Abstract
The predictive effect of quantitative anti-hepatitis B core on double-negative HBV DNA and RNA remains unstudied. We observed dynamic changes in this measure in chronic hepatitis B patients receiving entecavir for 10 years, evaluating its predictive value for double-negative HBV DNA and RNA. Twenty-seven chronic hepatitis B patients treated with entecavir for 10 years were enrolled in this study. Liver function, quantitative anti-hepatitis B core, hepatitis B surface and e antigens, HBV DNA and RNA were measured at baseline and each follow-up. Virological response was defined as double-negative HBV DNA and RNA; serological response was defined as hepatitis B e antigen seroconversion. After antiviral therapy, quantitative anti-hepatitis B core showed an overall downward trend. Patients with virological response had significantly higher quantitative anti-hepatitis B core levels than those without virological response at baseline. Patients with serological response also had higher quantitative anti-hepatitis B core levels than those without serological response at baseline and week 24. Baseline quantitative anti-hepatitis B core level was the only independent predictor for virological and serological responses. Baseline quantitative anti-hepatitis B core level was powerfully predictive of double-negative HBV DNA and RNA in chronic hepatitis B patients receiving long-term entecavir therapy.
Similar content being viewed by others
Introduction
Approximately 350 million people worldwide are infected with hepatitis B virus (HBV)1, which can lead to hepatitis, cirrhosis, hepatocellular carcinoma (HCC) and liver failure. Interferons (IFNs) and nucleotide analogue (NA) are the main anti-HBV drugs. For HBeAg-positive patients, virological response (VR) and serological response (SR) during therapy are defined as loss of serum HBV DNA and hepatitis B e antigen (HBeAg) seroconversion, respectively. Treatment discontinuation should not been considered until the patients have got alanine aminotransferase (ALT) normalization, VR and SR2,3,4,5. However, VR and SR don’t represent that HBV covalently closed circular DNA (cccDNA) has been cleared in hepatocytes. Patients with VR and SR still had high frequency of virological rebound and hepatitis relapse after discontinuation of NA.
Serum HBV RNA is an indicator of cccDNA activity in chronic hepatitis B (CHB) patients treated with NA6,7. Undetectable serum HBV RNA may indicate the transcriptional silencing of cccDNA8,9. Double-negative HBV DNA and RNA at end of NA treatment was considered as a potent marker for guiding discontinuation in HBeAg positive CHB patients by Fan et al.10. Accordingly, VR should be redefined as double-negative HBV DNA and RNA.
Hepatitis B core antibody (HBcAb) is an HBV-specific antibody that reflects the host immune response against HBV11,12. Yuan et al. first reported in 2013 that baseline quantitative anti-hepatitis B core (qAnti-HBc) levels may serve as a useful marker indicating an ongoing host immune activity against HBV13. Many studies have shown that baseline qAnti-HBc levels could serve as a useful marker for predicting SR in HBeAg-positive CHB patients during Peg-IFN and NA therapies14,15,16,17. In 2020, Fu et al. indicated that patients with baseline qAnti-HBc level ≥ 4.15log10 IU/mL and liver stiffness measurements ≥ 9.85 kPa had the highest rates of SR after 96 weeks of NA (entecavir, telbivudine or tenofovir disoproxil fumarate) therapy18. However, no studies have investigated the clinical value of qAnti-HBc levels for redefined VR (double-negative HBV DNA and RNA) following long-term NA therapy in CHB patients in real-life practice. Therefore, the aims of this study were to investigate dynamic changes of qAnti-HBc levels in CHB patients treated with entecavir for 10 years, and to evaluate its value in predicting redefined VR (double-negative HBV DNA and RNA).
Results
Demographic and clinical characteristics
Thirty-three CHB patients were enrolled in the study. In all, 27 patients with available serial samples were included in the analysis. The demographic, virological and clinical characteristics of the patients are summarized in Table 1. Patients were predominantly male (70.4%) with mean age of 32.41 ± 9.46 years, 77.8% were HBeAg positive, and 63% were genotype C. The means of baseline HBV DNA, HBV RNA, anti-HBc and ALT levels were 6.29 ± 1.21 log10 IU/mL, 5.39 ± 1.47 log10 copies/mL, 3.07 ± 0.87 log10 IU/mL and 104.73 ± 19.82 U/L, respectively.
Therapy efficacy
Of all 27 patients, 24 (88.9%) and one (3.7%) achieved ALT normalization and HBsAg loss, respectively, after 10 years of antiviral therapy. VR and SR during therapy increased from 25.9% and 4.8%, respectively, at week 48 to 63.0% and 71.4%, respectively, at year 10 (Fig. 1). The incidence of HCC, cirrhosis and death was 3.70% (1/27), 3.70% (1/27) and 0, respectively at year 10.
Kinetics of qAnti-HBc, HBV DNA and HBV RNA during 10 years of antiviral therapy
Serum qAnti-HBc, HBV DNA and HBV RNA were measured at baseline and after entecavir treatment at week 24, 48, and 96, and year 5 and 10 (Fig. 2a–c, Supplementary Table 1). Each parameter showed an overall significant downward trend with increasing duration of treatment (Fig. 2d, qAnti-HBc, p < 0.001; HBV DNA, p < 0.001; HBV RNA, p < 0.001).
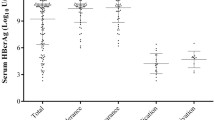
Kinetics of qAnti-HBc in patients with differing therapy responses
qAnti-HBc levels in patients stratified by treatment response were further analyzed (Fig. 3). Patients with VR had significantly higher baseline qAnti-HBc levels than those without VR (3.42 ± 0.71 log10 IU/mL vs 2.48 ± 0.84 log10 IU/mL, p = 0.005). In the HBeAg-positive cohort, patients with SR had significantly higher qAnti-HBc levels than those without SR at both baseline and week 24 (baseline, 3.17 ± 0.56 log10 IU/mL vs 2.24 ± 0.86 log10 IU/mL, p = 0.008; week 24, 3.05 ± 0.43 log10 IU/mL vs 2.29 ± 0.57 log10 IU/mL, p = 0.004).
Correlation between baseline characteristics and therapy efficacy at year 10
To further evaluate baseline characteristics in predicting VR and SR, multivariate analyses were conducted with inclusion of age, HBV genotype and baseline levels of ALT, HBV DNA, HBV RNA and qAnti-HBc in the model. Regression analysis showed that baseline qAnti-HBc level was the only independent predictor for VR (odds ratio [OR] 0.091, 95% confidence interval [CI] 0.003–0.843, p = 0.038; Table 2) and SR (OR 0.018, 95% CI 0.001–0.479, p = 0.016; Table 3).
Performance of baseline qAnti-HBc level in predicting VR and SR
To evaluate the performance of baseline qAnti-HBc levels in predicting VR and SR, we examined the areas under the receiver operator characteristic curve (AUROC). As shown in Fig. 4, the AUROC of baseline qAnti-HBc was higher in predicting VR (0.812, p = 0.008) and SR (0.844, p = 0.016) at year 10 than baseline HBV DNA (VR, 0.441, p = 0.616; SR, 0.511, p = 0.938), HBV RNA (VR, 0.329, p = 0.145; SR, 0.367, p = 0.350) and ALT (VR, 0.541, p = 0.725; SR, 0.656, p = 0.276).
Rates of VR and SR among patients with favorable baseline qAnti-HBc at year 10
The sum of sensitivity and specificity was maximal in predicting VR and SR at year 10 when the cut-off value was 3.1 log10 IU/mL. Patients were stratified into two groups according to the cut-off value. Eighty percent (8/10) and 100% (10/10) of patients with qAnti-HBc ≥ 3.1 log10 IU/mL achieved VR and SR, respectively, after 10 years of antiviral therapy. However, only 36.4% (4/11) and 45.5% (5/11) of patients in the group with qAnti-HBc < 3.1 log10 IU/mL achieved VR and SR, respectively, at year 10 (p = 0.006).
Discussion
Baseline qAnti-HBc could predict HBeAg seroconversion in CHB patients treated with IFNs or NA14,15,16,17,18. The present study evaluated dynamic changes in qAnti-HBc in CHB patients during 10 years of entecavir therapy. We demonstrated that the mean qAnti-HBc level decreased gradually, and that baseline qAnti-HBc could serve as an independent predictor for HBeAg seroconversion. To our knowledge, this is the longest comprehensive and definitive analysis to assess the performance of qAnti-HBc levels in CHB patients treated with entecavir.
HBeAg seroconversion and HBV DNA suppression at the end of post-antiviral therapy follow-up are the two major endpoints associated with favorable outcomes in HBeAg-positive patients. However, HBeAg seroconversion and HBV DNA suppression are not equivalent to HBV cccDNA elimination in hepatic cells. HBV RNA directly derived from cccDNA can reflect the intrahepatic cccDNA level. Recently, the use of the redefined VR (double-negative HBV DNA and RNA) has been suggested to be a safe rule for cessation of NA therapy in CHB patients. However, no data have been reported regarding the predictive value of baseline qAnti-HBc levels for redefined VR in an NA-treated cohort. We were the first to discover baseline qAnti-HBc could serve as an independent predictor for the redefined VR. In addition, a baseline qAnti-HBc level of ≥ 3.1 log10 IU/mL was associated with higher rates of VR and SR in CHB patients treated with entecavir. However, the levels were lower than the results reported by previous studies15,17,18. Serum qAnti-HBc levels are closely related to host immune status and are strongly associated with hepatitis activity in CHB patients. Song et al.11 showed that the mean qAnti-HBc levels in patients in the immune clearance and HBeAg-negative hepatitis phases were significantly higher than those in patients in both the immune tolerance and low replicative phases. Serum qAnti-HBc levels were also positively correlated with ALT levels, inflammatory activity, significant fibrosis, HBV DNA, HBsAg and hepatitis B core-related antigen19,20,21. Compared with patients in previous studies, the patients in this study had lower levels of ALT and HBV DNA, and most of them were HBeAg-positive. These factors may account for the low baseline qAnti-HBc levels in the patients in this study.
Baseline HBV DNA, HBV RNA and ALT levels have been proven to be independently associated with HBeAg seroconversion in previous studies15,22,23. However, in the present study, when anti-HBc was included in the multivariate analysis in combination with either VR or SR, baseline HBV DNA, HBV RNA and ALT showed no correlation with either VR or SR. The AUROC values of HBV DNA, HBV RNA and ALT for VR and SR were also less than that of anti-HBc, indicating that anti-HBc levels had better predictive value than baseline HBV DNA, HBV RNA and ALT. HBcAb is produced by hepatitis B core antigen-activated B-cells, which could inhibit HBV replication through hepatocytotoxic effects and regulate the activity of CD4+ and CD8+ T cells by producing cytokines such as IFN-γ or IL-624,25. Therefore, it is possible that a higher HBcAb level at baseline may reflect a better anti-viral response in CHB patients, which is associated with better prognosis after antiviral therapy. Baseline qAnti-HBc level may therefore be a potent biomarker for guiding NA discontinuation in CHB patients.
This study had several limitations. The major limitation was the relatively small sample size. Only 27 patients with CHB were included in this study, and therefore more patients are needed for future analyses. Furthermore, this was a single-center study; multi-center research should be conducted to explore in greater detail the clinical significance of qAnti-HBc in antiviral therapy. Additionally, we did not study the value of qAnti-HBc for the safe discontinuation of NA treatments.
In conclusion, our study showed that baseline serum qAnti-HBc was a powerful predictor of double-negative HBV DNA and RNA in CHB patients receiving long-term entecavir therapy.
Methods
Study population
CHB patients were given entecavir (0.5 mg/day, orally) after assigning informed consents and were followed between April 2007 and May 2018 at the department of infectious diseases of the First Affiliated Hospital of Xi’an Jiaotong University (Shaanxi, China). Serum samples of the patients were routinely collected and stored at -80℃. All patients were older than 16 years, with eGFR > 50 mL/(min × 1.73m2), had been positive for hepatitis B surface antigen (HBsAg) for longer than 6 months and had detectable serum HBV DNA. Reasons for exclusion were as follows: Compilated with A, C, D, E or other viral hepatitis; Compilated with acquired immunodeficiency syndrome; Decompensated liver cirrhosis (Child Pugh C); Taking other anti-HBV drug; Previous diagnosis of hepatocellular carcinoma; Compilated with autoimmune liver disease, alcoholic liver disease or cholestatic liver disease; With other serious medical conditions that affect follow-up compliance. The study was approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University and was performed in accordance with relevant guidelines and regulations. Informed consent was obtained from the parents legally authorized representatives of subjects that were under 18.
Clinical and laboratory evaluation
Routine demographic data collection (age, gender, family history of hepatitis B, etc.) was carried out for all the patients in the group. Liver function, blood routine, HBV DNA quantitative value, HBeAg quantitative value, HBsAg quantitative value and upper abdominal ultrasound were carried out at baseline, then liver function, blood routine, HBV DNA quantitative value, HBeAg quantitative/qualitative value, HBsAg quantitative/qualitative value and upper abdominal ultrasound were checked every 12 weeks at the first year, and 24 weeks from the second year to the end of study. HBV DNA was measured with COBAS TaqMan HBV test (ROCHE, USA, Lower limit of detection, 20 IU/mL). HBV genotype was determined by nested PCR using type-specific primers26.
Definitions
VR in this study was defined as double-negative serum HBV DNA and RNA. SR was defined as HBeAg seroconversion in HBeAg-positive patients. HBeAg seroconversion was defined as the loss of HBeAg accompanied by detection of anti-HBe antibodies.
Quantitative anti-HBc and HBV RNA evaluation
Serum samples collected at each visit (before treatment, week 24, week 48, week 96, year 5 and year 10) were stored at − 80 °C until analysis. Serum qAnti-HBc was measured using a commercial kit with a double-sandwich immunoassay (WANTAI, China) according to the manufacturer’s protocols and previous studies11,13,27. HBV RNA was measured with Diagnostic Kit for Hepatitis B virus pgRNA (PCR-Fluorescence Probing, Lower limit of detection, 300 copies/mL) according to the Manufacturer’s instruction (HOTGEN, China).
Statistical analysis
The non-normal distributions were showed as median values (Interquartile ranges, IQR), the continuous measurements of normal distribution were represented as means (standard deviations, SDs), and the categorical variables were represented as subject number (percentage). The associations between qAnti-HBc level and VR or SR were assessed using logistic regression analysis. AUROC were calculated for analyses of the performance of qAnti-HBc level in predicting VR and SR. Statistical analysis was performed using SPSS ver. 17.0 software (ISM, Armonk, NY). p < 0.05 was considered statistically significant.
References
Lee, H., Fawcett, J., Kim, D. & Yang, J. H. Correlates of hepatitis B virus-related stigmatization experienced by Asians: A scoping review of literature. Asia Pac. J. Oncol. Nurs. 3, 324–334 (2016).
Sarin, S. K. et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol. Int. 10, 1–98 (2016).
European Association for the Study of the Liver. European Association for the Study of the, EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 67, 370–398 (2017).
Terrault, N. A. et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 63, 261–283 (2016).
C. M. A. Chinese Society of Hepatology, C. M. A. Chinese Society of Infectious Diseases. The guideline of prevention and treatment for chronic hepatitis B: A 2015 update. Zhonghua Gan Zang Bing Za Zhi 23, 888–905 (2015).
Rokuhara, A. et al. Hepatitis B virus RNA is measurable in serum and can be a new marker for monitoring lamivudine therapy. J. Gastroenterol. 41, 785–790 (2006).
Huang, H. et al. Serum HBV DNA plus RNA shows superiority in reflecting the activity of intrahepatic cccDNA in treatment-naive HBV-infected individuals. J. Clin. Virol. 99–100, 71–78 (2018).
Wang, J. et al. Reply to: “Serum HBV pgRNA as a clinical marker for cccDNA activity”: Consistent loss of serum HBV RNA might predict the “para-functional cure” of chronic hepatitis B. J. Hepatol. 66, 462–463 (2017).
Giersch, K., Allweiss, L., Volz, T., Dandri, M. & Lutgehetmann, M. Serum HBV pgRNA as a clinical marker for cccDNA activity. J. Hepatol. 66, 460–462 (2017).
Fan, R. et al. Association between negative results from tests for HBV DNA and RNA and durability of response after discontinuation of nucles(t)ide analogue therapy. Clin. Gastroenterol. Hepatol. 18, 719–727 (2020).
Song, L. W. et al. Quantitative hepatitis B core antibody levels in the natural history of hepatitis B virus infection. Clin. Microbiol. Infect. 21, 197–203 (2015).
Zerbini, A. et al. The characteristics of the cell-mediated immune response identify different profiles of occult hepatitis B virus infection. Gastroenterology 134, 1470–1481 (2008).
Yuan, Q. et al. Quantitative hepatitis B core antibody level may help predict treatment response in chronic hepatitis B patients. Gut 62, 182–184 (2013).
Hou, F. Q. et al. Quantitative hepatitis B core antibody level is a new predictor for treatment response in HBeAg-positive chronic hepatitis B patients receiving peginterferon. Theranostics 5, 218–226 (2015).
Fan, R. et al. Baseline quantitative hepatitis B core antibody titre alone strongly predicts HBeAg seroconversion across chronic hepatitis B patients treated with peginterferon or nucleos(t)ide analogues. Gut 65, 313–320 (2016).
Xu, J. H. et al. Baseline hepatitis B core antibody predicts treatment response in chronic hepatitis B patients receiving long-term entecavir. J. Viral Hepat. 24, 148–154 (2017).
Cai, S., Li, Z., Yu, T., Xia, M. & Peng, J. Serum hepatitis B core antibody levels predict HBeAg seroconversion in chronic hepatitis B patients with high viral load treated with nucleos(t)ide analogs. Infect. Drug Resist. 11, 469–477 (2018).
Fu, X., Lou, H., Chen, F., Gao, X. & Lin, Z. Hepatitis B core antibody and liver stiffness measurements predict HBeAg seroconversion in HBeAg-positive chronic hepatitis B patients with minimally elevated alanine aminotransferase (ALT) levels. Clin. Exp. Med. 20, 241–248 (2020).
Li, M. R. et al. Quantitative hepatitis B core antibody level is associated with inflammatory activity in treatment-naive chronic hepatitis B patients. Medicine 95, e4422 (2016).
Li, M. R. et al. Serum hepatitis B core antibody titer use in screening for significant fibrosis in treatment-naive patients with chronic hepatitis B. Oncotarget 8, 11063–11070 (2017).
Caviglia, G. P. et al. Analytical and clinical evaluation of a novel assay for anti-HBc IgG measurement in serum of subjects with overt and occult HBV infection. Diagn. Microbiol. Infect. Dis. 96, 114985 (2020).
Wang, X. et al. Efficacy of a combination of HBV RNA and HBeAg in predicting HBeAg seroconversion in patients treated with entecavir for 144 weeks. Int. J. Infect. Dis. 99, 171–178 (2020).
van Bömmel, F. et al. Serum HBV RNA as a predictor of peginterferon alfa-2a response in patients with HBeAg-positive chronic hepatitis B. J. Infect. Dis. 218, 1066–1074 (2018).
Oliviero, B. et al. Enhanced B-cell differentiation and reduced proliferative capacity in chronic hepatitis C and chronic hepatitis B virus infections. J. Hepatol. 55, 53–60 (2011).
Zgair, A. K., Ghafil, J. A. & Al-Sayidi, R. H. Direct role of antibody-secreting B cells in the severity of chronic hepatitis B. J. Med. Virol. 87, 407–416 (2015).
Naito, H., Hayashi, S. & Abe, K. Rapid and specific genotyping system for hepatitis B virus corresponding to six major genotypes by PCR using type-specific primers. J. Clin. Microbiol. 39, 362–364 (2001).
Li, A. et al. Novel double-antigen sandwich immunoassay for human hepatitis B core antibody. Clin. Vaccine Immunol. 17, 464–469 (2010).
Acknowledgements
We thank Gillian Campbell, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
This work was supported by the National Science and Technology Projects (Project No. 2017ZX10203201, 2017ZX10201201, 2017ZX10202202, 2018ZX10302206), the National Natural Science Fund (Project No. 81700559).
Author information
Authors and Affiliations
Contributions
Dr. S.L. and F.Y. critically read this manuscript. X.Z. and X.A. wrote and edited the manuscript. X.L., L.S., Y.C., and J.L. conceived the study idea and revised the manuscript. X.Y. provided challenging and inspiring discussions, performed the statistical analyses, and participated in the manuscript revision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, X., An, X., Shi, L. et al. Baseline quantitative HBcAb strongly predicts undetectable HBV DNA and RNA in chronic hepatitis B patients treated with entecavir for 10 years. Sci Rep 11, 13389 (2021). https://doi.org/10.1038/s41598-021-92757-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-92757-0
- Springer Nature Limited