2 min), during (2 to maximal 6 min) and after execution of the Biering–Sorensen muscle endurance (BSME) test. The green lines indicate the start and the end of the BSME test.
There was no statistically significant difference between patients and controls in oxygenation of the M. longissimus or M. multifidus, irrespective of whether age, gender and BMI were controlled for in the analysis (ANCOVA) or not (unpaired t-test, Table 3).
To reproduce the analysis of Kell and Bhambhani13, only patients with pain intensity above 2 and the matched asymptomatic controls were included in a second analysis. This resulted in a reduced patient sample, which did not differ from the full sample with respect to any of the demographic or clinical variables, except for pain intensity (P 0.01). The reduced sample consisted of 24 CNSLBP subjects (mean age 30.88 (SD 9.24), 17 females, 7 males) and 24 asymptomatic controls (age 29.92 (SD 9.63), 17 females, 7 males)). Again, there were no statistically significant group differences in any of the NIRS parameters of the M. longissimus or M. multifidus in the unpaired t-test (Table 4) or the ANCOVA.
Multiple regression
The influence of muscle oxygenation of the M. longissimus and the M. multifidi (corrected SmO2) on BSME holding times was tested, in addition to the influence of sex, physical activity, BMI, group (LBP/asymptomatic controls), MAP, PP, HR and pain intensity. Because the residuals of the medians were highly correlated (Pearson correlation coefficients ≥ 0.7), separate models were calculated for each median. The model for median 1 showed a statistical trend (F(11,38) = 1.89, P = 0.072). The model for median 3 was statistically significant, the model for median 2 was neither statistically significant and did not show a trend. The amount of variance explained was around 37%, with significant predictions from the sport score and BMI (shown for median 3 in Table 5). In none the models did the muscle oxygenation parameters or systemic physiological measures significantly influence BSME holding times.
Power considerations
None of the SmO2 measures showed any significant difference between the groups in the present study. Previous studies examining differences in NIRS parameters between LBP patients and controls had relatively small sample sizes [29 (12 healthy/17 CLBP patients) and 68 (34 healthy/34 CLBP patients)]3,6. The sample of the current study was based on these previous studies with the goal of generously exceeding it. With a sample size of 90 (45 per group), a significance level of alpha = 0.5, and an observed effect size of 0.33, a power (1-beta) of 0.8 was reached (G*Power, Dusseldorf, Germany36).
Discussion
The first aim of this study was to compare BSME holding times as well as muscle perfusion and oxygenation during the BSME test between CNSLBP patients and asymptomatic controls individually matched for age, BMI, and physical activity. The second aim was to investigate the influence of muscle oxygenation, systemic physiological measures and subcutaneous tissue thickness on BSME holding times. Beforehand, reliability of the NIRS measurements was assessed in a separate sample to use the most reliable measures for analysis of the main study. The most reliable measures, achieving ‘excellent reliability’34, were muscle oxygenation of the M. longissimus and M. multifidi. Measures of the iliocostalis muscle were excluded from further analysis because of poor reliability and a considerable amount of missing data, in some instances due to space restrictions on the subjects’ back or because the data acquisition of the probe was arguable due to flatlined read-out during measurement. Muscle perfusion showed poor to moderate reliability for all three muscles and was therefore also excluded from further analyses34.Thus, only the muscle oxygenation data of M. longissimus and M. multifidi were used for further statistical analysis. Despite a statistically significant difference in BSME holding times between CNSLBP patients and asymptomatic controls, there was no difference in muscle oxygenation between the groups. This finding was confirmed in a sub-sample of CNSLBP patients with a pain intensity above 2. NIRS measurements were influenced by subcutaneous tissue thickness, which was addressed by correcting SmO2 for subcutaneous tissue thickness before using it in further analyses. Multiple regression analysis revealed that BSME holding times were influenced by BMI and sport scores, but not by muscle oxygenation.
Reliability
Kell et al. observed excellent reliability during the BSME for back muscle oxygenation and perfusion (ICC 0.96 and 0.95)37. The present study supports these findings by showing excellent reliability of muscle oxygenation in M. longissimus and M. multifidus. In contrast, the reliability of muscle perfusion was poor to moderate. Several differences between the previous reliability study and the present one might contribute to this discrepancy. Body and muscle composition, which itself is influenced by age and sex, is known to influence NIRS measurements30,38,39. Kell et al. only measured males, whereas the present sample was mixed. In addition, the sample in the present study was on average 7.6 years older. The technical set-up also differed slightly: in Kell et al., the probes were placed bilaterally at the height of L3 without specifying the underlying muscles. In contrast, the present study adhered to the SENIAM guidelines that were developed for the placement of surface electromyography22 and measured M. longissimus, M. multifidus and M. iliocostalis separately within an area from L5 to L1. Given that the oxygenation of the M. longissimus and the M. multifidus behaved differently, this approach seems justified. The poor reliability of the M. iliocostalis is in line with other surface measurement modalities40,41. These findings might generally be related to reduced reliability of palpation of M. iliocostalis because of e.g. variation of orientation42 and possibly missing the muscle when placing the NIRS probe. Furthermore, the present study used different parameters for analysis, i.e. AUC and medians. These measures are more robust with respect to outliers compared to using simply the minima and maxima as in Ref.43. Taken together, it seems important that reliability measurements are conducted in a sample of similar age and sex distribution and with an identical technical set-up as in the research study itself, as was the case here.
Main study
The findings of a shorter BSME holding time in CNSLBP patients is in line with current literature as the BSME is proposed to discriminate well between LBP subjects and asymptomatic controls10. However, none of the NIRS oximetry parameters that were found to be reliable differed between patients and controls. This was true irrespective of whether age, gender and BMI were corrected for or not. In accordance with this finding, none of the muscle oxygenation parameters explained a significant amount of variance of BSME holding times. These findings are identical to a previous study by McKeon et al.11, although in that study no matching for physical activity between patients and controls was performed. However, the results of the present study and the study by McKeon et al. conflict with the study by Kell and Bhambhani that investigated the BSME and back muscle oxygenation/perfusion in CNLBP patients13. In that study, a difference in recovery time was observed between healthy subjects and two groups of CNSLBP patients (active and sedentary)13. Surprisingly, BSME holding times did not differ in the study by Kell and Bhambhani, in contrast to a large body of literature, including one review12,14,16,25. A difference between the study by Kell and Bhambhani and the present one was that patients had to have pain greater than 2/10. Because this was not an inclusion criterion in the present study, the analysis was repeated using a sub-sample of patients with pain greater than 2/10. Similar to the full sample, the reduced patient sample had shorter BSME holding times and showed no difference in the NIRS oximetry parameters compared to the controls, thereby still not replicating the previous findings. It has to be noted, however, that recovery time was not analyzed in the present study, because its reliability was not found to be high enough (reliability was not assessed in the study by Kell and Bhambhani). This reiterates the importance of incorporating reliability measurements of the exact experimental set-up in NIRS muscle oximetry studies.
So how can reduced BSME holding times in NSCLBP patients be explained, when back muscle oxygenation does not differ from that of controls? Because physical activity levels are known to influence the holding capacity of the lumbar extensor muscles18, but are typically not assessed and/or not considered in the analysis of NIRS muscle oximetry studies of CLBP patients3,4,5,6,11,13, this study individually matched also for activity levels. We found a relationship between activity levels and BSME holding times in the regression analysis, but NIRS oximetry parameters did not differ between patients and controls. Additionally, we used systemic physiological measures in our regression models, but were not correlated with BSME holding times. Only a small percentage of variance was explained by the variables used in the regression analysis.
This points to two conclusions: First, BSME holding times are reduced in NSCLBP patients because of some other factor that was not measured in the present study, such as reduced motivation17,44 or different recruitment of muscles beyond back extensors10,45. Second, it appears unlikely that altered back muscle oxygenation and subsequent accumulation of metabolites is a major source of pain in patients with CNSLBP.
Two more aspects have to be kept in mind, when working with NIRS muscle oximetry measurements in general. First, measurements are influenced by the subcutaneous tissue thickness of the test subjects23,46,47. The present study adhered to the recommendation of the manufacturer, and only included test subjects with a subcutaneous tissue thickness of less than 13 mm. Nevertheless, there was a significant influence of tissue thickness on NIRS measures. It can therefore be concluded that it is not sufficient to adhere to the manufacturer’s guidelines. Interestingly, although patients and controls were matched for BMI, subcutaneous tissue thickness was greater in patients than in controls and it was therefore important to control the NIRS measures statistically for tissue thickness. Second, NIRS oximetry measurements also depend on the measurement device21. Both studies which preceded the present study used different NIRS devices11,13, possibly contributing to the divergent findings.
Limitations
In this study physical activity was assessed using a self-reported questionnaire, which might not reflect the real physical activity status of participants48. Additionally, the age of the participants was relatively young. Because muscle perfusion changes with age49, it is conceivable that differences in back muscle perfusion might only become apparent in older patients with CNSLBP. Furthermore, the SENIAM guidelines were developed for the use of electrode placements of surface electromyography and have not been validated for the use of NIRS probes. Lastly, because of the poor reliability of the NIRS measures of the M. iliocostalis, we cannot comment on any potential group differences of NIRS measures in this muscle.
Conclusion
NIRS oximetry measures of the M. longissimus and M. multifidus were found to be reliable measures during the BSME test. When comparing patients with CNSLBP to an individually-matched control sample, no difference in the oxygenation of back muscles during the BSME was detected. Thus, at this point, insufficient oxygenation during back muscle endurance does not seem to be a major pain mechanism in relatively young patients with CNSLBP. Furthermore, muscle oxygenation and systemic physiological measures did not influence BSME holding times. Therefore, future investigation should ensure to evaluate motivational determinants of BSME holding times as well as the recruitment of auxiliary muscles.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Maher, C., Underwood, M. & Buchbinder, R. Non-specific low back pain. The Lancet 389(10070), 736–747 (2017).
Golob, A. L. & Wipf, J. E. Low back pain. Med. Clin. N. Am. 98(3), 405–428 (2014).
Kankaanpaa, M. et al. Back extensor muscle oxygenation and fatigability in healthy subjects and low back pain patients during dynamic back extension exertion. Pathophysiology 12(4), 267–273 (2005).
Kovacs, K. M. et al. Localized oxygen use of healthy and low back pain individuals during controlled trunk movements. J. Spinal Disord. 14(2), 150–158 (2001).
Olivier, N. et al. An exercise therapy program can increase oxygenation and blood volume of the erector spinae muscle during exercise in chronic low back pain patients. Arch. Phys. Med. Rehabil. 94(3), 536–542 (2013).
Kell, R. T. & Bhambhani, Y. In vivo erector spinae muscle blood volume and oxygenation measures during repetitive incremental lifting and lowering in chronic low back pain participants. Spine 31(22), 2630–2637 (2006).
Light, A. R. et al. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X and TRPV1. J. Neurophysiol. 100, 1184–1201 (2008).
Pollak, K. A. et al. Exogenously applied muscle metabolites synergistically evoke sensations of muscle fatigue and pain in human subjects. Exp. Physiol. 99(2), 368–380 (2014).
Villafane, J. H. et al. Validity and everyday clinical applicability of lumbar muscle fatigue assessment methods in patients with chronic non-specific low back pain: A systematic review. Disabil. Rehabil. 38(19), 1859–1871 (2016).
Demoulin, C. et al. Spinal muscle evaluation using the Sorensen test: A critical appraisal of the literature. Jt. Bone Spine 73(1), 43–50 (2006).
McKeon, M. D., Albert, W. J. & Neary, J. P. Assessment of neuromuscular and haemodynamic activity in individuals with and without chronic low back pain. Dyn. Med. 5, 6 (2006).
Latimer, J. et al. The reliability and validity of the Biering–Sorensen test ins asymptomatic subjects and subjects reporting current or previous nonspecific low back pain. Spine 24(20), 2085–2090 (1999).
Kell, R. T. & Bhambhani, Y. Relationship between erector spinae static endurance and muscle oxygenation-blood volume changes in healthy and low back pain subjects. Eur. J. Appl. Physiol. 96(3), 241–248 (2006).
Moreau, C. E. et al. Isometric back extension endurance tests: A review of the literature. J. Manip. Physiol. Ther. 24(2), 110–122 (2001).
Kankaanpaa, M. et al. Age, sex and body mass index as determinants of back and hip extensor fatigue in the isometric Sorensen back endurance test. Arch. Phys. Med. Rehabil. 79(9), 1069–1075 (1998).
Süüden, E. et al. Low back muscle fatigue during Sorensen endurance test in patients with chronic low back pain: Relationship between electromyographic spectral compression and anthroppometric characteristics. Electromyogr. Clin. Neurophysiol. 48(3–4), 185–192 (2008).
Applegate, M. E. et al. Determining physiological and psychological predictors of time to task failure on virtual reality Sorensen test in participants with and without recurrent low back pain: Exploratory study. JMIR Serious Games 6(3), e10522 (2018).
Applegate, M. E. et al. Sorensen test performance in participants with and without recurrent low back pain. J. Electromyogr. Kinesiol. 44, 1–7 (2019).
Vrana, A. et al. Changes in spinal muscle oxygenation and perfusion during Biering-Sorensen test: Preliminary results of a study employing NIRS-based muscle oximetry. In Oxygen Transport to Tissue XL. Advances in Experimental Medicine and Biology (eds Thews, O. et al.) 103–109 (Springer, 2018).
Baecke, J. A., Burema, J. & Frijters, J. E. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am. J. Clin. Nutr. 36(5), 936–942 (1982).
Scholkmann, F. & Scherer-Vrana, A. Comparison of two NIRS tissue oximeters (moxy and nimo) for non-invasive assessment of muscle oxygenation and perfusion. In Oxygen Transport to Tissue XLI, Advances in Experimental Medicine and Biology (eds Ryu, P. et al.) 253–259 (Springer, 2020).
Hermens, H. & Freriks, B. Surface ElectroMyoGraphy for the Non-invasive Assessment of Muscles (The SENIAM Project) (2016).
Niemeijer, V. M. et al. The influence of adipose tissue on spatially resolved near-infrared spectroscopy derived skeletal muscle oxygenation: The extent of the problem. Physiol. Meas. 38(3), 539–554 (2017).
LLC, F.D. The Science Behind Moxy (2019). https://www.moxymonitor.com/ (Accessed 13 July 2021).
Biering–Sorensen, F. Physical measurements as risk indicators for low-back trouble over a one-year period. Spine 9(2), 106–119 (1984).
Hjermstad Jensen, M. et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: A systematic literature review. J. Pain Symptom Manage. 41(6), 1073–1093 (2011).
Ono, R. et al. Reliability and validity of the Baecke physical activity questionnaire in adult women with hip disorders. BMC Musculoskelet. Disord. 8, 8 (2007).
DeMers, D. & Wachs, D. Physiology, Mean Arterial Pressure (2021). https://www.ncbi.nlm.nih.gov/books/NBK538226/ (Accessed 15 May 2021).
Homan, T., Bordes, S. & Cichowski, E. Physiology, Pulse Pressure (2021) https://www.ncbi.nlm.nih.gov/books/NBK482408/ (Accessed 15 April 2021).
Ferrari, M., Muthalib, M. & Quaresima, V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: Recent developments. Philos. Trans. R. Soc. 2011(369), 4577–4590 (1955).
Shrout, P. E. & Fleiss, J. L. Intraclass correlation: Uses in assessing rater reliability. Psychol. Bull. 86(2), 420–428 (1979).
McGraw, K. O. & Wong, S. P. Forming interferences about some intraclass correlation coefficients. Psychol. Methods 1(1), 30–46 (1996).
De Vet, H. C. et al. Measurement in Medicine (Cambridge University Press, 2011).
Koo, T. K. & Li, M. Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropract. Med. 15(2), 155–163 (2016).
Wilcoxon, F. Individual comparison by ranking methods. Biometr. Bull. 1(6), 80–83 (1945).
Faul, F. et al. G*Power 3: A flexibel statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 29(2), 175–191 (2007).
Kell, R. T., Farag, M. & Bhambhani, Y. Reliability of erector spinae oxygenation in blood volume responses using near-infrared spectroscopy in healthy males. Eur. J. Appl. Physiol. 91(5–6), 499–507 (2004).
Mannion, A. F. et al. Fibre type characteristics of the lumbar paraspinal muscles in normal healthy subjects and in patients with low back pain. J. Orthop. Res. 15(6), 881–887 (1997).
Mazis, N. et al. The effect of different physical activity levels on muscle fibre size and type distribution of lumbar multifidus. A biopsy study on low back pain patient groups and healthy control subjects. Eur. J. Phys. Rehabil. Med. 4, 459–4567 (2009).
Mohseni Bandpei, M. A. et al. Reliability of surface electromyography in the assessment of paraspinal muscle fatigue: An updated systematic review. J. Manip. Physiol. Ther. 37(7), 510–521 (2014).
Farragher, J. et al. Reliability of lumbar multifidus and iliocostalis lumborum thickness and echogenicity measurements using ultrasound imaging. Australas. J. Ultrasound Med. 24(3), 151–160 (2021).
Macintosh, J. E. & Bogduk, N. The morphology of the lumbar erector spinae. Spine 12(7), 658–668 (1987).
Leys, C. et al. Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 49(4), 764–766 (2013).
Mannion, A. F. et al. The relationship between psychological factors and performance one the Biering-Soerensen back muscle endurance test. Spine J. 11(9), 849–857 (2011).
Muller, R., Strassle, K. & Wirth, B. Isometric back muscle endurance: An EMG study on the criterion validity of the Ito test. J. Electromyogr. Kinesiol. 20(5), 845–850 (2010).
Hamaoka, T. & McCully, K. K. Review of early development of near-infrared spectroscopy and recent advancement of studies on muscle oxygenation and oxidative metabolism. J. Physiol. Sci. 69(6), 799–811 (2019).
Craig, J. C. et al. Effect of adipose tissue thickness, muscle site, and sex on near-infrared spectroscopy derived total-[hemoglobin + myoglobin]. J. Appl. Physiol. 123(6), 1571–1578 (2017).
Marshall, G. The purpose, design and administration of a questionnaire for data collection. Radiography 11(2), 131 (2005).
Adlenia, F. et al. The role of muscle perfusion in the age-associated decline of mitochondrial function in healthy individuals. Front. Physiol. 10, 427 (2019).
Acknowledgements
The authors thank Sandra Rosser for her assistance during data acquisition.
Funding
Salaries of AV, AL and BW were partly supported by the Swiss Association of Chiropractic.
Author information
Authors and Affiliations
Contributions
A.L. analyzed data and wrote the manuscript. B.W. designed the study, helped with data analysis and wrote the manuscript, A.S.V. designed the study and acquired data. F.R. acquired data. K.G. conducted the reliability study. P.V. designed the study and supported data analysis. B.K.H. designed the study and supervised data acquisition. F.S. analyzed near-infrared spectroscopy raw data, supported the data analysis and reviewed the manuscript critically. M.F. designed the study and supervised data acquisition. P.S. supervised data analysis and wrote manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Langenfeld, A., Wirth, B., Scherer-Vrana, A. et al. No alteration of back muscle oxygenation during isometric exercise in individuals with non-specific low back pain. Sci Rep 12, 8306 (2022). https://doi.org/10.1038/s41598-022-11683-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11683-x
- Springer Nature Limited