Abstract
To assess whether a simplified cardiac magnetic resonance (CMR)–derived lung water density (LWD) quantification predicted major events in Heart Failure (HF). Single-centre retrospective study of consecutive HF patients with left ventricular ejection fraction (LVEF) < 50% who underwent CMR. All measurements were performed on HASTE sequences in a parasagittal plane at the right midclavicular line. LWD was determined by the lung-to-liver signal ratio multiplied by 0.7. A cohort of 102 controls was used to derive the LWD upper limit of normal (21.2%). The primary endpoint was a composite of time to all-cause death or HF hospitalization. Overall, 290 patients (mean age 64 ± 12 years) were included. LWD measurements took on average 35 ± 4 s, with good inter-observer reproducibility. LWD was increased in 65 (22.4%) patients, who were more symptomatic (NYHA ≥ III 29.2 vs. 1.8%; p = 0.017) and had higher NT-proBNP levels [1973 (IQR: 809–3766) vs. 802 (IQR: 355–2157 pg/mL); p < 0.001]. During a median follow-up of 21 months, 20 patients died and 40 had ≥ 1 HF hospitalization. In multivariate analysis, NYHA (III–IV vs. I–II; HR: 2.40; 95%-CI: 1.30–4.43; p = 0.005), LVEF (HR per 1%: 0.97; 95%-CI: 0.94–0.99; p = 0.031), serum creatinine (HR per 1 mg/dL: 2.51; 95%-CI: 1.36–4.61; p = 0.003) and LWD (HR per 1%: 1.07; 95%-CI: 1.02–1.12; p = 0.007) were independent predictors of the primary endpoint. These findings were mainly driven by an association between LWD and HF hospitalization (p = 0.026). A CMR-derived LWD quantification was independently associated with an increased HF hospitalization risk in HF patients with LVEF < 50%. LWD is a simple, reproducible and straightforward measurement, with prognostic value in HF.
Similar content being viewed by others
Introduction
Congestion is a central feature of Heart Failure (HF) often found in chronic stable disease1 and acute HF decompensation2. Pulmonary oedema plays a key role in several of the cardinal HF manifestations, namely dyspnoea and exercise intolerance. Albeit sensitive, these symptoms are not specific3 and, concurrently with left ventricular ejection fraction (LVEF) assessment, may influence crucial decisions in HF treatment (e.g., cardiac resynchronization therapy and/or implantable cardioverter defibrillator)4. Various fluid measuring methods have been proposed to accurately determine pulmonary extravascular water. Its quantification by thermodilution has been shown to correlate with increased left atrial, pulmonary wedge and/or diastolic pressures in series of patients with acute myocardial infarction5, chronic coronary syndrome6 and HF7. Imaging tests, such as chest radiography8 and lung ultrasonography9 are currently used in clinical practice as semi-quantitative measurements to assess the burden of pulmonary congestion and to discriminate it from non-cardiac causes of increased lung fluid. Whether routine evaluation, in addition to physical examination, may guide treatment in order to improve outcomes and whether these strategies may predict major events in the long-term (beyond 6 months) are yet to be thoroughly ascertained.
Lung Water quantification using Cardiac Magnetic Resonance (CMR) imaging has been shown to be feasible and is well correlated with the gold standard (post-mortem weighted gravimetric method)10,11. Added to its accuracy, CMR has the advantage of measuring lung water non-invasively and as a perfusion and ventilation-independent technique. Furthermore, it has been recently demonstrated that Lung Water Density (LWD) measured by this method independently predicts all-cause death, cardiovascular hospitalization or emergency department visit within 1-year in a cohort of patients with or at-risk of HF. However, the methodology proposed by Thompson et al.12 may be demanding and time-consuming, rendering CMR-derived LWD a less useful tool in everyday clinical practice. Thus, we aimed to assess whether a simplified CMR-derived LWD determination was feasible and correlated with major events in patients with HF and LVEF < 50%.
Methods
Study population
Consecutive patients with HF referred to CMR imaging were screened and those with a LVEF < 50% at CMR and follow-up in our centre from 2016 to 2018 were included. Patients aged < 18 years and those with known chronic lung disease and/or chronic liver disease (as determined by their physician) were excluded. Patients without available Half-Fourier Acquisition Single-shot Turbo spin Echo imaging (HASTE) sequences (n = 4), and those whose HASTE images had significant contribution from the heart, large blood vessels and/or hilar structures (n = 4) were also excluded. The study protocol was reviewed and approved by the local ethics' committee—Comissão de Ética para a Saúde do Centro Hospitalar de Lisboa Ocidental, with the Registry number 20170700050—, which waived the need for informed consent. This investigation was performed in accordance with the Declaration of Helsinki.
Demographic, clinical and laboratory data
Demographic, clinical and laboratory data were retrospectively collected from the patient chart and electronic medical records (within a 6-month window previous to CMR). HF diagnosis was defined according to the 2017 ACC/AHA/HFSA Guidelines13.
CMR data acquisition and lung water analysis
All subjects were imaged using a 1.5 T scanner (Siemens Avanto®, Siemens Healthineers, Erlangen, Germany). Cardiac function and structure were evaluated by using a balanced steady-state free precession cine sequence with retrospective ECG-gating. Ventricular volumes were measured by experienced Cardiologists and Radiologists using a dedicated software (Circle Cardiovascular Imaging® release 5.6.4, Calgary, Canada).
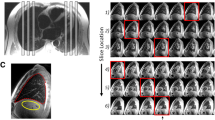
Lung water was measured using a HASTE pulse sequence. Typical imaging parameters included a field of view of 340 × 340 mm, 8 mm slice thickness, 5/8ths partial Fourier, 780 Hz/pixel bandwidth and a 120°–180° refocusing pulse flip angle and ECG-gated image acquisition during diastasis. A single sagittal HASTE slice at the right midclavicular line at end-expiratory breath-hold was used to measure lung and liver signal intensities. Two regions of interest (ROI) were manually drawn: one including all visible right lung tissue (excluding pleural effusion and any heart volume, when present), and another including the upper half of the liver. Finally, LWD (%) was determined as the lung-to-liver signal ratio multiplied by 0.714—Fig. 1. LWD measurements were performed by two independent observers (BR, GC) blinded to all patient data.
Simplified method for imaging Lung Water: Lung and Hepatic operator-selected region of interest (ROI) are outlined in the parasagittal plane at the larger cross-sectional lung area, usually at the right midclavicular line. Lung water density is calculated by the lung-to-liver signal ratio multiplied by 0.7.
Briefly, the major differences between this simplified method for LWD measurement and the previously reported one12 are: (1) lack of 30 min supine positioning before CMR; (2) single sagittal HASTE sequence acquired at end-expiratory breath-hold versus 20 repeats during free-breathing with subsequent retrospective selection of end-expiration images.
Control cohort
A retrospective cohort of patients undergoing CMR imaging for supraventricular or ventricular premature beats on 24-h Holter monitoring, doubtful structural heart disease on echocardiography and those prior to paroxysmal atrial fibrillation (AF) ablation were screened. Those without known cardiovascular risk factors other than age and sex, no demonstrable structural heart disease nor late gadolinium enhancement were included as controls (n = 102). This cohort was used to derive the upper limit of normal [i.e., two standard deviations (SD) above the mean] of the LWD (21.2%).
Follow-up and endpoint definitions
Hospitalization for acute HF was defined as per the ACC/AHA/HFSA Guidelines13. Patients were assessed at least annually and data on clinical status and HF-related events were documented in the electronic medical records. The primary endpoint was a composite of time to all-cause death or HF hospitalization. Three Cardiologists (BR, GC, PF) performed event adjudication and any disagreements were discussed within the panel and resolved by consensus.
Statistical analysis
Categorical values are presented as counts (and percentage) and continuous variables as mean ± SD (normal distribution) or median [interquartile range (IQR)] (nonparametric). Pearson’s Chi-squared (χ2) test, Mann–Whitney U and independent samples t-test were applied for comparison where appropriate. Bland–Altman analysis and Lin’s concordance correlation coefficient were used to evaluate the inter-observer agreement between LWD measurements as a continuous variable. Cohen’s κ was used to assess inter-observer reliability in categorizing LWD as increased.
Univariate analysis was applied to relate a broad range of clinical and CMR parameters to the study endpoint. After multicollinearity correction, variables with a p-value < 0.05 were then selected for multivariate analysis. LWD was imputed into the models as either a continuous variable or reclassified as the percentage (per 1%) above the upper limit of normal when it was increased (> 21.2%) and as a null value whenever within normal range (0 to 21.2%). The CMR time point served as the index time for time-to-event analyses which were performed using Cox-regression hazards model and Kaplan–Meier survival curves. Multivariate competing risk analyses of predictors for time to HF hospitalization was performed with the Fine and Gray proportional subdistribution hazard regression model. Subgroup analysis included: (1) HF with reduced LVEF (< 40%) vs. midrange LVEF (40–49%); (2) asymptomatic or mild HF symptoms [New York Heart Association (NYHA) I–II] vs. moderate or severe HF symptoms (NYHA III–IV); (3) increased NT-proBNP (> 600 or 900 pg/mL if AF) vs. “normal” NT-proBNP (≤ 600 or 900 pg/mL if AF); and (4) status at CMR acquisition (during HF hospitalization vs. outpatient).
A prespecified multivariate model adjusted for age and NT-proBNP was constructed with events right-censored at 1-year follow-up in order to ascertain whether results were similar to those previously reported in the literature12.
Statistical analysis was performed using SPSS v26.0 and STATA v13. Statistical significance was set at p-value < 0.05 (two-sided).
Ethics approval and consent to participate
The hospital ethics’ committee approved of this study at 07th September of 2020 (RNEC 20170700050). The requirement of informed consent to participate and publication was waived, based on the Portuguese Deliberation n.º 1704/2015 of the National Commission for the Protection of Data (CNPD) and the Legislation under the Laws n.º 58 and 59/2019, n.º 12/2005 and the Data Protection General Regulation (UE) 2016/679.
Results
Cohort demographics and clinical data
The overall cohort included 290 HF patients with a mean age of 64 ± 12 years, most of whom were male (74.8%). HF aetiology was mainly ischaemic (56.2%) and mean LVEF was 34 ± 10%. Baseline demographics are depicted in Table 1. CMR was acquired during hospitalization in 69 patients (23.7%).
LWD analysis
ROI tracing for LWD measurements lasted on average 35 ± 4 s. The simplified LWD quantification method was associated with good reproducibility: Lin’s concordance correlation coefficient of 0.95 [95% Confidence Interval (CI) 0.935–0.965; p < 0.001], with minimal bias according to the Bland–Altman analysis (bias 0.17%; 95% limits of agreement: -3.6% to 3.9%)—Fig. S1. Likewise, a good agreement (Cohen’s κ = 0.78; p < 0.001) was found between the two raters for the categorization of LWD as elevated.
LWD histogram is illustrated in Fig. S2. Increased LWD (> 21.2%) was observed in 65 (22.4%) patients. Even though this group of patients had a higher median NT-proBNP and a lower LVEF (Table 1 and Fig. S3), correlations between LWD and NT-proBNP or LVEF were weak (Spearman rho 0.26 and − 0.25, respectively—Fig. S3). Additionally, the percentage of patients with increased LWD—“wet lungs”—increased with worsening NYHA class (Fig. S4). Nevertheless, the number of patients with normal LWD—“dry lungs”—remains considerably high (> 30%) across the spectrums of NT-proBNP, LVEF and NYHA class. Overall, LWD appears to add incremental information that is relatively independent from NT-proBNP, LVEF and NYHA.
Primary composite endpoint
During a median follow-up of 21 (IQR 13–29) months, 20 patients (6.9%) died and 40 (13.8%) had one or more admissions for HF. Six of the patients who died were previously hospitalized for HF.
Compared to patients with normal LWD, those with increased LWD met more often the primary composite endpoint [25 (38.5%) vs. 29 (12.9%); p < 0.001], mostly due to HF hospitalization [24 (37.5%) vs. 16 (7.8%); p < 0.001). Univariate analyses to predict the primary endpoint are presented in Table 2. In the multivariate model (Table 3), LWD [Hazard ratio (HR) per 1%: 1.066; 95% CI: 1.018–1.115; p = 0.007] remained an independent predictor of the primary composite endpoint after adjusting for NT-proBNP, NYHA functional class, LVEF and serum creatinine. These findings were mainly driven by an association between increased LWD and time to first HF hospitalization (HR per 1%: 1.063; 95% CI: 1.007–1.122; p = 0.026), as adjusted for competing risks (Table S1). Event-free survival and time to HF hospitalization are depicted in Fig. 2.
(A) Kaplan–Meier curves for 290 HF patients and LVEF < 50% with increased (> 21.2%) or normal LWD (≤ 21.2%) for the primary composite endpoint (i.e., all-cause death or HF hospitalization) presented as event-free survival (%) at 30 months. Compared to normal LWD, those with increased LWD were significantly more likely to have an event (log rank p < 0.001). LWD lung water density. (B) Kaplan–Meier curves for 290 HF patients and LVEF < 50% with increased (> 21.2%) or normal LWD (≤ 21.2%) for HF hospitalization presented right-censored at 30 months. Compared to normal LWD, patients with increased LWD were significantly more likely to have at least one HF hospitalization (log rank p < 0.001). LWD lung water density.
Subgroup and sensitivity analyses
Multivariate analysis remained similar when evaluating LWD without imputing null values to LWD within the normal range (HR per 1%: 1.054; 95% CI: 1.018–1.092; p = 0.003) and when censoring follow-up at 1-year (HR for LWD > 21.2%: 2.851; 95% CI: 1.354–6.001; p = 0.006; HR per 1% LWD: 1.066; 95% CI: 1.026–1.109; p = 0.001).
LWD distribution throughout the subgroups showed increased lung water in patients with reduced LVEF (< 40%), NYHA III–IV, increased NT-proBNP and CMR during HF hospitalization (Fig. 3). Repeated subgroup multivariate analysis demonstrated consistent and similar results to the overall population (Fig. 4).
Distribution of LWD as per the following subgroups: (1) LVEF [reduced (< 40%) vs. midrange (40–49%) LVEF]; (2) functional class (NYHA I–II vs. III–IV); (3) NT-proBNP (> 600 or 900 pg/mL if AF vs. ≤ 600 or 900 pg/mL if AF); and (4) status at CMR acquisition (during HF hospitalization vs. outpatient); Box plots illustrating LWD median, 25th and 75th percentiles, and whiskers show the 10th and 90th percentiles; *p < 0.05 in comparison to control; p-values for LWD comparison between subgroups are shown in figure. CMR cardiac magnetic resonance, HF heart failure, LVEF left ventricular ejection fraction, NYHA New York Heart Association.
Primary composite endpoint analysis truncated at 1-year as per the following subgroups: (1) LVEF [reduced (< 40%) vs. midrange (40–49%) LVEF]; (2) functional class (NYHA I–II vs. III–IV); (3) NT-proBNP (> 600 or 900 pg/mL if AF vs. ≤ 600 or 900 pg/mL if AF, which is illustrated above as high vs. low NT-proBNP, respectively); and (4) and status at CMR acquisition (during HF hospitalization vs. outpatient). The HR (and 95% CI) of increased LWD (> 21.2%) for the primary endpoint is illustrated, adjusted to the variables used in the multivariate model (LVEF, serum creatinine, NT-proBNP and NYHA class). The results are consistent across different subgroups, particularly powerful in patients with LVEF 40–49% or whose MRI was performed in the outpatient setting. CMR cardiac magnetic resonance, LVEF left ventricular ejection fraction, NYHA New York Heart Association.
Discussion
We report the application of a simplified CMR-derived lung water quantification method in a cohort of patients with HF and LVEF < 50%. The major findings were as follows: (1) LWD is rapidly measurable in routine CMR without any additional technical procedure or incremental costs, with good inter-observer reproducibility and agreement; (2) the cut-off for “normal” LWD in our control population was similar to that reported in the study by Thompson et al. (20.8% vs. 21.2%)12; and (3) the simplified LWD measurement independently associated with an increased risk of the primary composite endpoint, with an early separation of the event-free survival curves, mainly due to an association between LWD and time to first HF hospitalization.
Persistent and recurrent congestion is associated with worse prognosis in HF15,16,17. Measuring lung water allows the detection and quantification of lung fluid, showing promise as both a prognostic marker and a potential therapeutic target to tailor diuretic treatment. Several methods have been proposed in clinical practice to extend and refine physical examination findings. Thermodilution is an accurate method compared to gold standard and, in addition, allows indirect measurement of lung injury. However, the technique is cumbersome and not widely available outside the critical care unit or the hemodynamic laboratory18,19. Similarly, tomographic bioimpedance-derived methods allow the accurate measurement of extravascular lung fluid20,21, but did not gain widespread use. In contrast, lung ultrasound, a method that allows water semi-quantification by assessing B-lines22,23, is being increasingly integrated into clinical practice in order to achieve euvolaemia in acute scenarios24. Moreover, ultrasound-guided diuretic treatment may facilitate symptom improvement and reduce the number of decompensations in chronic HF9. In comparison to other methods, LWD measured by CMR may allow a notably accurate and highly reproducible quantification of lung fluid that may be conceivably useful as a surrogate endpoint in studies focusing on strategies to tackle pulmonary congestion25.
Lung water content determination by CMR has been the focus of research for almost four decades10. Water was shown to be reliably measured by this method firstly in sponge phantoms, and later applied to lung measurements in animal models and humans26,27, and were correlated to invasively measured left-sided filling pressures in the latter12. Moreover, CMR has the advantage of accounting for topographical inhomogeneity in tissue water content28 that may go undetected by other means. Previously, Thompson et al. have investigated the prognostic role of LWD in patients with (n = 121) or at risk of HF (n = 82). Firstly, the derived upper limit of normal cut-off in the cohort was similar to the observed in our study (20.8% vs. 21.2%) which strengthens the clinical applicability of the simplified method. Secondly, increased LWD (> 20.8%) was an independent predictor of all-cause death, cardiovascular hospitalization or emergency department visit within 1-year in their cohort of patients with or at risk of HF and any LVEF12. In our study, we found similar results in a considerably larger cohort (n = 290) of patients with established HF and LVEF < 50% in whom LWD independently predicted all-cause death or HF hospitalization at a median follow-up of 21 months (HR per 1%: 1.07; p = 0.016). Thus, we further validate the prognostic value of a CMR-derived LWD determination in this population.
Thompson et al. were first to report the application of a CMR method for LWD estimation in a clinical population with established or at-risk of HF12. In the abbreviated protocol, measurements were performed in a single sagittal slice in the right lung at its largest cross-sectional area during free-breathing 20 repeats, separated for > 5 s (total scan time of about 2 min). Images were obtained approximately 30 min after supine positioning at the onset of the CMR exam12. In our study, we used routine HASTE images without any additional methodology concerns. Indeed, our study is retrospective in its design and CMR images were acquired without the specific purpose of measuring LWD. Despite this, images were appropriate for lung ROI outlining in all but four patients since slices had a significant contribution from the heart and/or hilar structures. Thus, not only does our investigation validates and strengthens the prognostic value of CMR-derived LWD determination in HF patients with LVEF < 50% but also simplifies the acquisition method. Notably, our findings suggest that CMR can be performed in the usual fashion regardless of LWD determination, allowing an accurate lung water quantification that has important prognostic value. Furthermore, lung and liver ROI outlining is a simple trainable task and an automated software tool may be easily applied.
Interestingly, we found that NT-proBNP, a strong established major outcome predictor in HF29,30, was not predictive of the primary endpoint in multivariate analysis once LWD was imputed into the model. Indeed, natriuretic peptides levels correlate with congestive symptoms and signs31, and, thus, LWD may represent a more refined measure of hypervolaemia. Nonetheless, the interpretation of these results may be complicated by the inclusion of patients whose CMR was performed during hospitalization, particularly as we collected NT-proBNP at the closest day available to the CMR and, while outpatient, we accepted a timeframe of 6 months.
We found that LWD was a strong predictor of meaningful events in HF. Of note, event-free survival curves diverged early, demonstrating LWD as a strong and early predictor of time to first HF hospitalization. Altogether, our investigation supports the systematic opportunistic measurement of LWD in HF with LVEF < 50% in order to further stratify patient risk. Whether LWD-targeted interventions are beneficial and whether LWD adds prognostic value to known and well-validated risk scores in HF, are interesting hypothesis worth being further investigated. Indeed, it would be appealing to test the predictive power of a CMR-HF score integrating LWD compared to the recommended risk score calculators. Finally, the targeted population in whom LWD measurement might be most useful (e.g. "subclinical congestion" detection at discharge or congestion in the outpatient setting) should be further investigated, as should one assess the clinical utility and cost-effectiveness of this method compared to others.
Limitations
Some limitations should be acknowledged. First, we assumed that the liver CMR signal corresponds to a water density of 70% as formerly reported32. Despite having excluded patients with known chronic liver disease, the presence of either congestion or fatty liver infiltration and/or subclinical liver disease may have led to LWD underestimation or overestimation. Similarly, albeit excluding those who had known chronic lung disease, pulmonary function tests and chest-computed tomography were not systematically available, hence variables other than lung congestion may have led to lung density miscalculations. Second, regional variations and positional redistribution in lung water content were not accounted for, and whether other parameters, such as maximal LWD, are of higher prognostic value, were not ascertained. Furthermore, we could not determine the therapeutic effect of diuretic treatment given the retrospective nature of our study and given that most patients had “normal” LWD at the time of CMR. Whether lung congestion detected by CMR should be a therapeutic target (e.g. diuretic adjustments) is a hypothesis worth exploring prospectively. Although patients with acute HF most often had increased LWD compared to those in the outpatient setting, subgroup analysis were consistent with the main findings. Moreover, we included patients who were referred to CMR (selection bias) as determined by their attending physician and whether findings can be extrapolated to the overall HF population is debatable. Limitations inherent to a retrospective single-centre study design are to be recognized. Finally, the control group, while not having overt structural heart disease, was not a healthy cohort and the true normal cut-off of LWD might even be lower than that considered here. Nonetheless, the prognostic value of LWD was confirmed in sensitivity analysis where all values were including, regardless of cut-off.
Conclusion
Lung water quantification by a simplified CMR-derived method independently associates with an increased risk of HF hospitalization in patients with HF with LVEF < 50%. LWD measurement is a simple, reproducible and straightforward method, further adding to the key prognostic role of CMR in HF. Future studies should prospectively assess the prognostic performance of LWD in comparison to and/or on top of known HF prognostic variables and well-validated risk scores.
Data availability
The datasets used and/or analysed for the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACEi:
-
Angiotensin-converting enzyme inhibitor
- AF:
-
Atrial fibrillation
- ARB:
-
Angiotensin II receptor blocker
- ARNi:
-
Angiotensin receptor-neprilysin inhibitor
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CMR:
-
Cardiac magnetic resonance
- HASTE:
-
Half-Fourier acquisition single-shot turbo spin echo imaging
- HF:
-
Heart failure
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- LVEDVi:
-
Left ventricle end-diastolic volume index
- LVEF:
-
Left ventricular ejection fraction
- LWD:
-
Lung water density (%)
- MDRD:
-
Modification of diet renal disease
- MI:
-
Myocardial infarction
- MRA:
-
Mineralocorticoid receptor antagonist
- NYHA:
-
New York Heart Association
- ROI:
-
Region of interest
- SD:
-
Standard deviation
References
Abraham, W. T. et al. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: Complete follow-up results from the CHAMPION randomised trial. Lancet 387(10017), 453–461 (2016).
Cooper, L. B. et al. The burden of congestion in patients hospitalized with acute decompensated heart failure. Am. J. Cardiol. 124(4), 545–553 (2019).
Mant, J. et al. Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care. Health Technol. Assess. 13(32), 1–207 (2009).
Ponikowski, P. et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 33, 1787–1847 (2016).
Biddle, T. L., Khanna, P. K., Yu, P. N., Hodges, M. & Shah, P. M. Lung water in patients with acute myocardial infarction. Circulation 49(1), 115–123 (1974).
McCredie, R. M. & Chia, B. L. Measurement of pulmonary oedema in ischaemic heart disease. Br. Heart J. 35(11), 1136–1140 (1973).
Bindels, A. J., van der Hoeven, J. G. & Meinders, A. E. Pulmonary artery wedge pressure and extravascular lung water in patients with acute cardiogenic pulmonary edema requiring mechanical ventilation. Am. J. Cardiol. 84(10), 1158–1163 (1999).
Kobayashi, M. et al. Chest X-ray quantification of admission lung congestion as a prognostic factor in patients admitted for worsening heart failure from the ICALOR cohort study. Int. J. Cardiol. 299, 192–198 (2020).
Rivas-Lasarte, M. et al. Lung ultrasound-guided treatment in ambulatory patients with heart failure: A randomized controlled clinical trial (LUS-HF study). Eur. J. Heart Fail. 21, 1605–1613 (2019).
Cutillo, A. G., Morris, A. H., Ailion, D. C., Durney, C. H. & Case, T. A. Determination of lung water content and distribution by nuclear magnetic resonance imaging. J. Thorac. Imag. 1(3), 39–51 (1986).
Hayes, C. E. et al. Lung water quantitation by nuclear magnetic resonance imaging. Science 216(4552), 1313–1315 (1982).
Thompson, R. B. et al. Quantification of lung water in heart failure using cardiovascular magnetic resonance imaging. J. Cardiovasc. Magn. Reson. 21(1), 58 (2019).
Yancy, C. W. et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation 136(6), e137–e161 (2017).
Allen, T. H., Kryzwicki, H. J. & Roberts, J. E. Density, fat, water and solids in freshly isolated tissues. J. Appl. Physiol. 14, 1005–1008 (1959).
Gargani, L. et al. Persistent pulmonary congestion before discharge predicts rehospitalization in heart failure: A lung ultrasound study. Cardiovasc. Ultrasound 13, 40 (2015).
Chioncel, O. et al. Acute heart failure congestion and perfusion status—Impact of the clinical classification on in-hospital and long-term outcomes; insights from the ESC-EORP-HFA heart failure long-term registry. Eur. J. Heart Fail. 21(11), 1338–1352 (2019).
Lala, A. et al. Relief and recurrence of congestion during and after hospitalization for acute heart failure: Insights from diuretic optimization strategy evaluation in acute decompensated heart failure (DOSE-AHF) and cardiorenal rescue study in acute decompensated heart failure (CARESS-HF). Circ. Heart Fail. 8(4), 741–748 (2015).
Jozwiak, M., Teboul, J. L. & Monnet, X. Extravascular lung water in critical care: Recent advances and clinical applications. Ann. Intensive Care 5(1), 38 (2015).
Chase, S. C. et al. The effect of diuresis on extravascular lung water and pulmonary function in acute decompensated heart failure. ESC Heart Fail. 5(2), 364–371 (2018).
Spinale, F. G., Reines, H. D., Cook, M. C. & Crawford, F. A. Noninvasive estimation of extravascular lung water using bioimpedance. J. Surg. Res. 47(6), 535–540 (1989).
Santarelli, S. et al. Usefulness of combining admission brain natriuretic peptide (BNP) plus hospital discharge bioelectrical impedance vector analysis (BIVA) in predicting 90 days cardiovascular mortality in patients with acute heart failure. Intern. Emerg. Med. 12(4), 445–451 (2017).
Platz, E. et al. Expert consensus document: Reporting checklist for quantification of pulmonary congestion by lung ultrasound in heart failure. Eur. J. Heart Fail. 21(7), 844–851 (2019).
Volpicelli, G. et al. Lung ultrasound predicts well extravascular lung water but is of limited usefulness in the prediction of wedge pressure. Anesthesiology 121(2), 320–327 (2014).
Shyamsundar, M., Attwood, B., Keating, L. & Walden, A. P. Clinical review: The role of ultrasound in estimating extra-vascular lung water. Crit. Care. 17(5), 237 (2013).
Bellenger, N. G., Davies, L. C., Francis, J. M., Coats, A. J. & Pennell, D. J. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2(4), 271–278 (2000).
MacLennan, F. M., Foster, M. A., Smith, F. W. & Crosher, G. A. Measurement of total lung water from nuclear magnetic resonance images. Br. J. Radiol. 59(702), 553–560 (1986).
Molinari, F., Madhuranthakam, A. J., Lenkinski, R. & Bankier, A. A. Ultrashort echo time MRI of pulmonary water content: Assessment in a sponge phantom at 1.5 and 3.0 Tesla. Diagn. Interv. Radiol. 20(1), 34–41 (2014).
Cutillo, A. G. et al. Determination of lung water content and distribution by nuclear magnetic resonance. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 57(2), 583–588 (1984).
Don-Wauchope, A. C. et al. Incremental predictive value of natriuretic peptides for prognosis in the chronic stable heart failure population: A systematic review. Heart Fail. Rev. 19(4), 521–540 (2014).
Sawano, M. et al. Performance of the MAGGIC heart failure risk score and its modification with the addition of discharge natriuretic peptides. ESC Heart Fail. 5(4), 610–619 (2018).
Korinek, J., Boerrigter, G., Mohammed, S. F. & Burnett, J. C. Insights into natriuretic peptides in heart failure: An update. Curr. Heart Fail. Rep. 5(2), 97–104 (2008).
Allen, T. H., Krzywicki, H. J. & Roberts, J. E. Density, fat, water and solids in freshly isolated tissues. J. Appl. Physiol. 14, 1005–1008 (1959).
Acknowledgements
We would like to thank all the technical support and enthusiastic collaboration of Carolina Padrão, Telma Lima, Ricardo Lopes, Afonso Grego, Fernando Marques and Patrícia Santim from the Radiology Department.
Author information
Authors and Affiliations
Contributions
Conception and design (B.R., G.C., P.F., A.F.); data collection (B.R., G.C.), image reading (B.R., G.C., P.F., J.A., A.F.), data analysis (B.R., G.C.), data interpretation and results (BR, GC, P.F., P.L., M.J.A., C.A., A.F.), drafting (B.R., G.C.), revising (P.F., A.S., S.G., A.T., A.V., M.J.A., C.A., J.A., M.M., A.F.); All authors read and approved the final manuscript; B.R. and G.C. have contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rocha, B.M.L., Cunha, G.J.L., Freitas, P. et al. Measuring lung water adds prognostic value in heart failure patients undergoing cardiac magnetic resonance. Sci Rep 11, 20162 (2021). https://doi.org/10.1038/s41598-021-99816-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-99816-6
- Springer Nature Limited