Abstract
Insecticides, especially pyrethroids, are the most important in the insect pest control and preventing insect vector-borne human diseases. However, insect pests, including mosquitoes, have developed resistance in the insecticides that used against them. Cytochrome P450s are associated with insecticide resistance through overexpression and detoxification mechanisms in insect species. In this study, we utilized a powerful tool, the RNAi technique, to determine the roles of key P450 genes overexpressed in permethrin resistant mosquitoes that confer insecticide resistance to unravel the molecular basis of resistance mechanisms in the mosquito Culex quinquefasciatus. The results showed that knockdown of 8 key P450 genes using RNAi techniques significantly decreased resistance to permethrin in resistant mosquitoes. In silico modeling and docking analysis further revealed the potential metabolic function of overexpressed P450 genes in the development of insecticide resistance in mosquitoes. These findings not only highlighted the functional importance of these P450 genes in insecticide resistance, but also revealed that overexpression of multiple P450 genes was responsible for the high levels of insecticide resistance in a mosquito population of Culex quinquefasciatus.
Similar content being viewed by others
Introduction
Mosquito-borne diseases are among the leading causes of human deaths worldwide1. Insecticides, especially pyrethroids, are the most important weapon in our arsenal for the control of mosquito vectors and mosquito-associated diseases, particularly those for which no vaccines have yet been developed2,3. Insecticides, especially pyrethroids, play a major role in campaigns to eradicate mosquito-borne diseases worldwide, however, the wide-spread development of resistance in mosquitoes to commonly used insecticides, in conjunction with the huge increase in human mobility seen in recent years, is leading to worldwide outbreaks of mosquito-related diseases2,4. Studies have revealed that mosquitoes can develop insecticide resistance through the transcriptional overexpression and/or enzymatic activity elevation of P450s2,5. P450s are critical for the detoxification and/or activation of xenobiotics, such as insecticides and plant toxins6,7. Overexpression of P450 genes can significantly affect the disposition of xenobiotics in the tissues of organisms, altering their pharmacological/toxicological effects8. New technologies such as whole-genome sequencing, RNA sequencing, deep targeted sequencing, and microarrays are allowing researchers to identify genes at a genome level that are potentially involved in insecticide resistance, through which multiple P450 genes are identified overexpressed mosquito species, including Cx. quinquefasciatus9,10,11,12, Anopheles gambiae13, and Aedes aegypti14.
To pinpoint the specific role of P450 genes in resistance, several biological technologies have been employed to investigate the gene functions, such as RNA interference (RNAi), a powerful and robust tool that discovers gene functions by using double-stranded RNA (dsRNA) to disrupt the target mRNA15, transcription activator-like effector nucleases (TALEN)s, and clustered regularly interspaced short palindromic repeats (CRISPR/Cas9)16. These techniques have made great contribution and applied successfully in many insect species to investigate the specific functions played by individual genes, including development17, metamorphosis18 and reproduction19, as well as to characterize the gene functions in the development of insecticide resistance in insect pests such as the mosquitoes Cx. quinquefasciatus16,20,21, Anopheles gambiae22 and Aedes aegypti23, the cockroach Blattella germanica24, the common bedbug Cimex lectularius25, and the aphid Sitobion avenae26.
Several P450 genes have been shown to be overexpressed in resistant Cx. quinquefasciatus9. In this study, we further characterized the function of these overexpressed P450 genes, revealing the association between changes in the expression levels of these P450 genes and the levels of insecticide resistance in mosquitoes using RNAi and in silico modeling and docking analyses. Our findings not only highlighted the functional importance of these P450 genes in insecticide resistance, but also revealed that the high levels of insecticide resistance in a single mosquito population were conferred by the increased expressed of multiple P450 genes their detoxification.
Results
Knockdown of the specific cytochrome P450 gene associated with larval resistance of Cx. quinquefasciatus to permethrin
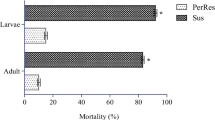
The functional study of the role played by the up-regulated P450 genes in the insecticide resistance of Culex mosquitoes was conducted by using the RNAi technique to knock-down each of the specific P450 genes in the late 3rd or early 4th instar larvae of HAmCqG8. RNAi on the 8 P450 genes in the larvae of HAmCqG8 mosquitoes showed that the RNAi reduced the mRNA levels of CYP9AL1 to 0.55-, CYP9J45 to 0.65-, CYP9J35 to 0.70-, CYP4C52V1 to 0.55-, CYP4D42V1 to 0.35-, CYP6BZ2 to 0.30-, CYP6P14 to 0.55-, CYP325Y6 to 0.60- and CYP6BY3 (negative control) to 0.70-fold in comparison with the effect of dsRNA on GFP injection mosquitoes (Fig. 1a), suggesting the expression of these P450 genes has been successfully suppressed by RNAi. A parallel RNAi functional study on a non-overexpressed P450 gene, namely CYP6BY3, was conducted as a negative control. The results showed a significant decrease in the expression of CYP6BY3, dropping to 0.70-fold in dsRNA-CYP6BY3 injected HAmCqG8 larvae.
Knockdown of P450 genes associated with permethrin resistance in larvae of Cx. quinquefasciatus. RNAi was conducted in the larvae of the permethrin resistant mosquito strain, HAmCqG8. DsRNA of P450 and GFP genes were individually injected into 3rd instar larvae of Culex mosquitoes. (A) Gene expression was detected by real-time PCR in 4th instar larvae, showing significantly decreased expression of P450 genes compared with GFP injection mosquitoes. (B) Permethrin sensitivity was determined by larval bioassay, indicating increased susceptibility to permethrin in dsRNA of 9 P450 gene injection mosquitoes compared with GFP-injection ones; the negative control showed no increased susceptibility to permethrin in the dsRNA of non-overexpressed P450 gene injection mosquitoes. The data shown are the mean ± SEM (n ≥ 3). The Student’s t-test was used for significance analysis. *P < 0.05; **P < 0.01; ***P < 0.001.
To determine the relationship between the knock-down of these P450 genes and permethrin resistance in the HAmCqG8 strain, we performed a larval bioassay to test the resistance levels in dsRNA for the P450 gene- and GFP-injection HAmCqG8 larvae. As shown in Fig. 1b, the LC50 of the dsRNA-P450 injected mosquitoes was significantly decreased in comparison with the dsRNA-GFP injected mosquitoes, with resistance ratios decreasing to 0.37 for CYP9AL1, 0.18 for CYP9J45, 0.60 for CYP9J35, 0.65 for CYP4C52V1, 0.34 for CYP4D42V1, 0.30 for CYP6BZ2, 0.49 for CYP6P14, and 0.40 for CYP325Y6. The negative control dsRNA-CYP6BY3 injected mosquitoes showed no change in their sensitivity to permethrin (Fig. 1b). These results demonstrate a strong association between the suppression of specific up-regulated P450 genes and increased susceptibility to permethrin in resistant Culex mosquitoes. The similar result has also been found in our previous study, in which RNAi reduced the mRNA level of CYP9M10 to 0.44-fold and resulted in the decreased level of resistance to 0.54-fold20,21. In contrast to the RNAi technique, which could only partially reduce the mRNA levels of genes and results in partial decreased in the levels of resistance, completely knockout gene function of CYP9M10 copy by ALEN and CRISPR/Cas9 had showed an approximately 110-fold reduction in permethrin susceptibility in resistant Culex mosquitoes16.
Knockdown of the specific cytochrome P450 gene associated with adult resistance of Cx. quinquefasciatus to permethrin
Since two P450 genes, namely CYP4C52v1 and CYP6AA7, were up-regulated in both the larval and adult stages of HAmCqG8, we then moved on to conduct RNAi in the female adults of the resistant mosquitoes. After injection of dsRNA-CYP4C52v1, -CYP6AA7, and the dsRNA-GFP control into adult mosquitoes, dynamic changes in the gene expression were detected and the adult resistance to permethrin assayed. The results again showed significantly reduced expression levels, with CYP4C52v1 dropping to 0.15 and CYP6AA7 to 0.59 (Fig. 2a). The suppression of the P450 gene by RNAi once again resulted in increased susceptibility to permethrin, with resistance ratios falling to 0.49 for both CYP4C52v1 and CYP6AA7 in comparison with the mosquitoes receiving a dsRNA-GFP injection (Fig. 2b). In the parallel RNAi study with CYP6BY3, the successful knockdown of CYP6BY3 had no effect on the level of resistance to permethrin in HAmCqG8 female adults (Fig. 2).
Knockdown of P450 genes associated with permethrin resistance in adults of Cx. quinquefasciatus. RNAi was conducted in the adults of the permethrin resistant mosquito strain, HAmCqG8. DsRNA of P450 and GFP genes were individually injected into 1-d-old female adults of Culex mosquitoes. (A) Gene expression was detected by real-time PCR in female adults, showing significantly decreased expression of P450 genes compared with GFP injection mosquitoes. (B) Permethrin sensitivity was determined by adult bioassay, indicating increased susceptibility to permethrin in dsRNA of 2 P450 gene injection mosquitoes compared with GFP-injection ones; the negative control showed no increased susceptibility to permethrin in the dsRNA of non-overexpressed P450 gene injection mosquitoes. The data shown are the mean ± SEM (n ≥ 3). The student’s t-test was used for significance analysis. *P < 0.05; **P < 0.01; ***P < 0.001.
Homology modeling and substrate docking
In a previous study, two P450 genes, CYP9M10 and CYP6AA7, was found to perform a critical function in the insecticide resistance21 and metabolism of permethrin in mosquitoes27. We, therefore, utilized in silico 3D modeling and docking to further confirm the metabolic function of CYP9M10 in insecticide resistance. The homology modeling and ligand docking studies for CYP9M10, CYP6BZ2, CYP9J35, CYP325Y6, and CYP4D42v1 showed several conserved P450 characteristics, including substrate recognition sites 1–6 (SRS1–SRS6), the heme-binding structure, a WXXXR motif of the C-helix, and an EXXRXXP motif of the helix k, as well as a PXRF motif (Fig. 3). SRS-2 was not present in either CYP325Y6 or CYP4D42v1, and the SDS-3 was also different in these two models compared with that in the other three P450 models. Applying the Autodock v1.5.6 tools to investigate the binding of permethrin within these five CYP450 models, comparing the estimated binding affinity and distance between the 4′-hydroxylation site of permethrin and the heme iron center for each predicted binding mode (Fig. 4), with larger binding energies indicating lower binding affinities for ligands and proteins. The docking results revealed favorable binding affinities (− 8.96 kcal/mol for CYP9M10, − 9.38 kcal/mol for CYP6BZ2 and − 9.17 kcal/mol for CYP9J35) and distances (≤ 6.0 Å) between the heme iron centers and permethrin 4′-carbon hydroxylation sites (2.91 Å for CYP9M10, 3.06 Å for CYP6BZ2 and 4.05 Å for CYP9J35), suggesting the strong binding affinity of these CYP450s towards permethrin insecticides and further demonstrating their close association with insecticide resistance in mosquitoes. However, the lowest binding affinities (− 6.34 kcal/mol) and greatest distance (9.96 Å) between the 4′ carbon hydroxylation site and heme iron center were observed for CYP4D42v1 and a relatively long binding distance (8.79 Å) was also found for CYP325Y6, indicating the weak binding affinity of permethrin within the structures of these two P450s. Their involvement in permethrin resistance may thus be due to an increase in the gene expression level rather than a change in binding affinity. Residues in the catalytic pocket of these five CYP450s were also examined using LigPlus (http://www.ebi.ac.uk/thornton-srv/software/LigPlus/)28. The amino acid residues Gly331/Ala301/Ala305/Ala311 and Leu400/Ala368/Val371/Val375 found in CYP9M10, CYP9J35, CYP325Y6, and CYP6BZ2, respectively, are highly conserved in all four of these CYP450s; other amino acids, such as Phe91/122 and E334/304/308, are conserved in either two or three of the CYP450s (Fig. 4). The active sites of these four CYP450s are rich in hydrophobic amino acids such as Valine, Phenylalanine, Alanine, and Leucine, providing a favorable chemical environment for hydrophobic insecticide binding. The amino acid residues in the catalytic pocket of CYP4D42v1 are not conserved, which may be due to its low binding affinity as well as the greater distance between the permethrin ligand and the heme center of the protein structure.
Homology modeling of CYP450s. The homology models for CYP9M10, CYP6BZ2, CYP9J35, CYP325Y6 and CYP4D42v1 were constructed with conserved SRSs. Motifs are labeled. Six putative SRSs are colored and predicted, according to Gotoh’s predicted models43. SRS1 to 6 are represented by green, orange, red, yellow, purple and ruby, respectively. The WXXXR motif of C-helix is shown in blue. The EXXRXXP motif of helix-k is shown in pale green. The PXRF motif is shown in pink and the Heme binding motif is shown in pale yellow. The HEME group is represented by cyan sticks.
Docking models of permethrin in the active sites of CYP450s. The predicted binding models of permethrin in the active sites of CYP9M10, CYP6BZ2, CYP9J35, CYP325Y6, and CYP4D42v1. The partial homology model of CYP450 is represented by white helices and sheets; the Heme group is represented by cyan sticks, and the permethrin ligands are shown as pink sticks. The conserved amino acids of the catalytic pockets are labeled and summarized.
Discussion
Cytochrome P450s are known to be members of a superfamily of metabolic enzymes that is found in all living organisms2. The overexpression of P450 genes, resulting in increased levels of P450 proteins and P450 activities, has been associated with enhanced metabolic detoxification of insecticides and has been implicated in the development of insecticide resistance in insects, including mosquitos2,4,6,29. Zhu et al.16 observed that several P450 genes, namely CYP4D4v2, CYP4G2, CYP6A38, and CYP6A36, were up-regulated by induction or constitutive expression in a permethrin resistant housefly strain ALHF. Cytochrome P450 genes have been identified as playing a role in pyrethroid resistance in mosquito species through gene overexpression mechanisms, including CYP6Z130 and CYP6P331 in An. gambiae, CYP9J32 in Ae. aegypti32, CYP4H34, CYP6F1, CYP9M10 and CYP6AA7 in Cx. quinquefasciatus9,11,33,34,35. More interestingly, overexpression of CYP9M10 gene has been identified in different strains of Cx. quinquefasciatus in different geography locations in Japan, USA, and South Africa9,10,11,12,36,37, indicating that some insect resistance P450 genes are global distributed and spread37. However, it is unclear how many P450 genes are involved in developing P450-mediated resistance in an organism such as the mosquito. The use of biological technologies, such as RNAi, TALENs, and CRISPR/Cas915,16,17,18,19,20,21,22,23,24,25,26, provides valuable tools for gene functional studies that has now been applied in many insect species. In this study, we employed RNAi and in silico modeling and docking analysis to determine the roles of overexpressed P450 genes known to overexpressed in insecticide-resistant mosquitoes9 to unravel the molecular basis of resistance mechanisms in the mosquito Cx. quinquefasciatus. Eight overexpressed P450 genes, CYP9AL1, CYP9J35, CYP9J45, CYP4C52v1, CYP4D42V1, CYP6BZ2, CYP6P14, and CYP6325Y6, were knocked down in 3rd/4th instar larvae of HAmCqG8 mosquitoes by RNAi, revealing that decreased expression of these P450 genes corresponded with the decreased level of insecticide resistance to permethrin. These results demonstrate that the decreased resistance to permethrin of the mosquito larvae is strongly associated with each of the target P450 genes knocked down by RNAi, thus indicating that multiple cytochrome P450 genes are likely involved in permethrin detoxification in Culex mosquitoes. While there is always a possibility of knockdown of off-target P450s that would alter the resistant level of the mosquitoes38, our further Baculovirus expression and HPLC based metabolism studies of these P450 suggest that these enzymes metabolize permethrin in Culex mosquitoes27 (some of the metabolism data by Gong et al. are not yet published), further confirming the involvement of these P450s in permethrin detoxification in mosquitoes.
Although previous studies showed Culex mosquitoes had a higher resistance level to permethrin in 4th instar larvae than in adults compared to susceptible mosquitoes39,40, we anticipated that overexpressed P450 genes in adult resistant mosquitoes may perform a similar function in permethrin resistance as that observed in the larvae. Two P450 genes, CYP4C52v1 and CYP6AA7, known to be overexpressed in HAmCqG8 adult mosquitoes9 were successfully knocked down by dsRNA injection in adult mosquitoes, again demonstrating a strong association of overexpressed P450 genes and levels of insecticide resistance. Similar results have been also reported in pyrethroid-resistant mosquito species such as An. gambiae for CYP6Z1 and CYP6P3 genes29,41 and Anopheles funestus for CYP6P9 genes42. In addition, in silico 3D modeling and docking analysis revealed that CYP9M10, CYP6BZ2, and CYP9J35 all exhibited high binding affinities to permethrin, indicating the high potential of these genes for metabolizing insecticide in mosquitoes. Although CYP325Y6 and CYP4D42v1 failed to show a high level of binding affinity to permethrin, suppression of the gene expression caused decreased resistance to insecticide in resistant mosquitoes. Taken together, our findings not only highlighted the functional importance of these P450 genes in insecticide resistance, but also revealed that the high levels of insecticide resistance in a single mosquito population were conferred by the accumulative detoxification mechanisms resulting from increased expression of multiple P450 genes.
Materials and methods
Mosquito strains
A mosquito strain, HAmCqG8, was used in the current study to character P450 gene function in insecticide resistance using RNAi. The HAmCqG8 is the 8th generation of permethrin-selected offspring of a field-collected parental strain, HAmCqG0 collected from Huntsville, Alabama9,34,39. After 8 generation-selection with permethrin, resistance level in HAmCqG8 was 2700-fold compared with those of a susceptible strain, S-Lab39. Permethrin selections on HAmCqG8 mosquitoes have been continually conducted for every 6 months and insecticide bioassays and gene expression measurements using CYP6AA7 and CYP9M10 as the target gene are performed after each permethrin selection to diagnose the levels of resistance and resistance P450 gene up-regulation in these resistant strains. While it had been showed that two major resistance mechanisms were involved in the permethrin resistance in HAmCqG8, namely P450 mediated detoxification9,33 and target site insensitivity (kdr)43,44, the current study has been focused on the P450 mediated resistance. All the mosquitoes were reared at 25 ± 2 °C under a photoperiod of 12:12 (L:D) h. Adult females were fed blood samples from horses for egg development (supplied by the Large Animal Teaching Hospital, College of Veterinary Medicine, Auburn University). To maintain the resistance level of HAmCqG8, resistance selection with permethrin in 4th instar larvae were applied every half year.
RNA extraction and cDNA preparation
Total RNAs were extracted from 4th instar larvae and female adults using the acidic guanidine thiocyanate (GIT)–phenol–chloroform method45. DNA in the RNA samples was removed using a TURBO DNA-free kit (Ambion) following the manufacturer’s instructions. cDNA was synthesized using the DNA-free RNA as a template and SuperScript II reverse transcriptase (Invitrogen), again following the manufacturer’s instructions. The quantity of cDNA was measured using a spectrophotometer prior to qRT-PCR. Each experiment was repeated more than 3 times with independent RNA preparation.
Quantitative real-time PCR (qRT-PCR)
Each qRT-PCR reaction (15 μl final volume) consisted of 1 × SYBR Green master mix (Roche), 1 μl of cDNA, and a P450 gene specific primer pair designed according to each of the P450 gene sequences (http://cquinquefasciatus.vectorbase.org/) at a final concentration of 3–5 μM. The primer pairs are listed in Table 1. All samples, including the ‘no-template’ negative control, were performed in triplicate. Relative expression levels for the P450 genes were calculated by the 2−ΔΔCT method using SDS RQ software46 and ABI7500 Real-Time PCR system (Applied Biosystems). The reaction cycle consisted of a melting step of 50 °C for 2 min, then 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The 18S ribosome RNA gene, an endogenous control, was used to normalize the expression of target genes20,21,47. Each experiment was repeated at least three times with independent biological samples. The statistical significance of the gene expression was calculated using a Student's t test for all 2-sample comparisons and one-way analysis of variance (ANOVA) for multiple sample comparisons (SAS v9.1 software); a value of P ≤ 0.05 was considered statistically significant.
Double-stranded RNA synthesis
Several P450 genes are up-regulated in resistant strains of Culex mosquitoes; eight P450 genes, namely CYP9AL1, CYP9J45, CYP9J35, CYP4C52V1, CYP4D42V1, CYP6BZ2, CYP6P14, and CYP325Y6 are up-regulated specifically in the larval stage of the permethrin-resistant HAmCqG8 strain in comparison with the lab susceptible strain (S-Lab) and field-collected parental strain (HAmCqG0), while two P450 genes, namely CYP4C52v1 and CYP6AA7, are up-regulated in both the larval and adult stages in HAmCqG8 mosquitoes9. For the functional study of these up-regulated P450 genes in insecticide-resistant mosquitoes, we utilized the RNAi technique to suppress the expression of these specific P450s in order to further test their involvement in the development of insecticide resistance in Culex mosquitoes. To avoid a potential false-positive result due to the dsRNA gene injection effect, the dsRNA of a green fluorescent protein (GFP-pMW1650) gene was injected to serve as the control20,21,47, non-overexpressed P450-CYP6BY3 served as the negative control. Briefly, a 300–500 bp PCR product was generated that was complementary to the cDNA sequences of the specific P450 gene or pMW1650 plasmid, with T7 promoter sequences (5′-TAATACGACTCACTATAGGG-3′) adjacent to the 5′ ends of both the sense and antisense of each PCR primer (Table 1). The dsRNAs of the specific P450 genes and the GFP gene were synthesized by in vitro transcription using a MEGAscrip T7 High Yield Transcription kit (AB, Applied Biosystems) following the manufacturer’s instructions. Phenol/chloroform extraction followed by ethanol precipitation was used for the dsRNA purification method45,46. Each of the dsRNA-P450s was diluted in nuclease-free water to 3–4 μg/μL for injection.
RNA interference conducted with dsRNA of specific P450 gene or GFP gene injection in HAmCqG8 larvae and adults
The 3rd instar larvae of HAmCqG8 were anesthetized in ice-cold water for about 5 min prior to injection. Anesthetized larvae were then placed on dry filter paper and 138 nl dsRNA (~ 400 ng) of the specific P450 or GFP gene was injected vertically to the body axis in the thoracic region using a capillary needle and Nanoject II (Drummond Scientific Company) under a dissection microscope. The capillary needle was pulled out using a needle puller (Sutter Instrument) following the program: Heat 545, Pull 30, Vel 120, Time 125. After injection, the larvae were immediately removed from the filter paper and placed back into distilled water under normal insectary rearing conditions and fed with yeast. For the mosquito adult microinjections, approximately 138 nl of dsRNA (~ 400 ng) was injected into the thorax of CO2-anesthetized 1-day-old female mosquitoes using a capillary needle, Nanoject II injector and Drosophila CO2 Fly Pads (Tritech Research, Inc.). Three day-post-injection, the living mosquitoes were collected and separated into two groups, the first of which was tested for dynamic changes of P450 gene expression using qRT-PCR detection system and the other was assayed for permethrin resistance using larva or adult bioassay. Each experiment was repeated > 3 times with independent microinjection and RNA isolation from other mosquitoes.
Mosquito larvae and adult bioassays
Stock and serial dilutions of permethrin (94.34%, supplied by FMC Corp., Princeton, NJ) for the insecticide bioassay were prepared in acetone. The bioassay methods used for the larvae and adults were as described in previous studies40,48. Briefly, each bioassay consisted of twenty 4th instar mosquito larvae or 15 adults using 4–5 concentrations that resulted in > 0 and < 100% mortality. Control groups received only 1% acetone. Mortality was assessed after 24 h. At least 3 replications of the bioassay were performed. Bioassay data were pooled and analyzed by standard probit analysis, as described by Liu et al.47. The resistance ratio was calculated based on the LC50 of dsRNA-P450-injected mosquitoes divided by the LC50 of GFP -injection mosquitoes.
In silico modeling and docking analysis
In silico 3D modeling was constructed by the I-TASSER server49,50 and five models were predicted for each P450. The top-scoring model for each P450 was then submitted to the FG-MD server for fragment guided molecular dynamics structure refinement51. Ligand permethrin structures were downloaded from the ZINC database (http://zinc.docking.org/) and prepared for docking using Autodock Tools v1.5.6 (http://mgltools.scripps.edu/downloads). Molecular docking was performed by Autodock 4.252. For all dockings, a search space with a grid box of 60 × 60 × 60A centered at the heme iron center was set corresponding to substrate recognition sites (SRSs) following those of the CYP2 family proposed by Gotoh53. The figures were generated by Pymol for publication (http://www.pymol.org)54.
References
Marshall, E. Malaria. A renewed assault on an old and deadly foe. Science 290, 428–430 (2000).
Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 60, 537–559 (2015).
Paine, M. J. I. & Brooke, B. Insecticide resistance and its impact on vector control. In Advances in Insect Control and Resistance Management 287–312 (2016). https://doi.org/10.1007/978-3-319-31800-4_15.
Hemingway, J., Field, L. & Vontas, J. An overview of insecticide resistance. Science 298, 96–97 (2002).
Hemingway, J., Hawkes, N. J., McCarroll, L. & Ranson, H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem. Mol. Biol. 34, 653–665 (2004).
Scott, J. G. Cytochromes P450 and insecticide resistance. Insect Biochem. Mol. Biol. 29, 757–777 (1999).
Schuler, M. A. & Berenbaum, M. R. Structure and function of cytochrome P450S in insect adaptation to natural and synthetic toxins: Insights gained from molecular modeling. J. Chem. Ecol. 39, 1232–1245 (2013).
Pavek, P. & Dvorak, Z. Xenobiotic-induced transcriptional regulation of xenobiotic metabolizing enzymes of the cytochrome P450 superfamily in human extrahepatic tissues. Curr. Drug Metab. 9, 129–143 (2008).
Yang, T. & Liu, N. Genome analysis of cytochrome P450s and their expression profiles in insecticide resistant mosquitoes, Culex quinquefasciatus. PLoS ONE 6, e29418 (2011).
Reid, W. R., Zhang, L., Liu, F. & Liu, N. The transcriptome profile of the mosquito Culexquinquefasciatus following permethrin selection. PLoS ONE 7, e47163 (2012).
Komagata, O., Kasai, S. & Tomita, T. Overexpression of cytochrome P450 genes in pyrethroid-resistant Culex quinquefasciatus. Insect Biochem. Mol. Biol. 40, 146–152 (2010).
Hardstone, M. C., Komagata, O., Kasai, S., Tomita, T. & Scott, J. G. Use of isogenic strains indicates CYP9M10 is linked to permethrin resistance in Culex pipiens quinquefasciatus. Insect Mol. Biol. 19, 717–726 (2010).
Bonizzoni, M. et al. Comparative transcriptome analyses of deltamethrin-resistant and -susceptible Anophelesgambiae mosquitoes from Kenya by RNA-Seq. PLoS ONE 7, e44607 (2012).
Faucon, F. et al. Identifying genomic changes associated with insecticide resistance in the dengue mosquito Aedesaegypti by deep targeted sequencing. Genome Res. 25, 1347–1359 (2015).
Zamore, P. D., Tuschl, T., Sharp, P. A. & Bartel, D. P. RNAi: Double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 101, 25–33 (2000).
Itokawa, K., Komagata, O., Kasai, S., Ogawa, K. & Tomita, T. Testing the causality between CYP9M10 and pyrethroid resistance using the TALEN and CRISPR/Cas9 technologies. Sci. Rep. 6, 24652 (2016).
Sim, C. & Denlinger, D. L. Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc. Natl. Acad. Sci. U.S.A. 105, 6777–6781 (2008).
Zhu, F., Li, T., Zhang, L. & Liu, N. Co-up-regulation of three P450 genes in response to permethrin exposure in permethrin resistant house flies, Musca domestica. BMC Physiol. 8, 18 (2008).
Xu, J., Tan, A. & Palli, S. R. The function of nuclear receptors in regulation of female reproduction and embryogenesis in the red flour beetle, Tribolium castaneum. J. Insect Physiol. 56, 1471–1480 (2010).
Li, T. et al. A G-protein-coupled receptor regulation pathway in cytochrome P450-mediated permethrin-resistance in mosquitoes, Culexquinquefasciatus. Sci. Rep. 5, 17772 (2015).
Li, T., Liu, L., Zhang, L. & Liu, N. Role of G-protein-coupled receptor-related genes in insecticide resistance of the mosquito, Culexquinquefasciatus. Sci. Rep. 4, 6474 (2014).
Lycett, G. J. et al. Anophelesgambiae P450 reductase is highly expressed in oenocytes and in vivo knockdown increases permethrin susceptibility. Insect Mol. Biol. 15, 321–327 (2006).
Lumjuan, N. et al. The role of the Aedesaegypti Epsilon glutathione transferases in conferring resistance to DDT and pyrethroid insecticides. Insect Biochem. Mol. Biol. 41, 203–209 (2011).
Revuelta, L. et al. RNAi of ace1 and ace2 in Blattellagermanica reveals their differential contribution to acetylcholinesterase activity and sensitivity to insecticides. Insect Biochem. Mol. Biol. 39, 913–919 (2009).
Zhu, F. et al. RNA interference of NADPH-cytochrome P450 reductase results in reduced insecticide resistance in the bed bug, Cimexlectularius. PLoS ONE 7, e31037 (2012).
Xu, L. et al. Silencing of an aphid carboxylesterase gene by use of plant-mediated RNAi impairs Sitobionavenae tolerance of Phoxim insecticides. Transgenic Res. 23, 389–396 (2014).
Gong, Y., Li, T., Feng, Y. & Liu, N. The function of two P450s, CYP9M10 and CYP6AA7, in the permethrin resistance of Culexquinquefasciatus. Sci. Rep. 7, 587 (2017).
Laskowski, R. A. & Swindells, M. B. LigPlot+: Multiple ligand–protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 51, 2778–2786 (2011).
Feyereisen, R. Insect cytochrome P450. Compr. Mol. Insect Sci. https://doi.org/10.1016/b0-44-451924-6/00049-1 (2005).
Nikou, D., Ranson, H. & Hemingway, J. An adult-specific CYP6 P450 gene is overexpressed in a pyrethroid-resistant strain of the malaria vector, Anophelesgambiae. Gene 318, 91–102 (2003).
Müller, P. et al. Field-caught permethrin-resistant Anophelesgambiae overexpress CYP6P3, a P450 that metabolises pyrethroids. PLoS Genet. 4, e1000286 (2008).
Stevenson, B. J., Pignatelli, P., Nikou, D. & Paine, M. J. I. Pinpointing P450s associated with pyrethroid metabolism in the dengue vector, Aedesaegypti: Developing new tools to combat insecticide resistance. PLoS Negl. Trop. Dis. 6, e1595 (2012).
Kasai, S., Weerashinghe, I. S., Shono, T. & Yamakawa, M. Molecular cloning, nucleotide sequence and gene expression of a cytochrome P450 (CYP6F1) from the pyrethroid-resistant mosquito, Culexquinquefasciatus Say. Insect Biochem. Mol. Biol. 30, 163–171 (2000).
Liu, N., Li, T., Reid, W. R., Yang, T. & Zhang, L. Multiple cytochrome P450 genes: Their constitutive overexpression and permethrin induction in insecticide resistant mosquitoes, Culexquinquefasciatus. PLoS ONE 6, e23403 (2011).
Gong, Y., Li, T., Zhang, L., Gao, X. & Liu, N. Permethrin induction of multiple cytochrome P450 genes in insecticide resistant mosquitoes, Culexquinquefasciatus. Int. J. Biol. Sci. 9, 863–871 (2013).
Itokawa, K., Komagata, O., Kasai, S., Masada, M. & Tomita, T. Cis-acting mutation and duplication: History of molecular evolution in a P450 haplotype responsible for insecticide resistance in Culex quinquefasciatus. Insect Biochem. Mol. Biol. 41, 503–512 (2011).
Itokawa, K. et al. Global spread and genetic variants of the two CYP9M10 haplotype forms associated with insecticide resistance in Culex quinquefasciatus Say. Heredity 111, 216–226 (2013).
Adams, R., Nicke, B., Pohlenz, H. D. & Sohler, F. Deciphering seed sequence based off-target effects in a large-scale RNAi reporter screen for E-cadherin expression. PLoS ONE 10(9), e0137640 (2015).
Li, T. & Liu, N. Inheritance of permethrin resistance in Culex quinquefasciatus. J. Med. Entomol. 47, 1127–1134 (2010).
Xu, Q. et al. Resistance in the mosquito, Culexquinquefasciatus (S.), and possible mechanisms for resistance. Pest Manag. Sci. 61, 1096–1102 (2005).
Djouaka, R. F. et al. Expression of the cytochrome P450s, CYP6P3 and CYP6M2 are significantly elevated in multiple pyrethroid resistant populations of Anophelesgambiae s.s. from Southern Benin and Nigeria. BMC Genomics 9, 538 (2008).
Amenya, D. A. et al. Over expression of a cytochrome P450 (CYP6P9) in a major African malaria vector, Anophelesfunestus, resistant to pyrethroids. Insect Mol. Biol. 17, 19–25 (2008).
Li, T. et al. Multiple mutations and mutation combinations in the sodium channel of permethrin resistant mosquitoes, Culex quinquefasciatus. Sci. Rep. 2, 781 (2012).
Xu, Q. et al. Kdr allelic expression of the sodium channel gene in insecticide resistance of Culex quinquefasciatus. Biochem. Biophys. Res. Commun. 345, 774–780 (2006).
Liu, N. & Scott, J. G. Phenobarbital induction of CYP6D1 is due to a trans acting factor on autosome 2 in house flies, Musca domestica. Insect Mol. Biol. 6, 77–81 (1997).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001).
Aerts, J. L., Gonzales, M. I. & Topalian, S. L. Selection of appropriate control genes to assess expression of tumor antigens using real-time RT-PCR. Biotechniques 36, 84–6, 88, 90–1 (2004).
Liu, H., Cupp, E. W., Guo, A. & Liu, N. Insecticide resistance in Alabama and Florida mosquito strains of Aedesalbopictus. J. Med. Entomol. 41, 946–952 (2004).
Roy, A., Kucukural, A. & Zhang, Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protoc. 5, 725–738 (2010).
Yang, J. et al. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods 12, 7–8 (2015).
Zhang, J., Liang, Y. & Zhang, Y. Atomic-level protein structure refinement using fragment-guided molecular dynamics conformation sampling. Structure 19, 1784–1795 (2011).
Morris, G. M. et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 30, 2785–2791 (2009).
Gotoh, O. Substrate recognition sites in cytochrome P450 family 2 (CYP2) proteins inferred from comparative analyses of amino acid and coding nucleotide sequences. J. Biol. Chem. 267, 83–90 (1992).
DeLano, W. L. et al. Pymol: An open-source molecular graphics tool. CCP4 Newslett. Protein Crystallogr. 40, 82–92 (2002).
Acknowledgements
This study was supported by AAES Hatch/Multistate Grants ALA08-045 and ALA015-1-10026, and ALA015-1-16009 to N.L and OUC-AU Joint Center Grants Program to SL and NL. We thank Dr. Nelson for the annotation of the Culex P450 genes. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Contributions
Conceived and designed the study: Y.T., T.L., M.L., X.F., S.L., N.L. Performed the experiments: Y.T., T.L., M.L., X.F. Prepare the materials: S.L., N.L. Wrote the paper: Y.T., T.L., M.L., X.F., S.L. N.L. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, T., Li, T., Feng, X. et al. Multiple cytochrome P450 genes: conferring high levels of permethrin resistance in mosquitoes, Culex quinquefasciatus. Sci Rep 11, 9041 (2021). https://doi.org/10.1038/s41598-021-88121-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-88121-x
- Springer Nature Limited
This article is cited by
-
Potential key genes involved in metabolic resistance to malathion in the southern house mosquito, Culex quinquefasciatus, and functional validation of CYP325BC1 and CYP9M12 as candidate genes using RNA interference
BMC Genomics (2023)
-
Overexpression of cytochrome P450 and esterase genes involved in permethrin resistance in larvae and adults of Culex quinquefasciatus
Parasitology Research (2023)
-
A chromosome-level genome of Semiothisa cinerearia provides insights into its genome evolution and control
BMC Genomics (2022)
-
Comparison of cytochrome P450 CYP332A1 gene in resistant and susceptible strains of the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae)
Applied Entomology and Zoology (2022)