Abstract
Virus-like particles (VLPs) are recognized as an alternative vaccine platform that provide effective protection against various highly pathogenic avian influenza viruses (HPAIVs). Here, we developed multi-clade VLPs expressing two HAs (a chimera of clade 2.3.2.1c and clade 2.3.4.4c HA) within a single vector. We then compared its protective efficacy with that of a monovalent VLP and evaluated its potency against each homologous strain. Chickens vaccinated with the multi-clade VLP shed less virus and were better protected against challenge than birds receiving monovalent vaccines. Single vaccination with a multi-clade VLP resulted in 100% survival, with no clinical symptoms and high levels of pre-challenge protective immunity (7.6–8.5 log2). Moreover, the multi-clade VLP showed high productivity (128–256 HAU) both in the laboratory and on a large scale, making it cheaper than whole inactivated vaccines produced in eggs. However, the PD50 (protective dose 50%) of the multi-clade VLP against clades 2.3.2.1c and 2.3.4.4c was < 50 PD50 (28 and 42 PD50, respectively), and effective antibody response was maintained for 2–3 months. This multi-clade VLP protects against both clades of HPAI viruses and can be produced in high amounts at low cost. Thus, the vaccine has potential as a pandemic preparedness vaccine.
Similar content being viewed by others
Introduction
Highly pathogenic avian influenza (HPAI) has been circulating in wild birds and poultry worldwide since the first H5N1 avian influenza virus, A/Goose/Guangdong/1/96, was identified in China in 19961,2. Novel HPAI H5Nx viruses, including various NA, have emerged continuously due to extensive genetic reassortment activity; indeed, various clades have emerged due to genetic evolution and antigenic drift3,4,5. As a result, persistent HPAI outbreaks on poultry farms in many countries have been caused by mutated viruses, resulting in serious economic losses1. Moreover, various subtypes of HPAI have emerged simultaneously in countries such as China (H5N6, H5N1), Vietnam (H5N6, H5N1), and Taiwan (H5N2, H5N5), making it difficult to eradicate the virus6.
Since the emergence of H5N1 on a poultry farm in 2003, H5 HPAI outbreaks have occurred continuously, and new influenza viruses and genotypes have been introduced into Korea7,8. Various clades of H5Nx, including H5N1, H5N6, and H5N8, have been identified9,10,11,12,13. In particular, outbreaks of HPAI subtypes H5N6 and H5N8 occurred simultaneously in 201714. During the unprecedented outbreaks of HPAI in 2016/2017, there has been increased demand for AIV vaccination by poultry producers and animal welfare organizations. Potential vaccines in the future should be considered with introduction of two or more viruses simultaneously around Korea.
Vaccination policy may be a supportable measure for preventing HPAI when implemented properly and in conjunction with accurate epidemiologic investigation and control measures7,15. Until recently, most AI vaccines used or registered in the field were whole inactivated AI vaccines16. VLPs are a vaccine platform that can respond effectively to a wide range of infectious viruses17,18. A previous study examined the efficacy of VLP vaccines against multi-clade H5N12, whereas another showed that VLP displaying H5, H7, H9, and N1 protect chicken from infection by heterologous virus19. However, preparation of a multivalent vaccine against various HPAI viruses by mixing monovalent whole inactivated vaccines raises biosecurity concerns and is economically unfeasible20. Therefore, a vaccine based on VLP requires stronger product development through further studies examining multi-subunit, chimeric, and other types of VLPs; such studies should focus on delivery of sufficient amounts of VLP antigen because these vaccines will not be competitive in the market unless they are both productive and cost-effective21.
Here, we constructed a single vector containing cassettes encoding multi-clade VLPs and then manufactured multi-clade VLPs as a vaccine capable of protecting against two separate clades of HPAI virus. We then compared its protective efficacy with that of a monovalent VLP and evaluated its potency against each homologous strain in specific pathogen free (SPF) chicken.
Results
Expression and preparation of monovalent and multi-clade VLPs
Monovalent VLP_ES2, VLP_KA435, and VLP_KA435chi, and multi-clade VLP_ES2/KA435chi vaccines, were produced and secreted successfully from Sf9 cells. The HA titer of VLP_ES2 in the culture supernatant ranged from 128 to 256 HAU. However, the HA titer of VLP_KA435 was as low as 32–64 HAU. The titer of VLP _KA435chi ranged from 128 to 256 HAU. The titer of the multi-clade VLP_ES2/KA435chi was 128–256 HAU. After large-scale production, the titer of the multi-clade VLP was also 128–256 HAU per 25 L of medium (the same as lab-scale production) (Table 1). The multi-clade VLP was purified from the culture supernatant and examined by transmission electron microscopy and western blot (Fig. 1).
Transmission electron microscopy (EM) and Western blot analysis of multi-clade VLPs. Negative stain (1% uranyl acetate) EM image of multi-clade VLPs (Scale bar is 100 nm, red arrow indicates multi-clade VLP and blue arrow indicate baculoviral particle) (A). Blots were incubated with chicken anti-HA antiserum and goat ant-chicken IgG (HRP). HA and M specific bands were detected in preparations of multi clade VLP infected cells (B).
Comparison of the efficacy between monovalent and multi-clade VLP vaccination
Next, we compared the protective efficacy of the monovalent and multi-clade VLP vaccines in SPF chickens infected with KA435/2.3.2.1c and ES2/2.3.4.4c. A 100% survival rate was observed in all groups of SPF chickens, except the group receiving the VLP_KA435 vaccine (90%), after challenge with homologous HPAI (Table 2). All SPF chickens vaccinated with VLPs had seroconverted before challenge, with a mean HI titer of 5.6–9.0 log2 against each antigen. SPF chickens vaccinated with the multi-clade VLP had a mean HI titer of 7.0–7.3 log2 against both antigens. In SN test, sera from the multi-clade VLP vaccinated chickens induced mean SN titer 72–524 (range from 20 to 1280) against both antigens before challenge, and sera from monovalent VLP vaccinated chickens induced mean SN titer 26 and 62 (range from 0 to 160) against each homologous antigen although they show no reaction in cross SN test. After challenge with KA435/2.3.2.1c and ES2/2.3.4.4c, all vaccinated chickens produced green stools and diarrhea, and some became lethargic. Virus shedding was detected from 1–5 dpi, with a viral titer of 101.3–102.2 TCID50/0.1 mL in OP swab samples and 101.6–102.0 TCID50/0.1 mL from 1 to 5 dpi in CL swab samples. In the multi-clade VLP vaccinated groups, virus shedding was detected at 1 dpi in an OP sample from one chicken, with viral titer of 101.3 TCID50/0.1 mL, after challenge with KA435/2.3.2.1c; no virus shedding was detected in birds challenged with ES2/2.3.4.4c. In the sham groups challenged with KA435/2.3.2.1c and ES2/2.3.4.4c, virus shedding was detected at 1 dpi in seven chickens, with a viral titer of 102.0–102.2 TCID50/0.1 mL only in the OP swab sample. There were significant differences (p < 0.05) at 1 dpi in the viral titers in OP swab samples from sham-vaccinated chickens and SPF chickens vaccinated with the multi-clade VLP vaccine against KA435/2.3.2.1c (Table 2).
Potency and efficacy of the chimeric multi-clade VLP vaccination against each homologous challenge
Clinical protection
For SPF chickens, vaccination with one dose conferred 100% clinical protection, with no clinical symptoms evident after challenge with KA435/2.3.2.1c and ES2/2.3.4.4c. However, the group vaccinated with one-tenth dose and then challenged with KA435/2.3.2.1c suffered 20% mortality by 6 dpi (Fig. 2A). Vaccination with one-hundredth dose resulted in even higher mortality (around 70%) at 6 dpi. Mortality in the group receiving one-hundredth dose of the multi-clade VLP vaccine and then challenged with KA435/2.3.2.1c was 100%, showing neurological signs and diarrhea; however, in the group challenged with ES2/2.3.4.4c, eight chickens died at 7–8 dpi post-challenge (Fig. 2B) after showing neurological signs and diarrhea. The mean time to death in one-hundredth dose vaccination groups challenged with either virus was 3.3–3.4 days. For sham-treated chickens, the mean time to death was 2.5–2.6 days.
Survival of vaccinated chickens challenged with each homologous HPAIV. Survival of sham-vaccinated chickens and chickens inoculated with one (1) dose, one-tenth (1/10) dose, or one-hundredth (1/100) dose of the multi-clade VLP and then challenged with each homologous H5 HPAIV: (A) KA435/2.3.2.1c. (B) ES2/2.3.4.4c.
Clinical protection was also indicated by the vaccine potency (PD50) results22, with SPF chickens challenged with the KA435 or ES2 viruses showing potency values of 28 and 42 PD50, respectively (Table 3).
Serology
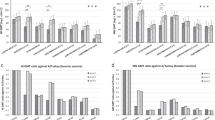
All vaccinated groups showed detectable antibody titers against ES2 and KA435 antigens pre-challenge and post-challenge; these titers increased over time (Fig. 3). In vaccinated SPF chickens (one dose or one-tenth dose), all those receiving the multi-clade VLP vaccine seroconverted before challenge, with a mean tier of 7.2–7.8 and 3.9–4.7 log2 after one dose and one-tenth dose, respectively. After challenge, the antibody titer against ES2 and KA435 antigens increased to 7.9–8.5 log2 and 6.5–7.6 log2 in the one dose and one-tenth dose groups, respectively. Unlike the one dose- and one-tenth dose-vaccinated groups, the one-hundredth dose-vaccinated groups showed a weaker antibody response (1.0 log2) prior to challenge. Following challenge, the HI titer of surviving chickens challenged with ES2 had more various antibody responses, with HI titers around 6.0 log2 (Fig. 3B). None of the sham-vaccinated chickens had detectable HI antibodies before challenge (data not shown).
Serological responses in vaccinated and challenged chickens. Hemagglutination inhibition (HI) assay titers in vaccinated chickens measured at different times post-vaccination and challenged with homologous virus. HI titers were assessed at 14 days post-vaccination (dpv), at 21 dpv, and at 14 days post-infection (dpi) with homologous virus. Chickens received one (1) dose, one-tenth (1/10) dose, or one-hundredth (1/100) dose. (A) Challenge with KA435/2.3.2.1c. (B) Challenge with ES2/2.3.4.4c. Individual data points are shown, along with mean and standard error.
Virus shedding
As shown in Fig. 4, little virus shedding was observed from 3 to 7 dpi in SPF chickens vaccinated with one dose of the multi-clade VLP. However, virus shedding was detected from 3 to 7 dpi in SPF chickens vaccinated with one-tenth dose of the multi-clade VLP and challenged with either KA435/2.3.2.1c or ES2/2.3.4.4c. In SPF chickens vaccinated with one-hundredth dose, virus shedding was detected in surviving birds, with a viral titer of 101.7–102.8 TCID50/0.1 mL in OP swab samples from 3–5 dpi and 102.4–104.0 TCID50/0.1 mL in CL swab samples from 3 to 5 dpi. Most birds in the sham-vaccinated groups were not tested because they died prior to sampling (Fig. 4).
Virus shedding in oropharyngeal (OP) and cloacal (CL) swab samples after inoculation with homologous HPAIV. Titers of virus shed in OP and CL samples from chicken sham-vaccinated or inoculated with vaccines at one (1) dose, one-tenth (1/10) dose, or one-hundredth (1/100) dose; titers were measured at 3, 5, and 7 days post-infection (dpi) with each homologous H5 HPAIV. Viral titers are expressed as log10TCID50 (50% tissue culture infectious dose)/0.1 mL, with error bars. (A) KA435/2.3.2.1c. (B) ES2/2.3.4.4c. The lower limit of detection was 1 log10TCID50/0.1 mL.
Antibody persistence
We measured the mean HI titers against ES2 and KA435 antigens in SPF chickens and layer chickens inoculated with one dose of the multi-clade VLP vaccine. In SPF chickens vaccinated with the multi-clade VLP, HI titers against the KA435 antigen were 9.3 log2 and those against the ES2 antigen were 7.8 log2 at 3 wpv. The mean HI titers peaked between 3 wpv, and remained above 7 log2 to provide reduction in challenge virus replication and shedding23 by 12 wpv (KA435) and 16 wpv (ES2) (Fig. 5A). In layer chickens vaccinated with the multi-clade VLP, HI titers against the KA435 antigen were 7.4 log2, and those against the ES2 antigen were 8.6 log2 at 3 wpv. The mean HI titers peaked at 3 wpv, and remained above 7 log2 by 8 wpv (KA435) and 3 wpv (ES2). None of the chickens showed HI titers above 7 log2 at 24 wpv (Fig. 5B).
Antibody persistence following administration of one dose (512 HAU) of vaccine. (A) SPF chickens. (B) Layer chickens. Hemagglutination inhibition (HI) assay titers were determined up to 24 weeks post-vaccination. Titers are expressed as log2 values. The horizontal dotted line indicates a HI titer of 7 log2, which is the standard threshold for preventing virus shedding.
Discussion
Here, we constructed a vector containing cassettes for multi-clade VLP into which two kinds of HA can be inserted. We then manufactured a multi-clade VLP vaccine by inserting the HA sequence of clade 2.3.2.1c(chimera) and 2.3.4.4c vaccine strains from the Korean AI national antigen bank. Finally, we compared the efficacy of monovalent and multi-clade VLP vaccines in SPF chickens challenged with homologous strains.
Chickens vaccinated with the multi-clade VLP shed less virus and were better protected against challenge than chickens vaccinated with the monovalent vaccine. This is likely because multivalent vaccines trigger stronger cellular and humoral responses, thereby inhibiting viral replication more effectively than the monovalent VLP vaccines24,25. Moreover, one chicken vaccinated with the monovalent VLP_KA435chi died, despite showing a higher HI titer (9.0 log2) (Table 2); this may be because the HA2 of KA435 was substituted with the HA2 of Buan2, coupled with challenge with the more virulent 2.3.2.1c strain as reported previously26, and induction of lower SN titer which more closely related to the capability of antibodies controlling the replication of virus comparing HI titer27.
For use in an antigen bank, an emergency vaccine should show a minimum 50 PD50 per dose, or antibody persistence above and HI of 1/128 HI for 6 months post-vaccination; in Korea, the guidelines are a minimum 80% protection from mortality and an HI titer of greater than 1/12814. In this study, the PD50 and antibody persistence of the multi-clade VLP did not meet these criteria or were lower than those of whole inactivated vaccinated chickens in comparison with the results of a previous study26. Whole inactivated vaccines induce a stronger immune response than subunit vaccines such as VLP vaccines28. Therefore, we believe that our results are due to the fact that the VLP vaccine is based on part (M and HA) of the virus. Indeed, other studies show that whole inactivated vaccines are more effective in potency tests than split, subunit vaccines and virosomes; also, whole inactivated vaccines have a significantly higher probability of inducing seroconversion than subunit vaccines29,30. Further study is needed on how multi-clade VLP increases protective efficacy comparing to whole inactivated vaccines.
Our multi-subtype vector includes a cassette into which two full HAs are co-localized within one VLP structure2; we then ensured high protein expression (128–256 HAU) by producing a chimeric VLP under optimized cell culture conditions. Indeed, productivity in cell culture was equal to that after large-scale (25 L) production (Table 2), and higher than that after large-scale production reported by other studies (e.g., 16–128 HAU in Sf9 cells)2,31. This process enables cost savings of approximately 0.02 dollars per dose when compared with mixing two types (0.068 dollars per dose) of whole inactivated vaccine using the egg product system of the Korean AI national bank (data not shown). This means that the multi-clade VLP we developed in this study may be economically more viable than vaccines cultured in eggs. In addition, multi-clade VLPs effectively induce Th1 type immune responses, and plasma and memory B cells32. Only single vaccination of multi-clade VLP with a dose of 512 HAU showed immunogenicity, survival rate and reduction of virus shedding in similar to results boosting other VLP vaccination2,19,33.
In conclusion, we constructed a single vector containing cassettes encoding multi-clade VLPs and produced a chimeric vaccine capable of protecting chickens against simultaneous challenge with clade 2.3.2.1c and 2.3.4.4c HPAIVs. Large amounts of vaccine were produced in cell culture, making the vaccine potentially cost-effective. However, the PD50 and antibody persistence were below national standards and lower than those of whole inactivated vaccines. Taken together, these results suggest that the multi-clade VLP vaccine is an economically effective vaccine that protects against infection by multiple HPAIVs.
Materials and methods
Cell and viruses
Sf9 insect cells (Gibco, Waltham, MA, USA) and Trichoplusia ni (Tni) insect cells were maintained in suspension in SF-900 III SFM medium (Gibco) and ESF 921 (Expression system, Davis, CA, USA), respectively, at 27 °C. Three different H5 HPAIVs from the Korean national antigen bank14 were used as seed strains and challenge strains. A/duck/Korea/ES2/2016(H5N6, clade 2.3.4.4c)12, hereafter called ES2/2.3.4.4c, and A/broiler duck/Korea/Buan2/2014(H5N8, clade 2.3.4.4a)11, hereafter called Buan2/2.3.4.4a, were isolated from poultry farms in Korea; A/chicken/Vietnam/NCVD-KA435/13(H5N1, clade 2.3.2.1c)14, hereafter called KA435/2.3.2.1c, was kindly provided by the National Center for Veterinary Diagnostics in Vietnam. A/Puerto Rico/8/34 (H1N1), hereafter named PR8, was used for cloning of the M1 gene. Viruses were propagated for 60 h in 10-day-old embryonated eggs from SPF chickens.
Cloning of HA and M genes
Viral RNA was extracted from ES2/2.3.4.4c, KA435/2.3.2.1c, Buan2/2.3.4.4a, and PR8 using RNeasy mini kit (Intron, Korea). To amplify DNA from extracted viral RNA, polymerase chain reaction (PCR) was performed using the RT kit (Intron) and specific primers containing restriction enzyme sequences. The following primer pairs were used for PCR amplification of modified HA genes with deletion of the polybasic amino acid region and the M1 gene: HA_ES2 (F) 5′-CGTGCGGGATCCATGGAGAAAATAGTGCTTCT-3′ and (R) 5′-AATAGGAAGCTTTTAAATGCAAATTCTTGCATT-3′; HA_KA435 (F) TGGACTACTAGTATGGAGAAGATCGTTCTTCT-3′ and (R) 5′-GAGTACGTCGACTTAAATGCAAATACGCACT-3′; M1_PR8 (F) CAATCGAGCATGCTCTCCCTCTTGAGCTTCCTA-3′ and (R) 5′-TGCCAGTCCCGGGATGAGTCTTCTAACCGAGGT-3′; HA1_KA435 (F) 5′-TGGACTACTAGTATGGAGAAGATCGTTCTTCT-3′ and (R) 5′-AGGAGTGGCGCCCCAAACAGTCCTCTTTTGCG-3′; HA2_Buan2 (F) 5′-TGTAAGCCTAGGATGGAGATAATTAAAATGAT-3′ and (R) 5′-AGCGGTCCTAGGATCAGATCCAGACATGATAA-3′. Amplified HA and M1 genes were cloned into the TA cloning vector pGEM®-T (Promega, Madison, WI, USA), and each sequence was confirmed by DNA sequencing.
Construction of expression vectors and generation of recombinant baculovirus
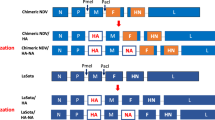
M1 of PR8 (M1_PR8) digested with SmaI/SphI and T4 DNA ligase (Takara, Japan) was used to ligate the resulting M1 DNA fragment into the SmaI/SphI-digested multi-cloning site (MCS) pFastBac™ dual vector (Invitrogen, Carlsbad, CA, USA) under the control of promotor10 (p10) (Fig. 6Aa). HA of ES2 (HA_ES2) and HA of KA435 (HA_KA435) were digested by BanH1/HindIII, and the resulting HA DNA fragments were each ligated into the BanH1/HindIII-digested polyhedron (PH) of the MCS pFastBac™ dual vector (Invitrogen) (Fig. 6Ab,Ac). To increase protein expression, HA_KA435 was constructed as a chimeric structure (HA_KA435chi). HA2 of KA435 and HA2 of Buan2 (HA_Buan2) were digested by KasI/HindIII, and HA2 of HA_KA435 was substituted with HA2 of HA_Buan2 (Fig. 6Ad). HA_KA435chi was digested by AvrII, and the resulting HA DNA fragment was ligated into the AvrII site of the pFastBac™ dual vector to generate a multi-clade VLP containing M1_PR8, HA_ES2, and HA_KA435chi (HA_ES2/KA435chi) (Fig. 6B).
Baculovirus construct for production of multi-clade VLPs. (A) (a) M1 of PR8 (M1_PR8) is inserted between restriction enzyme sites for SphI and SmaI, and the HAs of (b) ES2, (c) KA435 (HA_ES2 and HA_KA435), and (d) chimera of KA435 (HA_KA435chi) are inserted between restriction sites for BamHI and HindIII. (B) The M1 gene is under the control of the p10 promoter (PP10); the HA of ES2 (HA_ES2) is under the control of the polyhedron promoter (PPH); and the HA chimera of KA435 (HA_KA435chi), inserted into the AvrII restriction site, is under the control of the polyhedron promoter (PPH).
The constructed pFastBac™ dual vector plasmids pHA_ES2, pHA_KA435, pHA_KA435chi, and pHA_ES2/KA435chi were transformed into DH10Bac™ competent cells (Invitrogen) to generate recombinant bacmids. Recombinant bacmid DNA was transfected into Sf9 cells seeded in 6-well plates (8 × 105 cells/well) using Cellfectin® Reagent (Invitrogen), resulting in the release of recombinant baculovirus (rBV) into the culture medium. At 72 h post-transfection, culture medium was harvested and inoculated into Sf9 cells to generate a high titer rBV stock. Titration of the baculovirus stock was performed in a plaque assay on Sf9 cells.
Vaccine development
To express each monovalent VLP (VLP_ES2, VLP_KA435, and VLP_KA435chi) and multi-clade VLP (HA_ES2/KA435chi), Sf9 cells (2 × 106 cells/mL) were infected for 3 days with recombinant baculovirus expressing HA and M1 (for monovalent HA expression), or with two HAs and M1 (for multi-clade protein expression) at a multiplicity of infection of 1. The cultured medium containing VLPs was collected and centrifuged for 10 min at 2000 × g to remove cell debris. The culture supernatant was chemically treated with formalin (final concentration, 0.1%) to inactivate baculovirus. For large-scale production, Tni insect cells34 under optimized condition in similar with that of Sf9 cells were infected with recombinant baculovirus expressing VLPs. VLPs were harvested from the medium supernatant, purified on sucrose gradient and concentrated by pump system (100 kDa filtration) (Masterflex). VLP expression of influenza protein was confirmed by Coomassie staining of sodium dodecyl sulfate polyacrylamide gels, western blot carried out using chicken anti-HA antiserum and goat anti chicken IgG (HRP)(KPL) and by nucleotide sequence analysis and hemagglutinin inhibition (HI) tests against homologous antibody. Viral growth was tested in a hemagglutination activity (HA) assay, and titers were adjusted to yield 512 HA units per dose after emulsifying (30:70, w/w) in Montanide ISA VG70 oil adjuvant (SEPPIC, La Garenne-Colombes, France)35.
Transmission electron microscopy of VLPs
The VLP suspension was placed on formvar-coated (copper 300 mash) grids, negatively stained with 1% uranyl acetate, and dried by aspiration. The VLP particles were then examined under a transmission electron microscope (Hitachi7100FA, Tokyo, Japan).
Efficacy of the monovalent and multi-clade VLP vaccines
To compare the efficacy of the monovalent vaccines with that of the multi-clade VLP vaccine, 60 5-week-old SPF chickens were divided into six groups (10 chickens per group). The four groups used for vaccination comprised two groups of chickens receiving monovalent VLP vaccines (VLP_ES2 or VLP_KA435chi) and two groups receiving the multi-clade VLP (VLP_ES2/KA435chi) vaccine. All VLP vaccines were emulsified in Montanide ISA VG70 adjuvant and injected via the intramuscular route; each chicken received 0.5 mL. Another 20 SPF chickens were split into two sham groups and injected with an emulsified solution of PBS plus ISA VG70 in the same ratio as the VLP vaccines. Serum samples were collected from all chickens at weekly intervals post-vaccination and then at 14 days post infection (dpi). To determine the immunogenicity of the VLP vaccines, all sera were tested by HI test against homologous and heterologous, and Serum neutralizing antibody test (SN test) in Dermal Fibroblast 1 (DF1) cells, as described previously36. Three weeks after vaccination, two groups of vaccinated chickens were challenged with 0.1 mL ES2/2.3.4.4c and KA435/2.3.2.1c (106.0 EID50/0.1 mL). All birds were observed daily for 14 dpi to check mortality, clinical signs, and viral shedding. To determine viral shedding, oropharyngeal (OP) and cloacal (CL) swab samples were collected at 1, 3, 5, 7, 10, and 14 dpi. Each OP or CL sample was suspended in 1 mL of maintenance medium containing an antibiotic–antimycotic mixture (Invitrogen). Samples were used to inoculate cultures of Dermal Fibroblast 1 (DF1) cells, and virus growth was determined by detection of cytopathic effects and measurement of HA activity. Virus titers were calculated as described elsewhere22. The limit of virus detection was < 1 log10 TCID50/0.1 mL. All experiments with live H5 virus were performed in biosafety level 3 facilities and in accordance with guidelines approved by the Animal Ethics Committee of the Animal and Plant Quarantine Agency, Korea (approval number: 2019-492 and 2020-544). Our study is conducted in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Potency and efficacy of multi-clade VLP vaccination against each homologous challenge
To evaluate the potency (PD50, protective dose 50%) and efficacy of the multi-clade VLP vaccine, 40 6-week-old SPF chickens for each HA antigen (ES2/2.3.4.4c and KA435/2.3.2.1c) were divided into four groups (10 chickens per group): three immunization groups and one non-immunization (sham) group. Chickens were immunized intramuscularly with one dose, one-tenth (1/10) dose, or one-hundredth 1(1/100) dose of vaccine in Montanide ISA VG70. The sham group was inoculated with PBS in ISA VG70. At 3 weeks post-vaccination (wpv), chickens were challenged intranasally with 106 EID50 (in 0.1 mL) of a virus homologous to the vaccine strain. Post-challenge, chickens were monitored daily for clinical signs and mortality. The PD50 was calculated using mortality as the end point, as described previously22.
Serology and antibody assays
To determine the immunogenicity of the VLP vaccines, serum samples were collected from all chickens prior to vaccination and then at weekly intervals post-vaccination. Serum samples were also obtained from all living chickens at 14 days post-challenge. All sera were subjected to HI tests. The HI titer against clade 2.3.2.1c and clade 2.3.4.4c antigens was measured using OIE standard HI methods.
Post-challenge virus shedding
OP and CL swabs were collected from animals in all groups at 3, 5, 7, 10, and 14 dpi. Each sample was suspended in 1 mL of maintenance medium containing an antibiotic–antimycotic mixture (Invitrogen). Samples were treated and inoculated onto DF1 cells as mentioned above.
Antibody persistence
To determine the persistence of protection provided by the VLP vaccines, 10 6-week-old SPF chickens and 10 14-week-old unimmunized layer chickens (these chickens did not even have the H9N2 vaccine) from a poultry farm in Korea were immunized with a single dose. Blood samples were collected from all immunized chickens every month for 6 months post-vaccination.
Statistical analysis
Data were analyzed using Prism version 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA). Comparison of serum titers between the groups was made using one-way analysis of variance (ANOVA). Survival rates among groups were analyzed using the log-rank test. The statistical significance of differences between measurements was determined using Student’s t-test. A p-value < 0.05 was deemed significant.
Ethical statements
All experiments with live H5 virus were performed in biosafety level 3 facilities and in accordance with guidelines approved by the Animal Ethics Committee of the Animal and Plant Quarantine Agency, Korea (approval number: 2019-492 and 2020-544). Our study is conducted in accordance with ARRIVE guidelines (https://arriveguidelines.org).
References
Shen, H. et al. Assembly and immunological properties of a bivalent virus-like particle (VLP) for avian influenza and Newcastle disease. Virus Res. 178, 430–436 (2013).
Pushko, P. et al. Multi-clade H5N1 virus-like particles: Immunogenicity and protection against H5N1 virus and effects of beta-propiolactone. Vaccine 36, 4346–4353 (2018).
Dhingra, M. S. et al. Global mapping of highly pathogenic avian influenza H5N1 and H5Nx clade 2.3. 4.4 viruses with spatial cross-validation. Elife 5, e19571 (2016).
Lee, D. H., Bertran, K., Kwon, J.-H. & Swayne, D. E. Evolution, global spread, and pathogenicity of highly pathogenic avian influenza H5Nx clade 2.3. 4.4. J. Vet. Sci. 18, 269 (2017).
Claes, F., Morzaria, S. P. & Donis, R. O. Emergence and dissemination of clade 2.3. 4.4 H5Nx influenza viruses—How is the Asian HPAI H5 lineage maintained. Curr. Opin. Virol. 16, 158–163 (2016).
OFFLU avian influenza Report, September 2019 to February 2020.
Lee, C. W. et al. Characterization of highly pathogenic H5N1 avian influenza A viruses isolated from South Korea. J. Virol. 79, 3692–3702 (2005).
Yoon, H., Park, C. K., Nam, H. M. & Wee, S. H. Virus spread pattern within infected chicken farms using regression model: The 2003–2004 HPAI epidemic in the Republic of Korea. J. Vet. Med. Ser. B 52, 428–431 (2005).
Kim, H. R. et al. Highly pathogenic avian influenza (H5N1) outbreaks in wild birds and poultry, South Korea. Emerg. Infect. Dis. 18, 480 (2012).
Choi, J. G. et al. Characterization of clade 2.3. 2.1 H5N1 highly pathogenic avian influenza viruses isolated from wild birds (mandarin duck and Eurasian eagle owl) in 2010 in Korea. Viruses 5, 1153–1174 (2013).
Jeong, J. S. et al. Highly pathogenic avian influenza virus (H5N8) in domestic poultry and its relationship with migratory birds in South Korea during 2014. Vet. Microbiol. 173, 249–257 (2014).
Lee, E. K. et al. Multiple novel H5N6 highly pathogenic avian influenza viruses, South Korea, 2016. Infect. Genet. Evol. 51, 21–23 (2017).
Si, Y. J. et al. Genetic characterisation of novel, highly pathogenic avian influenza (HPAI) H5N6 viruses isolated in birds, South Korea, November 2016. Eurosurveillance 22, 30434 (2017).
Kang, Y. M. et al. Protective efficacy of vaccines of the Korea national antigen bank against the homologous H5Nx clade 2.3. 2.1 and clade 2.3. 4.4 highly pathogenic avian influenza viruses. Vaccine 38, 663–672 (2020).
Bertran, K. et al. Protection of White Leghorn chickens by US emergency H5 vaccination against clade 2.3. 4.4 H5N2 high pathogenicity avian influenza virus. Vaccine 35, 6336–6344 (2017).
Swayne, D. E. Impact of vaccines and vaccination on global control of avian influenza. Avian Dis. 56, 818–828 (2012).
Lua, L. H. et al. Bioengineering virus-like particles as vaccines. Biotechnol. Bioeng. 111, 425–440 (2014).
Frietze, K. M., Peabody, D. S. & Chackerian, B. Engineering virus-like particles as vaccine platforms. Curr. Opin. Virol. 18, 44–49 (2016).
Pushko, P. et al. Virus-like particles displaying H5, H7, H9 hemagglutinins and N1 neuraminidase elicit protective immunity to heterologous avian influenza viruses in chickens. Virology 501, 176–182 (2017).
Grgacic, E. V. & Anderson, D. A. Virus-like particles: Passport to immune recognition. Methods 40, 60–65 (2006).
Buckland, B. C. The process development challenge for a new vaccine. Nat. Med. 11, S16–S19 (2005).
Ramakrishnan, M. A. Determination of 50% endpoint titer using a simple formula. World journal of virology 5, 85 (2016).
OIE Terrestrial manual 2018. Chapter 3.3.4. Avian influenza (infection with avian influenza viruses).
Swayne, D., Beck, J., Garcia, M. & Stone, H. Influence of virus strain and antigen mass on efficacy of H5 avian influenza inactivated vaccines. Avian Pathol. 28, 245–255 (1999).
Quan, F. S. et al. A bivalent influenza VLP vaccine confers complete inhibition of virus replication in lungs. Vaccine 26, 3352–3361 (2008).
Kang, Y. M. et al. Protection of layers and breeders against homologous or heterologous HPAIv by vaccines from Korean national antigen bank. Sci. Rep. 10, 1–9 (2020).
Peipei, Wu. et al. Single dose od consensus hemagglutinin-based virus-like particles vaccine protects chickens against divergent H5 subtype influenza viruses. Front. Immunol. 8, 1649 (2017).
Geeraedts, F. et al. Whole inactivated virus influenza vaccine is superior to subunit vaccine in inducing immune responses and secretion of proinflammatory cytokines by DCs. Influenza Other Respir. Viruses 2, 41–51 (2008).
Stephenson, I. et al. Safety and antigenicity of whole virus and subunit influenza A/Hong Kong/1073/99 (H9N2) vaccine in healthy adults: Phase I randomised trial. The Lancet 362, 1959–1966 (2003).
Hagenaars, N. et al. Head-to-head comparison of four nonadjuvanted inactivated cell culture-derived influenza vaccines: Effect of composition, spatial organization and immunization route on the immunogenicity in a murine challenge model. Vaccine 26, 6555–6563 (2008).
Krammer, F. et al. Trichoplusia ni cells (High Five TM) are highly efficient for the production of influenza A virus-like particles: A comparison of two insect cell lines as production platforms for influenza vaccines. Mol. Biotechnol. 45, 226–234 (2010).
Song, J. M. et al. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology 405, 165–175 (2010).
Kapczynski, D. R. et al. Vaccination with virus-like particles containing H5 antigens from three H5N1 clades protects chickens from H5N1 and H5N8 influenza viruses. Vaccine 34, 1575–1581 (2016).
Vicente, T., Roldão, A., Peixoto, C., Carrondo, M. J. & Alves, P. M. Large-scale production and purification of VLP-based vaccines. J. Invertebr. Pathol. 107, S42–S48 (2011).
Liu, C. G. et al. Evaluation of several adjuvants in avian influenza vaccine to chickens and ducks. Virol. J. 8, 1–6 (2011).
Kim, H. M. et al. Immunogenicity and protective efficacy of clade 2.3.2.1c and clade 2.3.4.4c H5Nx avian influenza antigen bank vaccines in mice, Korea. Vaccine 38(39), 6080–6087 (2020).
Acknowledgements
This research was supported by a grant from the Animal and Plant Quarantine Agency (M-1543418-2019-20-01) of the Republic of Korea.
Author information
Authors and Affiliations
Contributions
All authors approved the final article. K.H.-M. and K.M.-C. are corresponding authors. K.Y.M. is the first author and wrote main manuscript text with C.H.K. and P.S.J. K.J.H., L.S.J. and P.J.W. prepared figures and K.D.Y., K.S.Y. and L.M.H. prepared tables. All authors reviewed the manuscript. All authors have approved the final article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, YM., Cho, HK., Kim, J.H. et al. Single dose of multi-clade virus-like particle vaccine protects chickens against clade 2.3.2.1 and clade 2.3.4.4 highly pathogenic avian influenza viruses. Sci Rep 11, 13786 (2021). https://doi.org/10.1038/s41598-021-93060-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93060-8
- Springer Nature Limited