Abstract
Patient–ventilator asynchrony (PVA) is commonly encountered during mechanical ventilation of critically ill patients. Estimates of PVA incidence vary widely. Type, risk factors, and consequences of PVA remain unclear. We aimed to measure the incidence and identify types of PVA, characterize risk factors for development, and explore the relationship between PVA and outcome among critically ill, mechanically ventilated adult patients admitted to medical, surgical, and medical-surgical intensive care units in a large academic institution staffed with varying provider training background. A single center, retrospective cohort study of all adult critically ill patients undergoing invasive mechanical ventilation for ≥ 12 h. A total of 676 patients who underwent 696 episodes of mechanical ventilation were included. Overall PVA occurred in 170 (24%) episodes. Double triggering 92(13%) was most common, followed by flow starvation 73(10%). A history of smoking, and pneumonia, sepsis, or ARDS were risk factors for overall PVA and double triggering (all P < 0.05). Compared with volume targeted ventilation, pressure targeted ventilation decreased the occurrence of events (all P < 0.01). During volume controlled synchronized intermittent mandatory ventilation and pressure targeted ventilation, ventilator settings were associated with the incidence of overall PVA. The number of overall PVA, as well as double triggering and flow starvation specifically, were associated with worse outcomes and fewer hospital-free days (all P < 0.01). Double triggering and flow starvation are the most common PVA among critically ill, mechanically ventilated patients. Overall incidence as well as double triggering and flow starvation PVA specifically, portend worse outcome.
Similar content being viewed by others
Introduction
Invasive mechanical ventilation is essential for the treatment of critically ill patients with respiratory failure, yet a double-edged sword with potential to harm if ventilator settings do not meet patient demand. Suboptimal or mismatched ventilation selection may lead to patient ventilator asynchrony (PVA). PVA is common and may occur at any point during the course of mechanical ventilation. Its reported incidence varies widely, ranging from 10 to 85%1,2,3,4,5.
The factors that affect the occurrence of PVA can be related to the patient, the ventilator, or both. Patient factors include severity of illness, underlying diagnosis, indication for mechanical ventilation, and patient response to medical treatments3,6. Several studies3,7,8,9,10 have shown that patients with chronic obstructive pulmonary disease (COPD), in the presence of intrinsic PEEP, experience ineffective triggering or wasted efforts, delayed triggering, or prolonged cycling asynchronies. Another common PVA, double triggering, more frequently occurs in acute respiratory distress syndrome (ARDS) patients ventilated with low tidal volume11. The choice of ventilator mode and settings may also play a role3.
PVA may result in adverse consequences including increased respiratory workload, patient discomfort, deterioration in gas exchange, diaphragmatic injury, and/or patient self-inflicted lung injury. PVA is associated with worse clinical outcomes objectively identified by increased duration of mechanical ventilation, length of intensive care unit (ICU) and hospital stay, and mortality12,13,14,15,16,17,18. To date studies of PVA have mainly examined specific patient populations and/or ventilator modes and been limited by the timing and length of observations. The risk factors for, and clinical consequences of, PVA remain unclear. Though some may be benign, growing evidence suggests that certain PVAs may be more deleterious in the ventilatory management of patients with acute respiratory failure12,16,18,19.
Advanced methods of detecting PVA include esophageal pressure monitoring, diaphragm electrical activity, and software algorithms to continuously and automatically detect PVA. These techniques can provide robust information and help clinician detection of PVA but are quite complex to employ, requiring dedicated equipment and specialized expertise. As such these tools are not routinely used in clinical practice6,20,21,22,23,24. Furthermore, automated PVA detection has not been validated in large, heterogeneous populations and remains restricted mostly to research protocols6,21,24,25.
In contrast, ventilator waveforms require no additional equipment and are readily available for real-time, examination and interpretation by experienced clinicians26,27. In current clinical practice, the most frequent and practical approach to detect PVA remains the evaluation of airway pressure, flow, and volume tracings on the ventilator display. Utilizing our local standardized mechanical ventilation practice and incorporating an established, routine PVA management by bedside respiratory therapists, we aimed to systematically investigate the prevalence of and identify risk factors for PVA, as well as define associations between PVA and clinical outcome in critically ill mechanically ventilated patients.
Methods
Study design and patients
This was a single-center, retrospective cohort study. From December 2018, through May 2019, adult patients admitted to the medical and surgical ICUs, Mayo Clinic, Rochester, MN, and requiring invasive mechanical ventilation for at least 12 h, were included. Patients less than 18 years of age, with no evidence of spontaneous breathing, in a moribund state or palliative care were excluded. The study was reviewed and approved by the Mayo Clinic Institutional Review Board. All research was performed in accordance with relevant guidelines/regulations, and informed consent was obtained from all participants and/or their legal guardians.
Definition of PVA and counting PVA events
In October 2018, before study commencement, all Respiratory Therapists received PVA identification training consisting of analysis of the ventilator’s pressure, flow, and volume waveforms. Following the training, RTs were required to pass a PVA identification competency exam with a minimum score of 85% detailed in Supplementary Information S1. Our institution has a standardized mechanical ventilation guideline which includes respiratory therapists performing a 10-min assessment of all mechanically ventilated spontaneously breathing patients to identify the occurrence of PVA. Assessments were conducted every 4 h during routine ventilator checks or sooner if the RT felt an assessment was warranted. The presence and type of PVA were documented in the electronic medical record (EMR).
Definitions of various types of PVA were derived from descriptions in the literature as follows12,16,27,28,29,30. Double-triggering: Sustained patient inspiratory effort beyond the ventilator inspiratory time resulting in the triggering and delivery of all or part of a second ventilator breath. Flow starvation: Ventilator inspiratory flow rate does not meet patients’ inspiratory demand. In volume controlled ventilation flow starvation is represented by a concave deflection (scalping out) of the pressure–time tracing during early inspiration with a square flow profile or a concave deflection in late inspiration with a decelerating flow profile. In pressure controlled ventilation flow starvation is due to a slow pressurization rate (rise time) and represented by a distortion (rounding off or shoulder) of early part of the pressure waveform. Ineffective triggering (wasted effort): Patient inspiratory effort prior to complete exhalation that does not initiate a ventilator breath. Represented by a temporary decrease in expiratory flow prior to the next delivered breath. Cycle mismatch including premature cycling and prolonged cycling. Premature cycling: Inspiratory effort continues beyond ventilator inspiratory time represented by a decrease in expiratory flow and airway pressure immediately after the onset of expiration; Prolonged cycling: Ventilator inspiratory time is longer than the patient’s effort, represented as a sharp spike at the end of inspiration. Delayed Triggering: A delayed response of the ventilator output to patient inspiratory effort. Represented by a marked decrease in pressure before the ventilator output begins. Examples of asynchronies are available in Supplementary Information S1. Overall PVA included any type of PVA listed as above.
Data collection
Demographics, mechanical ventilation data, and clinical outcomes were extracted from EMR, listed as the following: (1) Demographics, chronic comorbidities; (2) Reasons for initial mechanical ventilation; (3) PVA assessment: the prevalence of any type of PVA; (4) Ventilator mode and ventilator settings at the time of PVA assessment, and the classification of ventilator modes listed in Supplementary Information S2; (5) Clinical outcomes: mechanical ventilation duration, ventilator-free days at day 28, length of ICU and hospital stay, ICU and hospital mortality. Ventilator free days at day 28 were defined as the number of ventilator-free days and alive through 28 days after mechanical ventilation initiation31. All data were collected during the first episode of mechanical ventilation during the first ICU stay, whether or not multiple episodes occurred during the same hospital stay.
Statistical analysis
Incidence of PVA (overall, and by type) is described using the observed percentage of subjects with the event and using cumulative incidence curves, estimated where death and weaning from ventilator are competing risks. Assessments of the association of PVA with baseline patient characteristics and ventilator settings using a recurrent events analysis Prentice, Williams, and Peterson gap time (PWP-GT) model32, and the correlation of PVA event with hospital mortality and hospital-free days using adjusted logistic regression and multivariable-adjusted linear regression respectively are described in Supplementary Information S3.
Ethics approval and consent to participate
This study was approved by the Mayo Clinic Institutional Review Board, under the approval number #18-0098365, June 27th, 2019. All research was performed in accordance with relevant guidelines/regulations, and where applicable informed consent was obtained from all participants and/or their legal guardians.
Results
Baseline patient characteristics and incidence of PVA throughout the course of mechanical ventilation
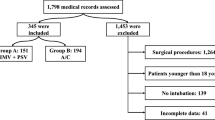
A total of 676 patients who underwent 696 episodes of mechanical ventilation were included. Thirteen patients had more than one episode of hospital stay (Supplementary Fig. S1). Demographics, comorbidities, reasons for initial mechanical ventilation, and initial ventilator mode are shown in Table 1. During the entire course of 696 episodes of mechanical ventilation, overall PVA occurred in 170 (24%) episodes (Supplementary Fig. S1). The most common PVA was double triggering 92 (13%) episodes, followed by flow starvation 73 (10%) episodes. The incidence of cycle mismatch 29 (4%) was similar to ineffective effort 27 (4%). Delayed triggering rarely occurred;7 (1%) episodes (Table 1). Double triggering (2.11% vs. 1.09%) and flow starvation (1.63% vs.0.76%) occurred more frequently in volume targeted ventilation (VC) than in pressure targeted ventilation (PC) (both P < 0.01) Supplementary Table S1.
The cumulative incidences of patients experiencing all types of PVA at day 12 in Supplementary Table S2, were similar to those during the whole period of mechanical ventilation in Table 1. Figure 1 presents the cumulative incidence of PVA over the first 12 days of mechanical ventilation. The first 5 days were associated with a higher rate of cumulative incidence for double triggering, and the first 8 days with a higher rate for flow starvation. Incidences of cycle mismatch and ineffective effort increased gradually through the first 12 days. Occurrence of delayed triggering started after the third day.
Association of PVA with baseline patient characteristics and ventilator settings
PWP-GT model was used to analyze the association of time to PVA event with patient characteristics (Table 2) and ventilator settings (Table 3). History of smoking (HR 1.46, 95% CL 1.13–1.89; P = 0.004), cirrhosis (HR 1.57, 95% CL 1.06–2.32; P = 0.024), and of reasons for initial mechanical ventilation, pneumonia/sepsis/ARDS (HR 1.48, 95% CL 1.01–2.16) and other (HR 1.52, 95% CL 1.02–2.27) compared to post-surgery (P = 0.007) were risk factors for overall PVA. However, heart disease (HR 0.65, 95% CL 0.50–0.85; P = 0.002) was negatively associated with overall PVA. When analysis for the specific type of PVA, history of smoking, kidney disease, and pneumonia/sepsis/ARDS in comparison with post-surgery were risk factors for double triggering, while heart disease and immunosuppression were associated with decreased risk of this event; cirrhosis and ideal body weight (per 10 kg) were associated with increased risk of flow starvation.
Adjusting for baseline characteristics, chronic comorbidities, and reasons for initiation of mechanical ventilation, in patients on synchronized intermittent mandatory ventilation with volume-controlled ventilation (VC-SIMV), increasing peak inspiratory flow setting (HR 1.12, 95% CL 1.04–1.20; P = 0.002) was associated with increased risk of overall PVA, while increasing PEEP (HR 0.82, 95% CL 0.70–0.95; P = 0.009) was associated with decreased risk of PVA event. In PC ventilation, an inspiratory pressure above PEEP of 12cmH2O, was associated with increased risk of PVA event (HR 1.10 per cmH2O, 95% CI 1.03–1.17, P = 0.006). However, in VC ventilation, no association was observed between ventilator settings and overall PVA event.
Patient outcome: correlation of PVA with hospital mortality and hospital-free days
Patients with overall PVA, double triggering, and flow starvation were associated with fewer ventilator-free days, longer duration of mechanical ventilation, longer ICU and hospital stay, and higher ICU and hospital mortality than those without (all P < 0.01) in Supplementary Table S3.
In the analysis of the effect of PVA on subsequent outcome (length of hospital stay and hospital mortality post-invasive ventilation), 97 patients who died on ventilator were excluded and a total of 599 patients were analyzed. 128 (21.4%) patients experienced at least one PVA event, hospital-free days at day 28 were 15.2 (0.1–21.2) vs. 19.7 (11.8–23.5) days, hospital mortality 14% vs. 7% in patients with and without PVA respectively (Table 4). After adjustment for history of smoking, heart disease, reasons for mechanical ventilation and initial ventilation modes, overall PVA (RR − 4.00; 95% CL − 5.81 to − 2.18; P < 0.001), double triggering (RR − 3.12; 95% CL − 5.43 to − 0.81; P = 0.008) and flow mismatch (RR − 4.24; 95% CL − 7.01 to − 1.47; P = 0.003) were each associated with fewer hospital-free days, but no association was observed between PVA and hospital mortality (P > 0.05) in Table 5.
Discussion
The current study examines the incidence and consequences of PVA in medical, surgical, and medical-surgical patients managed across a number of general and subspecialty ICUs staffed by providers of varying background (internal medicine/Pulm CC, anesthesia, surgery). The main findings include: (1) Overall, PVA was common. Double triggering was most prevalent, followed by flow starvation; (2) Risk factors for the development of PVA—and double triggering specifically—include a history of smoking, sepsis, pneumonia, or ARDS as etiology of respiratory failure. PC ventilation was associated with a lower overall incidence of PVA, double triggering, and flow starvation compared to VC; (3) Double triggering, flow starvation, and the total number of PVA per patient were associated with worse outcome and fewer hospital-free days.
In our study, the overall prevalence of PVA in adult, critically ill patients over their entire course of mechanical ventilation was 24%. The most prevalent PVA was double triggering, followed by flow starvation. This finding is, to some extent, consistent with previous studies reporting that double triggering occurs in most mechanically ventilated patients11,13,15,33. However, others have shown that the incidence of PVA varies widely with the most common being ineffective effort1,2,4,5,7. This may be explained by differences in study population (e.g., COPD, trauma, medical or surgical patients), observation time (e.g., 1–10 min, 30 min, or one day), detection method (e.g., clinical assessment, waveform continuously monitored, detection of esophageal pressure and electrical activity of the diaphragm), and ventilator settings1,2,4,5,7.
PVA can occur throughout the course of mechanical ventilation and varies widely over time15,33. In a recent proof of concept study, Marchuk et al.34 developed a Hidden Markov model to predict the time course of PVA and inferred the probability that the number of PVA events would be above a given threshold, based on discrete time-series data in 51 mechanically ventilated patients. Here we report the cumulative incidence of PVA, identifying the first 12 days after mechanical ventilation initiation as a critical period over which the risk for development of any PVA event increases. The first 5 days appear to be a critical time with a high likelihood of developing double triggering; Over the first 8 days, flow starvation. This finding may suggest that critically ill, mechanically ventilated patients could benefit from closer monitoring of those with a higher risk of PVA over this time period, to enable early identification and intervention upon PVA to improve patient–ventilator interaction.
Factors associated with overall PVA
Patient factors may predict PVA. A history of smoking, cirrhosis, and pneumonia/sepsis/ARDS as etiology of respiratory failure, as opposed to a post-surgery status, were positively associated with overall PVA events. Conversely, heart disease was negatively associated with overall PVA. Several studies reported that COPD, ARDS, and greater severity of illness favor the occurrence of PVA3,11,33. In PC ventilation, higher inspiratory pressure (> 12 cmH20 above PEEP), and in VC-SIMV mode, higher inspiratory flow were associated with a higher risk of PVA, while higher PEEP levels were associated with lower risk. During VC ventilation, no association was observed between ventilator settings and overall PVA event. Robinson et al.24 found ventilator asynchrony was more common in SIMV with set breathing frequencies of > 10 breaths/min in trauma patients. Similar to the previous studies30,35,36, the use of PC ventilation was associated with better patient–ventilator interaction than VC ventilation, but requires careful monitoring to avoid delivery of larger than targeted volumes.
Factors associated with double triggering and flow starvation
Double triggering occurs when there is a mismatch between set tidal volume or inspiratory time and patient’s ventilatory demand16,29,31,37. Risk factors include a history of smoking, chronic kidney disease, and pneumonia/sepsis/ARDS, while chronic heart disease and immunosuppression had a reduced risk of double triggering. We speculate that kidney disease may cause acidosis, resulting in increased central respiratory drive. Pulmonary function impairment might be more severe in patients with pneumonia/sepsis/ARDS than those patients intubated in the postoperative period, and this may lead to high ventilatory demand.
Flow starvation occurs when ventilator flow rate is less than patient demand. Our results demonstrate a positive correlation between cirrhosis and ideal body weight with flow starvation. We speculate that patients with greater ideal body weight may need higher flow and that cirrhosis might cause increased ventilatory demand or neural drive through liver-lung cross talk.
We confirm previous reports12,30,35,36 that VC ventilation is associated with more frequent double triggering and flow mismatch events, perhaps due to inadequate tidal volume or flow as a result of strict limitation by operators. However, de Haro et al. found a higher percentage of double cycling occurred in PCV than in VCV with constant flow or decelerated flow33. A plausible explanation for this discrepancy could be related to differences in study population and ventilator settings. We did not analyze the influence of the ventilator mode-specific settings on the occurrence of double triggering and flow mismatch due to the limited number of events.
Outcome
Patients in our cohort who developed PVA were associated with fewer ventilator-free days, longer ICU and hospital stay, and higher ICU and hospital mortality than those without. However, after patients who died on ventilator were excluded, with the analysis of the effect of PVA on subsequent outcome (length of hospital stay and hospital mortality post-invasive ventilation), overall PVA, double triggering and flow mismatch each independently predicted fewer hospital-free days, but no association was observed between PVA and hospital mortality after adjustment for history of smoking, heart disease, reasons for mechanical ventilation and initial ventilation modes.
Nevertheless, examining PVA in trauma patients24 or in the early phase of weaning38, with a short observation showed that asynchrony index (number of PVA events/total respiratory rate × 100) > 10% was not associated with adverse clinical outcome; Rue et al. using Bayesian analysis found that patient ventilator asynchrony was positively associated with alive discharge39. What’s more, Thille and Blanch, et al.12,15 showed that asynchrony index > 10% was associated with prolonged duration of mechanical ventilation, or higher ICU and hospital mortality. Additionally, de Wit and Vaporidi et al.17,18 found that ineffective triggering index ≥ 10% or clusters of ineffective triggering were correlated with a worse outcome. Given the differences in population, timing and duration of observation, and the definition of asynchrony employed, the varying outcomes and the erratic results40, the relationships between asynchronies and outcomes still need to be further validated.
Our study examined the relationships of double triggering, flow starvation, and patient outcome. Both were associated with worse outcomes. As expected, the total delivered volume during double triggering events was much larger than the set/targeted tidal volume, often double or more a normal breath30,33, which could lead to overinflation. Stronger spontaneous inspiratory effort during flow starvation can cause harmful transpulmonary pressure swings, which might lead to occult pendelluft and consequent regional lung overdistension41,42. Those mechanisms might cause ventilator induced lung injury and worsen outcomes42. Our study indicated the association of PVA with a poorer prognosis, but whether the relationship between PVA and outcome is causative or associative requires further investigation.
Strengths
To our knowledge, this is the first and largest study to systemically investigate the incidence of overall and specific types of PVA, their risks, and associated outcomes among a heterogeneous population of ICU patients in a large, academic institution, where well trained RTs routinely manage PVA per local standardized clinical practice guidelines. This study used a PWP-GT approach to estimate the correlation of time to PVA events with factors related to patient characteristics, ventilator settings—time-dependent covariates throughout the entire course of mechanical ventilation; and analyzed the prediction of time to PVA events on the subsequent outcomes after extubation or weaning success.
Limitations
Our study has several limitations. First, this is a single-center, retrospective cohort study, which may limit generalizability. Second, PVA detection relied on ventilator waveform analysis by RT’s at designated timepoints, thus the incidence of PVA is likely underestimated. Third, reverse triggering, the activation of diaphragm muscles by a ventilator initiated breath, may produce several cycling desynchronies including double triggering or double cycling, which causes breath stacking. As reverse triggering was not evaluated as a specific PVA, its contribution to the incidence of double triggering was not evaluated. Though software that provides continuous monitoring and automatic detection of PVA may be available in the near future, it is not currently part of routine clinical practice. This study analyzed a real-world method for identifying PVA applicable to any bedside intensive care practice. Lastly, for reasons of statistical power, factors associated with double triggering and flow starvation related to ventilator settings were not analyzed.
Conclusion
PVA are common, with double triggering and flow starvation encountered most often among critically-ill patients undergoing at least 12 h of invasive mechanical ventilation. Occurrence is associated with worse outcome, including fewer hospital free days. Patient characteristics including day of mechanical ventilation and etiology of respiratory failure may predict development of PVA and help to identify at-risk individuals for closer monitoring and early intervention. Further investigations are needed to determine whether the relationship between PVA and outcome is associative or causal.
Abbreviations
- PVA:
-
Patient–ventilator asynchrony
- PEEP:
-
Positive end of expiratory pressure
- ARDS:
-
Acute respiratory distress syndrome
- COPD:
-
Chronic obstructive pulmonary disease
- PWP-GT:
-
Prentice, Williams, and Peterson gap time
- EMR:
-
Electronic medical record
- VC:
-
Volume targeted ventilation
- PC:
-
Pressure targeted ventilation
- VC-SIMV:
-
Synchronized intermittent mandatory ventilation with volume-controlled ventilation
- PC-SIMV:
-
Synchronized intermittent mandatory ventilation with pressure controlled ventilation
References
Chao, D. C., Scheinhorn, D. J. & Stearn-Hassenpflug, M. Patient–ventilator trigger asynchrony in prolonged mechanical ventilation. Chest 112(6), 1592–1599 (1997).
Thille, A. W., Rodriguez, P., Cabello, B., Lellouche, F. & Brochard, L. Patient ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med. 32(10), 1515–1522 (2006).
Holanda, M. A., Vasconcelos, R. D. S., Ferreira, J. C. & Pinheiro, B. V. Patient–ventilator asynchrony. J. Bras. Pneumol. 44(4), 321–333 (2018).
de Wit, M., Pedram, S., Best, A. M. & Epstein, S. K. Observational study of patient–ventilator asynchrony and relationship to sedation level. J. Crit. Care. 24(1), 74–80 (2009).
Colombo, D. et al. Efficacy of ventilator waveforms observation in detecting patient–ventilator asynchrony. Crit. Care Med. 39(11), 2452–2457 (2011).
de Haro, C. et al. Patient–ventilator asynchronies during mechanical ventilation: Current knowledge and research priorities. Intensive Care Med. Exp. 7, 1–14 (2019).
Alexopoulou, C., Kondili, E., Plataki, M. & Georgopoulos, D. Patient–ventilator synchrony and sleep quality with proportional assist and pressure support ventilation. Intensive Care Med. 39(6), 1040–1047 (2013).
Branson, R. D. Patient–ventilator interaction: The last 40 years. Respir. Care. 56(1), 15–24 (2011).
Nava, S., Bruschi, C., Fracchia, C., Braschi, A. & Rubini, F. Patient–ventilator interaction and inspiratory effort during pressure support ventilation in patients with different pathologies. Eur. Respir. J. 10(1), 17783 (1997).
Vasconcelos, R. S. et al. Influences of duration of inspiratory effort, respiratory mechanics, and ventilator type on asynchrony with pressure support and proportional assist ventilation. Respir. Care. 62(5), 550–557 (2017).
Pohlman, M. C. et al. Excessive tidal volume from breath stacking during lung-protective ventilation for acute lung injury. Crit. Care Med. 36(11), 3019–3023 (2008).
Thille, A. W., Rodriguez, P., Cabello, B., Lellouche, F. & Brochard, L. Patient–ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med. 32, 1515–1522 (2006).
Beitler, J. R. et al. Quantifying unintended exposure to high tidal volumes from breath stacking dyssynchrony in ARDS: The BREATHE criteria. Intensive Care Med. 42(9), 1427–1436 (2016).
Yonis, H., Gobert, F., Tapponnier, R. & Guérin, C. Reverse triggering in a patient with ARDS. Intensive Care Med. 41(9), 1711–1712 (2015).
Blanch, L. et al. Asynchronies during mechanical ventilation are associated with mortality. Intensive Care Med. 41(4), 633–641 (2015).
Gilstrap, D. & MacIntyre, N. Patient–ventilator interactions. Implications for clinical management. Am. J. Respir. Crit. Care Med. 188(9), 1058–1068 (2013).
de Wit, M. et al. Ineffective triggering predicts increased duration of mechanical ventilation. Crit Care Med. 37, 2740–2745 (2009).
Vaporidi, K. et al. Clusters of ineffective efforts during mechanical ventilation: Impact on outcome. Intensive Care Med 43, 184–191 (2017).
Colombo, D. et al. Physiologic response to varying levels of pressure support and neurally adjusted ventilatory assist in patients with acute respiratory failure. Intensive Care Med. 34(11), 2010–2018 (2008).
Murias, G., Villagra, A. & Blanch, L. Patient–ventilator dyssynchrony during assisted invasive mechanical ventilation. Minerva Anestesiol. 79, 434–444 (2013).
Dres, M., Rittayamai, N. & Brochard, L. Monitoring patient–ventilator asynchrony. Curr. Opin. Crit. Care. 22(3), 246–253 (2016).
Branson, R. D., Blakeman, T. C. & Robinson, B. R. Asynchrony and dyspnea. Respir. Care. 58(6), 973–989 (2013).
Gentile, M. A. Cycling of the mechanical ventilator breath. Respir. Care 56(1), 52–60 (2011).
Robinson, B. R. et al. Patient–ventilator asynchrony in a traumatically injured population. Respir. Care. 58(11), 1847–1855 (2013).
Subirà, C., de Haro, C., Magrans, R., Fernández, R. & Blanch, L. Minimizing asynchronies in mechanical ventilation: Current and future trends. Respir. Care. 63(4), 464–478 (2018).
Ramirez, I. I. et al. Ability of ICU health-care professionals to identify patient–ventilator asynchrony using waveform analysis. Respir. Care. 62(2), 144–149 (2017).
Pham, T., Telias, I., Piraino, T., Yoshida, T. & Brochard, L. J. Asynchrony consequences and management. Crit. Care Clin. 34(3), 325–341 (2018).
Chacón, E. et al. Nurses’ detection of ineffective inspiratory efforts during mechanical ventilation. Am. J. Crit. Care. 21(4), e89-93 (2012).
Figueroa-Casas, J. B. & Montoya, R. Effect of tidal volume size and its delivery mode on patient–ventilator dyssynchrony. Ann. Am. Thorac. Soc. 13(12), 2207–2214 (2016).
Georgopoulos, D., Prinianakis, G. & Kondili, E. Bedside waveforms interpretation as a tool to identify patient–ventilator asynchronies. Intensive Care Med. 32, 34–47 (2006).
Writing Group for the PReVENT Investigators et al. Effect of a low vs intermediate tidal volume strategy on ventilator-free days in intensive care unit patients without ARDS: A randomized clinical trial. JAMA 320(18), 1872–1880 (2018).
Amorim, L. D. A. F. & Jianwen, C. Modelling recurrent events: A tutorial for analysis in epidemiology. Int. J. Epidemiol. 44(1), 324–333 (2015).
de Haro, C. et al. Double cycling during mechanical ventilation: Frequency, mechanisms, and physiologic implications. Crit. Care Med. 46(9), 1385–1392 (2018).
Marchuk, Y. et al. Predicting patient–ventilator asynchronies with hidden markov models. Sci. Rep. 8(1), 17614 (2018).
Yang, L. Y., Huang, Y. C. & Macintyre, N. R. Patient–ventilator synchrony during pressure-targeted versus flow-targeted small tidal volume assisted ventilation. J. Crit. Care 22(3), 252–257 (2007).
MacIntyre, N. R., McConnell, R., Cheng, K. C. & Sane, A. Patient–ventilator flow dyssynchrony: Flow-limited versus pressure-limited breaths. Crit. Care Med. 25(10), 1671–1677 (1997).
Kallet, R. H., Campbell, A. R., Dicker, R. A., Katz, J. A. & Mackersie, R. C. Effects of tidal volume on work of breathing during lung-protective ventilation in patients with acute lung injury and acute respiratory distress syndrome. Crit. Care Med. 34(1), 8–14 (2006).
Rolland-Debord, C. et al. Prevalence and prognosis impact of patient–ventilator asynchrony in early phase of weaning according to two detection methods. Anesthesiology 127, 989–997 (2017).
Rué, M. et al. Bayesian joint modeling of bivariate longitudinal and competing risks data: An application to study patient–ventilator asynchronies in critical care patients. Biom. J. 59(6), 1184–1203 (2017).
Bruni, A. et al. Patient–ventilator asynchrony in adult critically ill patients. Minerva Anestesiol. 85(6), 676–688 (2019).
Yoshida, T. et al. Spontaneous effort during mechanical ventilation: Maximal injury with less positive end-expiratory pressure. Crit. Care Med. 44, e678–e688 (2016).
Yoshida, T. et al. Fifty years of research in ARDS. Spontaneous breathing during mechanical ventilation. Risks, mechanisms, and management. Am. J. Respir. Crit. Care Med. 195, 985–992 (2017).
Acknowledgements
We appreciate the respiratory therapists of Mayo Clinic for their dedication to patient care and their support of this study. Lei Fan, IT Analyst/Programmer in Department of Information Technology, and Grant D Wilson for their help with this study.
Funding
This trial was funded by the Critical Care Research Committee at Mayo Clinic, Rochester, Minnesota, USA.
Author information
Authors and Affiliations
Contributions
Y.F.Z., R.A.O., G.A.C.P. and S.R.H. conceived and designed the protocol, Y.F.Z., S.R.H., M.L., T.J.M. and R.A.O. were involved in acquisition of funding and data. A.C.H. and P.J.S. organized and performed the analysis of the data, and wrote the statistical methodology. All authors participated in the interpretation of the data. Y.F.Z. drafted the manuscript, and G.A.C.P., S.R.H., R.A.O. participated in the critical revision of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, Y., Holets, S.R., Li, M. et al. Etiology, incidence, and outcomes of patient–ventilator asynchrony in critically-ill patients undergoing invasive mechanical ventilation. Sci Rep 11, 12390 (2021). https://doi.org/10.1038/s41598-021-90013-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-90013-z
- Springer Nature Limited
This article is cited by
-
Knowledge and associated factors of healthcare professionals in detecting patient-ventilator asynchrony using waveform analysis at intensive care units of the federal public hospitals in Addis Ababa, Ethiopia, 2023
BMC Nursing (2024)
-
Brain–lung interactions and mechanical ventilation in patients with isolated brain injury
Critical Care (2021)