Abstract
Streptococcus mutans, a major pathogen of dental caries, is also known as a causative agent of cardiovascular disease. A 120 kDa collagen-binding protein (Cnm) of S. mutans is an important contributor to the pathogenicity of cardiovascular disease. Although dead bacteria have been detected in cardiovascular specimens by molecular biological methods, the pathogenicity of the bacteria remains unknown. Here, we analyzed the pathogenicity of killed S. mutans by focusing on collagen-binding ability and the effects on silkworms. In live S. mutans, Cnm-positive S. mutans had high collagen-binding activity, while Cnm-negative S. mutans had no such activity. After treatment with killed Cnm-positive S. mutans, amoxicillin-treated bacteria still had collagen-binding ability, while lysozyme-treated bacteria lost this ability. When live and amoxicillin-treated S. mutans strains were administered to silkworms, the survival rates of the silkworms were reduced; this reduction was more pronounced in Cnm-positive S. mutans infection than in Cnm-negative S. mutans infection. However, the administration of any of the lysozyme-treated bacteria did not reduce the survival rate of the silkworms. These results suggest that amoxicillin-killed Cnm-positive S. mutans strains maintain collagen-binding properties and pathogenicity in the silkworm model, and are possibly associated with pathogenicity in cardiovascular diseases.
Similar content being viewed by others
Introduction
The oral cavity contains not only live bacteria, but also bacteria that have been killed by the administration of antibiotics or by antimicrobial substances in saliva1,2. Live and dead bacteria present in the oral cavity can infiltrate the bloodstream when bleeding occurs following invasive dental treatment or daily tooth brushing1. Such bacterial invasion into the blood can induce cardiovascular diseases such as infective endocarditis (IE) or arteriosclerosis when bacterial adhesion occurs because of bacterial infections on the vascular walls, especially under abnormal conditions3,4. In Gram-positive bacteria, the adhesion properties of live bacteria are considered to be an important risk factor for IE5,6,7, while the pathogenicity of dead bacteria is not well understood.
Streptococcus mutans, a major pathogen of dental caries, is also known as a causative agent of IE8. Cnm, a 120 kDa collagen-binding protein, is expressed on the cell surface of S. mutans at a frequency of approximately 10–20% in bacteria isolated from the oral cavity9,10. Cnm-positive S. mutans strains can adhere to the vascular wall by virtue of their collagen-binding properties11, which are closely associated with the pathogenicity of IE. It has also recently been reported that Cnm-positive S. mutans strains are frequently isolated from the oral cavity of patients with some cerebrovascular diseases, such as cerebral microbleeds and intracerebral hemorrhage12,13.
A blood culture method is widely used for culturing live bacteria that cause IE4. Recently, molecular biological methods that can detect the bacterial DNA of live bacteria as well as dead bacteria have been developed4. Previous studies reported that bacterial DNA of S. mutans was frequently detected in extirpated heart valve specimens from patients with IE using molecular biological methods, even when no live S. mutans was isolated from the patients by the blood culture method14. Additionally, genes encoding collagen-binding proteins were frequently detected in these S. mutans-positive heart valve specimens15. These results led us to hypothesize that dead S. mutans may be a possible virulence factor for cardiovascular diseases. In the present study, we analyzed the pathogenicity of Cnm-positive and Cnm-negative S. mutans strains killed by amoxicillin, a major antibiotic used for the prevention of IE, and lysozyme, an antimicrobial substance in saliva and serum, using a collagen-binding assay and a silkworm model.
Results
Morphological evaluation of live S. mutans and killed S. mutans (treated with amoxicillin or lysozyme)
We used S. mutans strain TW295, a Cnm-positive strain isolated from individuals with bacteremia after tooth extraction, TW295CND, a Cnm-defective isogenic mutant strain of TW295, and TW295comp, a Cnm-complemented mutant strain of TW295. The killed S. mutans strains were prepared by treatment with either amoxicillin or lysozyme. Scanning electron microscopy (SEM) images showed slight damage in the cell surface layer of S. mutans treated with amoxicillin (Fig. 1A), and transmission electron microscopy (TEM) images showed abnormal changes in the cytoplasm of the bacteria (Fig. 1B). After the lysozyme treatment, S. mutans was lysed in SEM and TEM images (Fig. 1A,B).
Representative electron microscopy images of live S. mutans, amoxicillin-killed S. mutans, and lysozyme-killed S. mutans. (A) Scanning electron microscopy images. Scale bar, 500nM. White and black arrowheads indicate damage to the bacterial cell surface layer and lysis of the bacteria, respectively. (B) Transmission electron microscopy images. Scale bar, 500 μm. White and black arrowheads indicate abnormal changes in the cytoplasm and lysis of the bacteria, respectively.
Collagen-binding activity of live S. mutans, and killed S. mutans (treated with amoxicillin or lysozyme)
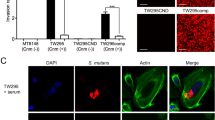
In live S. mutans, TW295 displayed high collagen-binding activity. This binding activity was dependent on Cnm expression, as shown by the lack of binding by TW295CND, and the recovered binding demonstrated by TW295comp (Fig. 2A,B). After amoxicillin treatment, collagen-binding ability was also observed in the Cnm-positive strains, although the collagen-binding ability was lower than that of live bacteria. In contrast, the collagen-binding ability of all these bacteria was lost following lysozyme treatment.
Collagen-binding properties of live S. mutans, amoxicillin-killed S. mutans, and lysozyme-killed S. mutans. (A) Collagen-binding rates of S. mutans strains. Significant differences were observed using analysis of variance with Bonferroni correction (**P < 0.01 and ***P < 0.001). (B) Representative confocal laser scanning microscopy images of S. mutans binding to collagen. Bacterial cells are stained red. Scale bar, 50 μm.
Silkworm larvae virulence assay with different doses of live S. mutans
Silkworm (Bombyx mori) larvae have similar susceptibility to bacterial infections as humans, and have been used to assess the pathogenicity of bacteria16. As a quantitative analysis, different amounts of live S. mutans (1 × 105 colony-forming units [CFU], 1 × 106 CFU, and 1 × 107 CFU) were administered to the silkworms. When 1 × 105 CFU and 1 × 106 CFU of TW295 were administered, silkworm mortality commenced late in the experimental period, reaching 50% at 120 h (Fig. 3A). When 1 × 107 CFU of TW295 was administered, silkworm mortality commenced at 12 h after the start of the experiment and reached 50% within 72 h. When 1 × 105 CFU and 1 × 106 CFU of the TW295CND was administered, 50% mortality has not been reached at the end of the experimental period (Fig. 3B). When 1 × 107 CFU of TW295CND was administered, 50% mortality was reached 84 h after the start of the experiment, which was later than that of TW295 for the same dose of bacteria. TW295comp administration resulted in a similar survival curve of mortality as TW295 administration for each dose of bacteria (Fig. 3C).
Survival curve for silkworm larvae infected with different doses of live S. mutans. Silkworm larvae infected with (A) TW295 (Cnm +), (B) TW295CND (Cnm−), and (C) TW295comp (Cnm +). Survival rates in each group were evaluated in a Kaplan–Meier plot, which was analyzed by a log-rank test. *P < 0.05 and ***P < 0.001 versus phosphate-buffered saline group; #P < 0.05, ##P < 0.01, and ###P < 0.001 versus 1 × 107 CFU of S. mutans administration group.
Silkworm larvae virulence assay of live S. mutans and killed S. mutans (treated with amoxicillin or lysozyme)
Live S. mutans and killed S. mutans treated with amoxicillin or lysozyme were all adjusted to 1 × 107 CFU and administered to silkworms. The silkworms administered with amoxicillin-killed TW295 showed delayed mortality compared with those administered with live bacteria (Fig. 4A). However, the survival rate of silkworms administered with amoxicillin-treated TW295 was significantly lower than that of silkworms administered with phosphate buffered saline (PBS) (P < 0.001), and 50% of the silkworms died 84 h after the start of the experiment. In contrast, administration of lysozyme-killed TW295 did not reduce the survival rate of the silkworms. TW295CND administration also resulted in a significant decrease in survival rate in the groups treated with live and amoxicillin-killed bacteria compared with the control group (P < 0.05) (Fig. 4B). However, the survival rate of silkworms treated with TW295CND was higher than that of silkworms treated with TW295 for both live and amoxicillin-killed bacteria. Administration of lysozyme-killed TW295CND did not reduce the survival rate of silkworms. Furthermore, TW295comp administration restored the virulence of the silkworms in both live and amoxicillin-killed bacteria compared with those administered with TW295CND (Fig. 4C).
Survival curve for silkworm larvae administered with live S. mutans, amoxicillin-killed S. mutans, and lysozyme-killed S. mutans. Silkworm larvae infected with 1 × 107 CFU of (A) TW295 (Cnm +), (B) TW295CND (Cnm−), and (C) TW295comp (Cnm +). Survival rates in each group were evaluated in a Kaplan–Meier plot, which was analyzed by a log-rank test. *P < 0.05, **P < 0.01 and ***P < 0.001 versus phosphate-buffered saline group; #P < 0.05, ##P < 0.01, and ###P < 0.001 versus live S. mutans administration group.
Histopathological evaluation of silkworms administered with live S. mutans and killed S. mutans (treated with amoxicillin or lysozyme)
To confirm the presence of S. mutans in silkworm tissues after administration of the bacteria, histopathological evaluation was performed by preparing tissue sections from euthanized silkworms. In silkworms administered with live TW295, bacteria were found in each organ except the cocoon gland, and the highest numbers of bacteria were found in the intestinal tract (Fig. 5A,B). Silkworms administered with amoxicillin-killed TW295 had fewer bacteria than silkworms administered with live TW295, but bacteria were confirmed in all organs. In contrast, no bacteria were found in any of the organs of silkworms administered with lysozyme-killed TW295. In silkworms treated with live TW295CND, bacteria were found in all organs except the cocoon gland. However, silkworms treated with amoxicillin-killed TW295CND showed only a few bacteria in the intestinal tract and interstitial tissue, and those treated with lysozyme-killed TW295CND had no bacteria in any organ. Furthermore, bacteria were detected in all organs except the cocoon gland in silkworms treated with live and amoxicillin-killed TW295comp, whereas no bacteria were detected in any organ when lysozyme-killed bacteria were administered. Histopathological evaluation of each organ of the silkworms, except for bacterial localization, is shown in Supplementary Tables 1–3.
Histopathological evaluation of silkworm larvae administered with live S. mutans, amoxicillin-killed S. mutans, and lysozyme-killed S. mutans. (A) Scoring for the localization of bacteria in tissue sections of each organ. Significant differences were observed using analysis of variance with Bonferroni correction (*P < 0.05, **P < 0.01, and ***P < 0.001). (B) Representative histopathological images following Gram staining of tissue sections of the intestinal tract from silkworms euthanized 72 h after S. mutans administration. Lower panels show high-magnification images of the boxes on the upper images. White arrowheads indicate bacterial masses. Bar = 1 mm (upper images) and bar = 100 μm (lower images).
Collagen-binding activity and silkworm larvae virulence assay of live and killed S. mutans clinical isolates
The collagen-binding activity of 10 Cnm-positive and 10 Cnm-negative S. mutans clinical isolates was evaluated. The Cnm-negative group had almost no collagen-binding activity in live S. mutans, amoxicillin-killed S. mutans, or lysozyme-killed S. mutans (Fig. 6A). In contrast, high collagen-binding rates were observed in live bacteria in the Cnm-positive group, whose collagen-binding rate was significantly higher than that of the Cnm-negative group (P < 0.001). Collagen-binding activity was also observed in the amoxicillin-killed Cnm-positive group, but not in the lysozyme-killed group.
Collagen-binding properties and survival curve for silkworm larvae induced by live S. mutans clinical isolates and killed S. mutans clinical isolates (treated with amoxicillin or lysozyme). (A) Collagen-binding rates of S. mutans strains. Each closed circle represents the mean value for each bacterial strain. Significant differences were observed using analysis of variance with Bonferroni correction (***P < 0.001). Silkworm larvae infected with (B) Cnm-negative clinical isolates, and (C) Cnm-positive clinical isolates. Survival rates in each group were evaluated in a Kaplan–Meier plot, which was analyzed by a log-rank test. **P < 0.01 and ***P < 0.001 versus phosphate-buffered saline group; ##P < 0.01 and ###P < 0.001 versus live S. mutans administration group. (D) Correlation between collagen-binding rates and survival rates of silkworms analyzed by regression analysis. Each point represents the mean value for each bacterial strain.
The live bacteria in the Cnm-negative group killed half of the silkworms in 84 h, but more than 70% and 80% of the amoxicillin-killed and lysozyme-killed silkworms survived throughout the experiment, respectively (Fig. 6B). The live and amoxicillin-killed bacteria in the Cnm-positive group killed more than half of the silkworms in 60 h (Fig. 6C). However, the lysozyme-killed bacteria did not show any decrease in survival rate compared with the control. In these clinical strains, there was a correlation between collagen-binding activity and reduced survival rate of the silkworms (P < 0.001) (Fig. 6D).
Discussion
S. mutans is involved in the development of cardiovascular diseases such as IE and intracerebral hemorrhage, when they infiltrate into the bloodstream from the oral cavity8,17. Although bacterial adherence of S. mutans to cardiac tissue is important for the development of cardiovascular disease18, it remains unknown whether dead S. mutans have these adhesive properties. In the present study, we found that the collagen-binding ability and the adhesion and virulence to silkworms were present in bacteria killed by amoxicillin treatment, especially Cnm-positive S. mutans. In contrast, lysozyme treatment was found to be effective in eliminating the virulence of all S. mutans strains.
First, we killed S. mutans strains with amoxicillin, which is a major antibiotic widely used to prevent IE during invasive dental treatment4. Electron microscopic images revealed abnormal findings on the cell surface and cytoplasm of S. mutans killed with amoxicillin. However, we found that amoxicillin-treated Cnm-positive S. mutans retained its collagen-binding ability and virulence to silkworms. These results suggest that preoperative administration of amoxicillin during invasive dental treatment reduces, but does not eliminate, the pathogenicity of S. mutans against cardiovascular diseases. Based on the results obtained from our study, the effects of other antibiotics on the pathogenicity of S. mutans should be analyzed.
Lysozyme is known to be a major antimicrobial agent in saliva and serum, and lysozyme has also been applied in food and pharmaceuticals2,19. Cnm-positive S. mutans lysed with lysozyme lost its collagen-binding ability and virulence against silkworms, and the inhibitory effect of lysozyme on S. mutans was greater than that of amoxicillin. Therefore, lysozyme may be more effective than antibiotics in inhibiting cardiovascular diseases caused by Cnm-positive S. mutans. In addition to lysozyme, there are other antimicrobial substances such as lactoferrin and lactoperoxidase in saliva and serum20,21,22 that are effective in inhibiting the cariogenicity of S. mutans23. Therefore, the inhibitory effect of these antimicrobial substances derived from humans on the pathogenicity of S. mutans in cardiovascular diseases should be analyzed.
In some patients with cardiovascular diseases including IE, the bacterial DNA of S. mutans has been detected in heart valve specimens, even though live S. mutans was not isolated by blood culture methods14. However, it was unclear whether the detection of dead S. mutans DNA meant that S. mutans was attached to the tissue surface via collagen or that there were dead nonpathogenic S. mutans that were captured and lysed by immune cells. In the present study, we evaluated the adhesion of S. mutans to silkworm organs histopathologically. The results showed that Cnm-positive S. mutans killed with amoxicillin had the ability to adhere to silkworm organs, while lysozyme-treated bacteria were not found in silkworm organs. This suggests that the pathogenicity of dead S. mutans detected in cardiovascular disease lesions may vary depending on how they were killed. However, it is known that silkworm hemoproteins can bind to various bacteria and form nodules24. Therefore, it is necessary to use silkworms to analyze in detail whether the ability of killed S. mutans to adhere to tissues is due to the collagen-binding ability or to the cellular immune response.
Staphylococcus aureus and Enterococcus faecalis are the major causative agents of IE4. S. aureus has adhesion properties to osteoblasts and kidney cells, even when the bacteria are killed by ultraviolet rays or formalin25,26. Additionally, E. faecalis killed by antibiotic treatment was able to adhere to the artificially injured heart valves of rats, resulting in the development of IE27. These bacteria express collagen-binding proteins homologous to Cnm on the cell surface10, and may be involved in bacterial adhesions. Although one study focused on the presence of fibronectin-binding activity in killed S. aureus treated with formalin25, no study to date has focused on the collagen-binding activity of killed bacteria. Thus, to the best of our knowledge, this is the first study to clearly show that the collagen-binding protein of killed bacteria is involved in bacterial adhesion.
The strength of the collagen-binding ability of Cnm-positive S. mutans varied among strains10, and this was also observed in killed bacteria. The strength of the collagen-binding ability is related to the strength of the expression of mRNA encoding Cnm9. Additionally, the collagen-binding ability of Cnm-positive S. mutans is affected by PA, a 190 kDa cell surface protein antigen11. Therefore, it is necessary to focus on the proteins that affect the expression of Cnm such as PA as well as Cnm, and to analyze the expression pattern of Cnm in each S. mutans strain in detail, not only in live bacteria but also in dead bacteria.
S. mutans of different genotypes are present in the oral cavity28, and there may be some participants in whom both Cnm-positive and Cnm-negative S. mutans are present in the oral cavity, but the details remain unknown. There are some reports of increased aggregation ability as a result of co-cultivation of several different bacterial species29,30. Cnm-positive-S. mutans may bind to collagen and serve as a scaffold for other Cnm-negative S. mutans, or an aggregation reaction may occur between dead and live bacteria. In future research, it will be necessary to clarify how often Cnm-positive S. mutans and Cnm-negative S. mutans are mixed, and in what proportion each bacterium exists in the oral cavity. Additionally, collagen-binding assays and silkworm models using multiple S. mutans strains should be evaluated.
Recently, it has been shown that Cnm-positive S. mutans in the oral cavity affect not only cardiovascular diseases, but also inflammatory bowel disease, non-alcoholic steatohepatitis, and IgA nephropathy31,32,33. In these systemic diseases, Cnm-positive S. mutans in the oral cavity infiltrating into the bloodstream is considered to be a common trigger for the onset of the disease. According to the results of the present study, dead Cnm-positive S. mutans in the oral cavity may be a risk factor for the development of systemic diseases via the blood vessels, although the pathogenicity may be lower than that for live bacteria.
In summary, Cnm-positive S. mutans killed with amoxicillin has collagen-binding ability and virulence in silkworms, although these pathogenicities are lost in the bacteria killed with lysozyme. These results suggest that antimicrobial substances derived from humans may be more effective than antibiotics in preventing the development of cardiovascular diseases caused by Cnm-positive S. mutans.
Methods
Ethics statement
This study was conducted in full adherence to the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of Osaka University Graduate School of Dentistry (approval no. 04382). All S. mutans strains have been used in our previous studies, and informed consent was obtained from participants (and parents, if necessary) at the time of oral specimen collection, which could be referred in the previous manuscripts28,34,35,36,37,38.
Bacterial strains and growth conditions
S. mutans strains used in the present study are listed in Supplementary Table 4. S. mutans TW295 (Cnm +) isolated from a patient with bacteremia after tooth extraction were used28. TW295CND, a Cnm-knockout mutant strain of TW295, and TW295comp, a Cnm-complemented strain of TW295, were also used, and were generated as previously described37,39. Furthermore, a total of 20 S. mutans clinical strains selected from our laboratory stock (10 each displaying Cnm-positive and Cnm-negative phenotypes) were used. All strains were confirmed to be S. mutans based on observation of rough colony morphology on Mitis-salivarius agar plates (Difco Laboratories, Detroit, MI, USA) containing bacitracin (0.2 U/ml; Sigma-Aldrich Co., St. Louis, MO, USA) and 15% (wt/vol) sucrose (MSB agar) as well as 16S rRNA sequence analysis with the primers 8UA (5′-AGA GTT TGA TCC TGG CTC AG-3′) and 1540R (5′-AAG GAG GTG ATC CAG CC-3′), as described previously40. For routine growth, all strains were cultured overnight in brain heart infusion broth (Difco Laboratories). When the mutant strains of TW295 were cultured, TW295CND was supplemented with erythromycin (10 µg/ml), and TW295comp was supplemented with erythromycin (10 µg/ml) and spectinomycin (10 mg/ml).
Preparations of killed bacteria
Cultured bacteria were collected by centrifugation at 3,000 × rpm at 4 °C for 10 min. For the lysozyme and amoxicillin treatment, the cultures were washed and resuspended in PBS containing 10 mg/mL lysozyme (FUJIFILM Wako Pure Chemical Corporation, Tokyo, Japan) and 1.0 mg/mL amoxicillin (FUJIFILM Wako Pure Chemical Corporation), respectively, and incubated at 37 °C for 18 h. Successful killing of the bacteria was confirmed by the absence of colonies on MSB agar after culturing at 37 °C for 48 h. After the lysozyme and amoxicillin treatment, each bacterial suspension was washed twice and resuspended in PBS to reach an optical density at 550 nm (OD550) of 1.0, which was equal to 1 × 109 CFU/ml41. The bacterial suspension was diluted and used in the following study.
Electron microscopy observations
Observation using electron microscopy was performed in accordance with the method previously described34,42. As preparation for SEM imaging, each bacterial sample was washed and fixed with 2% osmium tetroxide and 1% glutaraldehyde, dehydrated with ethanol, and then dried with t-butyl alcohol by the freeze-drying method. The dried samples were mounted on the stage and coated with osmium for conductive processing and then observed with SEM. In the TEM analysis, each bacterial sample was washed and fixed with 2% glutaraldehyde adjusted with PBS. After dehydration, bacterial cells were embedded in Epon, then cut into ultrathin sections, and the bacterial structure of these samples was observed by TEM.
Collagen-binding assay
The collagen-binding properties of the S. mutans strains were evaluated according to methods described previously, with some modifications9. A 10 mg/ml sample of type I collagen (Sigma-Aldrich Co.) prepared in 0.25 M acetic acid was coated onto 96-well tissue culture plates (Becton Dickinson, Franklin Lakes, NJ, USA) and incubated overnight at 4 °C. The plates were then washed three times with PBS and blocked for 1.5 h with bovine serum albumin (BSA; Sigma-Aldrich Co.) in PBS at 37 °C. Cultured bacteria was collected by centrifugation and washed and diluted with PBS. Then, 100 µL of the bacterial suspension was added to the wells (1 × 109 CFU per well). After 3 h of incubation at 37 °C, adherent cells were washed three times with PBS and fixed with 100 µL of 25% formaldehyde at room temperature for 30 min. After another three washes with PBS, adherent cells were stained with 100 µL of 0.05% crystal violet (FUJIFILM Wako Pure Chemical Corporation, Tokyo, Japan) in water for 1 min, washed three times with PBS, and the dye was dissolved by adding 7% acetic acid (100 µL) before determining the OD595 values. Results are expressed as OD595 values following subtraction of those from BSA-coated wells. The results for each strain are expressed as a percentage compared with the binding property of SA83 (Cnm +), which was defined as 100%. Data are expressed as the mean ± standard deviation of four independent experiments using three wells for each sample.
Fluorescence microscopy observations
Observation of S. mutans strains binding to type I collagen using confocal laser scanning microscopy was performed by a method described previously43, with some modifications. The collagen-binding assay described above was performed using a chambered cover glass system (CultureWell™, Grace Bio Labs, Bend, OR, USA) instead of a 96-well plate. After binding the bacteria to collagen for 3 h, bacterial cells were stained with 5 µl of 10 mM hexidium iodide (Invitrogen, Carlsbad, CA, USA) in 1 ml of Hanks’ balanced salt solution (Lonza, Walkersville, MD, USA) for 15 min at room temperature in the dark. Stained bacteria were observed by confocal scanning laser microscopy using a TCS-SP5 microscope (Leica Microsystems GmbH, Wetzlar, Germany) with reflected laser light at 543 nm, as well as a DMI6000 B fluorescence microscope (Leica Microsystems GmbH) and a 63 × oil immersion objective.
Silkworm larvae virulence assay
A silkworm larvae virulence assay was performed by a method described previously34, with some modifications. B. mori larvae aged 10 days were purchased (Kougensya, Nagano, Japan), and then stored at 25 °C in the dark. At 18 days of age, larvae with body weights in the range of 150–250 mg were randomly divided into each group (10 larvae per group). The larvae were inoculated on the dorsal surface with 50 µL of bacterial suspension containing different amounts of live S. mutans (1 × 105 CFU, 1 × 106 CFU, and 1 × 107 CFU, respectively). After injection, the silkworms were incubated at 37 °C for 120 h. Live S. mutans and killed S. mutans treated with amoxicillin or lysozyme, adjusted to 1 × 107 CFU, were also administered to silkworms in the same manner. The larvae were checked every 12 h and were considered dead if they did not move in response to touch. In addition, 20 S. mutans clinical isolates were inoculated into n = 3 silkworms each and the average time of death of the three silkworms was calculated. All the experiments were performed three times in each group to ensure reproducibility.
Histopathological evaluation of silkworm larvae
Silkworms euthanized 72 h after S. mutans administration were fixed in 10% formalin neutral buffer solution (FUJIFILM Wako Pure Chemical Corporation) and divided into five sections from head to tail at intervals of 7 mm. These specimens were embedded in paraffin, and cut into 3 μm sections. Gram staining and hematoxylin–eosin staining were then performed using these sections, followed by evaluation of histopathological features as shown in Supplementary Tables 1–3.
For scoring of bacterial infection and pigmentation, the entire specimens of five sections of the silkworm were observed, and the area with the highest bacterial accumulation or pigmentation in each organ was extracted. Then, the area was observed under a 10 × field of view of an objective lens, and the number of sites with bacterial mass or pigmentation was counted to obtain the following scores: score 0 (no bacterial mass or pigmentation), score 1 (5 or less), score 2 (6 to 10), score 3 (11 or more). The scoring of degenerative lesions was based on the number of sections with lesions among the five sections, as follows: score 0 (no lesions), score 1 (1 section), score 2 (2 to 3 sections), score 3 (4 to 5 sections). Necrosis was scored based on the number of sections with lesions among the five cross-sections as follows: score 0 (no lesions), score 1 (1 section), score 2 (2 sections), score 3 (3 to 5 sections). All scoring evaluations were performed in a double-blinded fashion by a pathologist (Sept. Sapie Co. Ltd, Tokyo, Japan).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 9 (GraphPad Software Inc., La Jolla, CA, USA) by a method described previously44, with some modifications. Intergroup differences were compared using analysis of variance (ANOVA). Bonferroni correction was used for post hoc analyses. Differences with P < 0.05 were considered statistically significant. Survival rates in the silkworm larvae virulence assay in each group were evaluated with a Kaplan–Meier plot, which was analyzed using a log-rank test.
References
Tawakoli, P. N., Al-Ahmad, A., Hoth-Hannig, W., Hannig, M. & Hannig, C. Comparison of different live/dead stainings for detection and quantification of adherent microorganisms in the initial oral biofilm. Clin. Oral Investig. 17, 841–850 (2013).
Tonguc Altin, K. et al. Antibacterial effects of saliva substitutes containing lysozyme or lactoferrin against Streptococcus mutans. Arch. Oral Biol. 129, 105183 (2021).
Koren, O. et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl. Acad. Sci. U.S.A. 108, 4592–4598 (2011).
Nakatani, S. et al. JCS 2017 Guideline on prevention and treatment of infective endocarditis. Circ. J. 83, 1767–1809 (2019).
Nobbs, A. H., Lamont, R. J. & Jenkinson, H. F. Streptococcus adherence and colonization. Microbiol. Mol. Biol. Rev. 73, 407–450 (2009).
Paharik, A. E. & Horswill, A. R. The staphylococcal biofilm: Adhesins, regulation, and host response. Microbiol. Spectr. 4, 10 (2016).
Abranches, J. et al. Biology of oral streptococci. Microbiol. Spectr. 6, 10 (2018).
Nakano, K. & Ooshima, T. Common knowledge regarding prevention of infective endocarditis among general dentists in Japan. J. Cardiol. 57, 123–130 (2011).
Nomura, R. et al. Molecular and clinical analyses of the gene encoding the collagen-binding adhesin of Streptococcus mutans. J. Med. Microbiol. 58, 469–475 (2009).
Nomura, R. et al. Identification and characterization of a collagen-binding protein, Cbm Streptococcus mutans. Mol. Oral Microbiol. 27, 308–323 (2012).
Nomura, R. et al. Potential high virulence for infective endocarditis in Streptococcus mutans strains with collagen-binding proteins but lacking PA expression. Arch. Oral Biol. 58, 1627–1634 (2013).
Tonomura, S. et al. Intracerebral hemorrhage and deep microbleeds associated with cnm-positive Streptococcus mutans; a hospital cohort study. Sci. Rep. 6, 20074 (2016).
Watanabe, I. et al. Oral Cnm-positive Streptococcus mutans expressing collagen binding activity is a risk factor for cerebral microbleeds and cognitive impairment. Sci. Rep. 6, 38561 (2016).
Nomura, R. et al. Molecular analyses of bacterial DNA in extirpated heart valves from patients with infective endocarditis. Oral Microbiol. Immunol. 24, 43–49 (2009).
Nomura, R. et al. Potential involvement of collagen-binding proteins of Streptococcus mutans in infective endocarditis. Oral Dis. 19, 387–393 (2013).
Kaito, C., Akimitsu, N., Watanabe, H. & Sekimizu, K. Silkworm larvae as an animal model of bacterial infection pathogenic to humans. Microb. Pathog. 32, 183–190 (2002).
Nakano, K. et al. The collagen-binding protein of Streptococcus mutans is involved in haemorrhagic stroke. Nat. Commun. 2, 485 (2011).
Avilés-Reyes, A., Miller, J. H., Lemos, J. A. & Abranches, J. Collagen-binding proteins of Streptococcus mutans and related streptococci. Mol. Oral Microbiol. 32, 89–106 (2017).
Proctor, V. A. & Cunningham, F. E. The chemistry of lysozyme and its use as a food preservative and a pharmaceutical. Crit. Rev. Food Sci. Nutr. 26, 359–395 (1988).
Hof, W., Veerman, E. C., Nieuw Amerongen, A. V. & Ligtenberg, A. J. Antimicrobial defense systems in saliva. Monogr. Oral Sci. 24, 40–51 (2014).
Wally, J. & Buchanan, S. K. A structural comparison of human serum transferrin and human lactoferrin. Biometals 20, 249–262 (2007).
Fernández-Espejo, E. et al. Cerebrospinal fluid lactoperoxidase level is enhanced in idiopathic Parkinson’s disease, and correlates with levodopa equivalent daily dose. Brain Res. 1761, 147411 (2021).
Pinheiro, S. R. L., da Silva, C. C., da Silva, L. A. & Cicotti, M. P. Antimicrobial capacity of a hydroxyapatite-lysozyme-lactoferrin-lactoperoxidase combination against Streptococcus mutans for the treatment of dental caries. Indian J. Dent. Res. 31, 916–920 (2020).
Watanabe, A. et al. Characterization of a novel C-type lectin, Bombyx mori multibinding protein, from the B. mori hemolymph: Mechanism of wide-range microorganism recognition and role in immunity. J. Immunol. 177, 4594–4604 (2006).
Sinha, B. et al. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell Microbiol. 1, 101–117 (1999).
Somayaji, S. N., Huet, Y. M., Gruber, H. E. & Hudson, M. C. UV-killed Staphylococcus aureus enhances adhesion and differentiation of osteoblasts on bone-associated biomaterials. J. Biomed. Mater. Res. A 95, 574–579 (2010).
Augustin, P. et al. Predominant role of host proteases in myocardial damage associated with infectious endocarditis induced by Enterococcus faecalis in a rat model. Infect. Immun. 81, 1721–1729 (2013).
Nakano, K., Nomura, R., Nakagawa, I., Hamada, S. & Ooshima, T. Demonstration of Streptococcus mutans with a cell wall polysaccharide specific to a new serotype, k, in the human oral cavity. J. Clin. Microbiol. 42, 198–202 (2004).
Lang, C. et al. Specific lactobacillus/mutans streptococcus co-aggregation. J. Dent. Res. 89, 175–179 (2010).
Kitada, K. & Oho, T. Effect of saliva viscosity on the co-aggregation between oral streptococci and Actinomyces naeslundii. Gerodontology 29, e981–e987 (2012).
Kojima, A. et al. Infection of specific strains of Streptococcus mutans, oral bacteria, confers a risk of ulcerative colitis. Sci. Rep. 2, 332 (2012).
Naka, S. et al. Contributions of Streptococcus mutans Cnm and PA antigens to aggravation of non-alcoholic steatohepatitis in mice. Sci. Rep. 6, 36886 (2016).
Naka, S. et al. Streptococcus mutans induces IgA nephropathy-like glomerulonephritis in rats with severe dental caries. Sci. Rep. 11, 5784 (2021).
Nomura, R. et al. Contribution of Streptococcus mutans serotype k strains interaction with fibrinogen to the pathogenicity of infective endocarditis. Infect. Immun. 82, 5223–5234 (2014).
Ooshima, T., Izumitani, A., Sobue, S. & Hamada, S. Cariostatic effect of palatinose on experimental dental caries in rats. Jpn. J. Med. Sci. Biol. 36, 219–223 (1983).
Lapirattanakul, J. et al. Multilocus sequence typing analysis of Streptococcus mutans strains with the cnm gene encoding a collagen-binding adhesin. J. Med. Microbiol. 60, 1677–1684 (2011).
Otsugu, M. et al. Contribution of Streptococcus mutans strains with collagen-binding proteins in the presence of serum to the pathogenesis of infective endocarditis. Infect. Immun. 85, e00401-e417 (2017).
Lapirattanakul, J. et al. Variation of expression defects in cell surface 190-kDa protein antigen of Streptococcus mutans. Int. J. Med. Microbiol. 305, 383–391 (2015).
Nakano, K. et al. Molecular characterization of Streptococcus mutans strains containing the cnm gene encoding a collagen-binding adhesin. Arch. Oral Biol. 55, 34–39 (2010).
Nomura, R. et al. Isolation and characterization of Streptococcus mutans in heart valve and dental plaque specimens from a patient with infective endocarditis. J. Med. Microbiol. 55, 1135–1140 (2006).
Nomura, R., Morita, Y., Matayoshi, S. & Nakano, K. Inhibitory effect of surface pre-reacted glass-ionomer (S-PRG) elute against adhesion and colonization by Streptococcus mutans. Sci. Rep. 8, 5056 (2018).
Fujita, K., Matsumoto-Nakano, M., Inagaki, S. & Ooshima, T. Biological functions of glucan-binding protein B of Streptococcus mutans. Oral Microbiol. Immunol. 22, 289–292 (2007).
Nomura, R. et al. Inhibitory effects of flavedo, albedo, fruits, and leaves of Citrus unshiu extracts on Streptococcus mutans. Arch. Oral Biol. 124, 105056 (2021).
Nomura, R. et al. Contribution of Streptococcus mutans to Helicobacter pylori colonization in oral cavity and gastric tissue. Sci. Rep. 10, 12540 (2020).
Acknowledgements
This work was supported by JSPS KAKENHI Grant numbers 18H03010, 18K09831 and 21H03149. All authors read and approved the final manuscript. We thank Ms Rewa Yanagisawa, Department of Pediatric Dentistry, Osaka University Graduate School of Dentistry, for technical support with analysis of the silkworm larvae virulence assay.
Author information
Authors and Affiliations
Contributions
R.N. designed the study under the supervision of K.N. Y.S., R.N., S.M., and M.O. performed the experiments. Y.S., and N.I. performed histopathological analysis. Data interpretation was conducted by Y.S., R.N. and K.N. R.N. and K.N. wrote the manuscript, which all authors read and approved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suehiro, Y., Nomura, R., Matayoshi, S. et al. Evaluation of the collagen-binding properties and virulence of killed Streptococcus mutans in a silkworm model. Sci Rep 12, 2800 (2022). https://doi.org/10.1038/s41598-022-06345-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-06345-x
- Springer Nature Limited
This article is cited by
-
Alternatives to animal models to study bacterial infections
Folia Microbiologica (2023)
-
Clinical characteristics of children and guardians possessing CBP-positive Streptococcus mutans strains: a cross-sectional study
Scientific Reports (2022)