Abstract
The excessive usage of antibiotics in humans and veterinary medicine has lead to the emergence of antibiotic resistance and now requires the use of novel antibiotics. There has been increased interest towards plants as source of drugs because of their pharmacological potency and long traditional usage. The aim of the current study was to evaluate bioactive components, antioxidant, and anti-inflammatory activities of the leaf extracts of Murraya paniculata, a plant traditionally used in Indian medicinal system. Evaluations were made for phytochemical analysis, antioxidant, membrane stabilizing, and antimicrobial activities. The methanol extract displayed the highest flavonoid and phenolic content, the acetone extract demonstrated considerable ABTS inhibitory activity (IC50value:555.18 ± 1.68 µg/mL) and the hexane extract exhibited highest H2O2 radical scavenging activity (IC50value: 509.84 ± 3.03 µg/mL). The aqueous extract displayed 19.4 ± 0.66% RBC hemolysis and 80.5 ± 0.66% protection caused by hypotonic solution at high concentration of the extract. The fractions of hexane extract revealed a higher zone of inhibition than crude extract. The major components found in the fractions were cyclohexane (40.11%) and 3-(6-Methoxy-3-methyl-2-benzofuranyl) Cyclohexanone (13.68%) as analyzed by GC–MS/MS technique. The current results validate the traditional use of the M. paniculata and warrant its potential in drug development programs in further investigations.
Similar content being viewed by others
Introduction
The discovery of compounds with antimicrobial properties has revolutionized modern medicine systems. Use of antibiotics has provided successful interventions in surgical procedures, organ transplants, and patient care. However,reports of antimicrobial resistance amongst common bacterial pathogens are now threatening this therapeutic aid1. A large number of diseases such as pneumonia, tuberculosis, gonorrhoea, and salmonellosis are becoming harder to treat with the currently available antibiotics. It has been estimated that around 300 million premature deaths will occur by 2050 with a global economic loss of up to $100 trillion2. The resistance against antibiotics is an immediate threat to the treatment of infectious diseases as the development of new antimicrobial agents is declining and very few drugs have been approved in the recent years3. Phytochemicals have displayed potential antibacterial properties against sensitive and resistant pathogens. Thus,it is a priority in medical research to screen plant extracts for novel antimicrobial properties as a possible source for a new drug development4, 5. Plants possess a variety of secondary metabolites that can be utilized for the development of biocompatible therapeutics. Many traditional medicinal systems practiced in major parts of the world are based largely on therapeutic use of such medicinal plants. The World Health Organization (WHO) has also emphasized that several countries are gradually accepting the contribution of traditional medicine to the health and well-being of individuals, and the need to integrate it to their healthcare systems6. Thus, a logical application is that plants be selected based on traditional medicinal knowledge and screened for characterization of natural products as novel antimicrobials. Free radicals play a central role in various physiological conditions and have been linked to a majority of diseases and disorders7. Free radicals can be generated in living organisms as a normal part of metabolism by oxidation–reduction reactions catalyzed by enzymes such as catalase and peroxidase or due to exposure of biological systems to external factors such as chemical carcinogens or ionizing radiation. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) may cause disintegration of cell membranes or adversely affect nucleic acids, lipids, and proteins, thereby altering the normal redox status leading to increased oxidative stress8. This results in development of various diseases such as cancer, arteriosclerosis, diabetes mellitus, Alzheimer’s disease, liver and skin damage, coronary heart diseases, arthritis, and inflammation9.
Inflammation is a non-specific response employed by both innate and adaptive immune systems against pathogenic intruders, hazardous stimuli such as allergens or tissue injury. Unregulated inflammatory responses are the underlying cause of several disorders including allergies, cardiovascular dysfunctions, metabolic syndrome, cancer, and autoimmune diseases10. Synthetic chemical drugs, including steroids, non-steroid anti-inflammatory drugs, and immunosuppressant, are used for regulating inflammatory crises; however, there are associated adverse effects related to gastrointestinal, cardiovascular and kidney functions11. Alternatively, natural compounds, dietary supplement, and herbal remedies are known to be beneficial and are gaining importance for the prevention and treatment of inflammatory diseases. Some medicinal plants which have anti-inflammatory properties associated with the presence of naturally occurring antioxidants, such as polyphenols, carotenoids, flavonoids, ascorbic acid, and tocopherols, have been reviewed thoroughly12,13,14. Vitamins A, C, and E are known to terminate lipid peroxidation chain reactions, and bioflavonoids that are widely distributed in fruits and vegetables function by scavenging the free radicals;exerting a protective effect on DNA damage possibly by chelating metal ions. Carotenoids, the most common lipid soluble phytonutrient, protect cellular membranes and lipoproteins against the ROS by scavenging peroxyl radicals15,16.
One plant of interest in the search for plant-based therapeutics and antimicrobial compounds is Murraya paniculata (also known as Cholcas paniculata L, Chalcas exotica L, and Murraya exotica L.).Commonly known as Orange Jasmine, mock orange, Chinese box, or Kamini, is a tropical, evergreen plant with tiny, white, scented flowers, and is cultivated as an ornamental tree or hedge17. The leaves have been used as a food additive in many Indian and Malay dishes due to their strong fragrance. The plant is known for its therapeutic usage and has been traditionally used for the treatment of tuberculosis, diarrhea, abdominal pain, dysentery, headache, edema, thrombosis, and stasis of blood. More than 120 secondary metabolites have been reported from M. paniculata, polymethoxylated flavonoids and their glycosides being the prominent ones18,19. The plant is also known to contain coumarins20,21 indole alkaloids22,23,24, and essential oils25,26,27. M. paniculata has been reported to display several ethno-pharmacological activities such as anti-anxiety, anti-depression28 antioxidant17,29,30,31, antimicrobial30,32,33,34,35, anti-inflammatory, antifungal36,37,38, antifeedent, toothache 39, diarrhea, asthama and hypertension40, nematicidal and toxicity analysis41,42 diabetic nephropathy and cardiomyopathy43,44. Traditional medicinal healers of Amarkantak region utilize medicinal plants for the treatment of various diseases, including M. paniculata. In our initial studies we found M. paniculata to be one such promising plant used by the healers for the successful treatment of wounds, tuberculosis, malaria, and as an antidote for snakebite. The different parts of the plant such as leaves, roots, and stem are used in the preparation of herbal remedies. To the best of our knowledge, there are no reports of systematic validation of the medicinal use of M. paniculata in traditional medicine. Thus, it was critical to define its pharmacological properties, such as antioxidant, antimicrobial and anti-inflammatory capabilities. The present study was undertaken to compare efficiency of different leaf extracts to find out the phyto-constituents, total phenolic, total flavonoid content, total reducing power and antimicrobial property against selected microbial strains. To our knowledge, this is the first reporting of the comparative account of the pharmacological efficiency of different leaf extracts of M. paniculata.
Results
Extract preparation
The extracts were prepared using dried leaves (100 g) of the plant in different solvents such as hexane, acetone, chloroform, methanol, and water. The results display that maximum yield per gram is obtained from methanol (4.8 g) followed by acetone (3.7 g), water (3.47 g), hexane (1.78 g), and chloroform (1.07 g). These data highlight methanol as an efficient solvent for extraction of phytochemicals from leaves of M. paniculata.
Phytoconstituents analysis
Alkaloids, flavonoids, steroids, and terpenoids were detected as major secondary metabolites in the plant extracts. Tannins, saponins and glycosides were present in minor quantities while phlobatanins and terpenoids were not present in any of the extracts (Table S1).
Total phenolic and total flavonoid content
The total phenolic and total flavonoid content in the different extracts of M. paniculata is represented in Table 1. Among the five extracts, methanol extract possesses highest phenolic content (1060 ± 52.83 mg gallic acid equivalent/g dry material) followed by acetone (849.8 ± 49.2 mg/g), water (114.4 ± 10.49 mg/g), chloroform (21.32 ± 3.6 mg/g), and hexane (22.17 ± 5.5 mg/g). The total flavonoid concentration in different solvent extracts was estimated using quercetin as a standard and values expressed in mg quercetin equivalent /g dry wt. Out of five extracts the methanol (318.4 ± 9.16 mg) contains highest flavonoid content followed by water (244.8 ± 7.98 mg), acetone (121.8 ± 4.42 mg), hexane (43.75 ± 1.5 mg), and chloroform (42.83 ± 6.66 mg).
Antioxidant activity
Supplementary figure (Fig. S1) shows the concentrations of plant extract with percentage inhibition of ABTS radical. The acetone extract demonstrated elevated ABTS+ inhibition with increased concentration. For ABTS+ inhibition the IC50 valuesranged between 500–800 µg/mL (Table 2). The IC50value of acetone (555.18 ± 1.68 µg/mL) were less than the standard (ascorbic acid) used. The percentage of H2O2 inhibition by different extracts was found to be concentration dependent (Fig. S2). The hexane extracts exhibited highest H2O2 scavenging ability compared to other extracts. The IC50 values ranged between 500–800 µg/mL (Table 2). The IC50value of hexane (509.84 ± 3.03 µg/mL) was lowest as compared to the standard (ascorbic acid) or other extracts that were analyzed. These result demonstrate that hexane and acetone have better antioxidant property as compared to the standard.
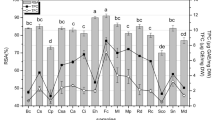
The analysis of total reducing capacity of extracts was carried out using FRAP and reducing power assays. FRAP value of methanolic extract (0.085 ± 0.002 Fe2+/gram sample) showed highest value, followed by acetone, water, chloroform, and hexane (Table 2) The reducing power property of compound/extracts indicates its electron donating capacity. In reducing power assay, the M. paniculata methanol extract displayed maximum absorbance followed by water, acetone, ascorbic acid, hexane, and chloroform extracts (Fig. 1).
Membrane stabilizing property
The results of membrane stabilization assays are presented in Table 3. It was observed that all the extracts of M. paniculata exhibited concentration-dependent anti-inflammatory properties as compared to the standard drug, indomethacin. The maximum concentration analyzed was 5000 µg/mL and extracts were found to possess anti-inflammatory properties in the order MPW > MPM > MPH > MPA > MPC. The aqueous extract showed the 80% protection, whereas the standard drug showed 83% protection at highest concentration tested. Pearson’s correlation was done with concentration against percent protection provided by different extracts (Table S2), where we found strong positive correlation with concentration and percent protection with all extracts.
Antimicrobial activity
Table 4 presents antimicrobial activity against tested bacterial (E. coli, S. aureus, P.aeruginosa, B. subtilis) and fungal (C. albicans, P. chrysogenum, A. niger, A. flavus) strains using agar well diffusion method (Fig. 2). The growth of P. aeruginosa was inhibited by all extracts of M. paniculata. Growth inhibition was observed in standard ciprofloxacin (MIC of 25 µg/mL) against all tested bacterial strains. The MIC for different extracts was as 25 µg/mL for aqueous extract while 250 µg/mL for hexane, methanol and chloroform extracts and 500 µg/mL for acetone extract. The hexane extract displayed good inhibition activity against all the bacterial strains as compared to other tested extracts. All extracts were found inactive against fungi as we did not observe any zone of inhibition even after repeated experiments.
Fractionization of hexane extract
13 fractions were collected initially using column chromatography. The results of TLC showed that F1- F3 contain single spot and were eluted using hexane and dichloromethane (5:5) as the solvent. The fractions were pooled and renamed as PC1. The F9 & 10 showed similar spots and were pooled as PC3. Further F11-13 had similar spots and was renamed as PC4. The F4 to F8 displayed 3 spots on TLC (2 spots in UV and 1 spot in Vis) which were concentrated and further allowed for column chromatography separation using mixture of hexane and acetone as solvent. From the repeated chromatography we obtained fraction PC2 (in visible region), PC11, and PC22 (in UV region). Finally, 6 pooled fractions were obtained, named as PC1, PC2, PC3, PC4, PC11, and PC22, and were used for further antibacterial activities.
Antibacterial activity
Table 5 presents antimicrobial activity against selected multi resistance bacteria (E. coli, S. aureus, P. aeruginosa, B. subtilis) using fractions obtained from column chromatography. Growth inhibition was observed in standard ciprofloxacin (MIC of 25 µg/mL) against all the tested bacterial strains. The B. subtilis and P. aeruginosa were inhibited by all fractions of hexane extract, out of which PC4 showed MIC of 250 and 25 µg/mL respectively (Fig. 3).
GC–MS/MS of PC4 fraction of Hexane extract
The PC4 fraction from the hexane extract exhibited significant antibacterial activity was subjected to analysis by GC–MS/MS technique. The major antibacterial components found in the hexane fractions were Cyclohexane (40.11%) and 3-(6-Methoxy-3-methyl-2-benzofuranyl)Cyclohexanone (13.68%) is shown in Table 6 and Fig. 4. The supplementary figure (Fig. S3) shows mass spectrum of major components identified through GC–MS/MS analysis.
Discussion
Amarkantak is located in an ecologically diverse region in the states of Chhattisgarh and Madhya Pradesh,India. Several tribal and non-tribal communities reside in this area and utilize the forest produce for their livelihood and other needs. Being biodiversity rich, Amarkantak is a potential source of hundreds of medicinal plants that are being utilized by the tribal communities for the treatment of various health problems. M. paniculata is used by them for treatment of tuberculosis, malaria, wound healing, and as an antidote for snakebite. Therefore, M. paniculata leaves were selected for investigation of antioxidant, membrane stabilizing, and antimicrobial properties stemming from phytochemical features. Phytochemical screening determines the presence of alkaloids, flavonoids, steroids, tannins, saponins and glycosides that are known to have protective or disease preventive properties. Several phytochemicals play vital roles in alleviating the several health problems like malaria, asthma, cancer, arthritis, jaundice, diabetes, dengue, diarrhea, dysentery, and microbial infections45. In the current study, secondary metabolites such as alkaloids, flavonoids, steroids andtannins were present in majority of extracts, corroborated by earlier studies46 Alkaloids, tannins flavonoids and other metabolites are reported to have antioxidant, antifungal, anticancer, antiviral, anti-inflammatory and antiophidic activities47. Alkaloids have been the basis of several antibacterial drugs and serve as scaffolds for important antibacterial drugs such as metronidazole, quinolones, linezolid, and trimethoprim48,49. More than 8000 flavonoids have been reported from different plants species50. That play vital role in stimulation, protection, flavoring, communication, and pigmentation51. Flavonoids are rich source of antioxidants mainly due to their free radical scavenging activity by the transfer of an H atom or of a single electron to the radical stabilizing it or due to their metal chelating activity52,53. The flavonoids are known to possess anti-allergic, hepatoprotective anticancer, antibacterial, anti-inflammatory, anti-diabetic, and anti-viral properties54,55,56,57,58. Saponins, a vast group of compounds are characterized by their foam forming and detergent properties59,60,61. They are known to have several biological properties like antimicrobial, immunomodulatory, anti-malarial, anti-allergic, anti-diabetic, insecticidal, and anti-inflammatory62,63,64,65,66. The tannins are water soluble polyphenolic compounds synthesized by plants62 and are responsible to protect plants against herbivores and insects67,68. Tannins have also been reported to have anti-cancer, anti mutagenic, anti-oxidant, antibacterial, anti-viral, anti-tumor, anti-inflammatory effects and wound healing capabilities68,69,70,71,72,73. The presence of all these phytochemicals makes it understandable why M. paniculuta leaves are a prominent medicine for treatment of various diseases within tribal communities.
Here, the total phenolic content was present in high concentrations in methanolic extract followed by acetone, water, chloroform, and hexane while the total flavonoid content was highest in methanol extract followed by water, acetone, hexane and chloroform. Antioxidant activity of flavonoids is due to their ability to reduce free radical formation and to scavenge free radicals. Previously, Zhang et al. (2011) reported the presence of seventy polymethoxylated flavonoids (PMF) in the leaves extract of M. paniculata74; which are responsible for a number of biological activities including antioxidant75,76 and antimicrobial properties77,78. Phenolic compounds are good antioxidants as their hydroxyl groups can directly contribute to antioxidant action leading to a wide range of biological effects including antibacterial, anti-inflammatory, anti-allergic, hepato-protective, antithrombotic, antiviral, anticarcinogenic, and vasodilatory actions79. Some phenolic compounds can stimulate the synthesis of endogenous antioxidant molecules in the cell80. The results of antioxidant activities correlated well with the presence of phenolic and flavonoids in different extracts.
Significant concentration-dependent inhibition of ABTS free radicals was observed in all the tested extracts. The IC50 value observed for acetone extract was less than the standard ascorbic acid, indicating the strong ability of the extracts to act as ABTS and H2O2 scavenger. Significant dose-dependent H2O2 scavenging activities were observed in all the extracts analyzed. The lowest IC50 (509.84 μg/mL) was observed for the hexane extract that was lower than that of ascorbic acid (528.01 μg/mL) although it was observed that at low concentrations ascorbic acid was found to be more efficient. The reducing capacity of antioxidant is mainly because of their electron transfer property for instance polyphenols and flavonoids 81. The FRAP assay is a relatively simple, quick, and inexpensive direct method of measuring the combined antioxidant activity of reductive antioxidants in a test sample 82. The higher values shows, higher antioxidant property of extracts. Our study clearly indicated that the methanolic extract of the leaves exhibits high scavenging capacity, demonstrating that the high antioxidant capacity of the methanol extract correlates with the total content of phenolic and flavonoid content (i.e., higher the absorbance shows higher antioxidant properties). The present study also demonstrated the results that total antioxidant reducing power of compound/extract is depending on the amount of phenolic and flavonoid content. The comparison between the results of total phenolic content and reducing power assay is found to be in similar order: MPM > MPW > MPA > MPH > MPC. Whereas the results of total flavonoid content and FRAP values found to be in order of: MPM > MPA > MPW > MPC > MPH. Many studies have demonstrated a strong correlation between the phenolic content and reducing property of compounds. The redox properties of phenolic are major reason for the antioxidant property of an extract83,84.
Low-grade inflammatory state is correlated with various disorders and chronic health conditions, such as obesity, diabetes, cancer, and cardiovascular diseases. Living tissues respond to injury, infection or irritation by releasing inflammatory lysosomal enzymes that may damage macromolecules and cause lipid peroxidation of membranes. Plant extracts can be utilized for stabilization of lysosomal membranes or control of major pro-inflammatory mediators by inhibiting the release of lysosomal constituents of activated neutrophil such as bactericidal enzymes and proteases. Human red blood cell (HRBC) or erythrocyte membranes are analogous to lysosomal membranes and have been used in stabilization assays. The antioxidant activity of the M. paniculata extracts correlated well with the RBC membrane stabilization properties. At the concentrations of 4000–5000 µg/mL, 60–80% protection was observed, which was comparable to the standard drug indomethacin in RBC hemolysis with water and methanol extract. Non-steroidal anti-inflammatory drugs (NSAIDs), such as indomethacin, acts by inhibiting the enzyme cyclooxygenase, but the use of NSAIDs is questionable due to the emerging evidence suggesting a high risk of acute myocardial infarction, stroke, heart failure, renal failure, and arterial hypertension85. Several medicinal plants have been reported to have phytoconstituents such steroids, flavonoids, alkaloids, polyphenols, glycosides, terpenoids, curcumins, GLA, linear aliphatic alcohols, harpagoside, phenolic diterpenes, which have anti-inflammatory properties with minimal side effects14. The results of our study clearly suggest that M. paniculata extracts may provide us with some potent compounds for the management of inflammatory conditions. The presence of saponins and flavonoids in the methanolic and water extracts also support the current findings as they have been known to have profound stabilizing effect on lysosomal membrane86. It has been reported that flavonoids isolated from M. paniculata displayed anti-inflammatory activity by inhibition of NO and IL-6 production87. The development of flavonoid based phytotherapeutic anti-inflammatory agents, Acheflan and Daflon, gives us hope for the development of drugs without adverse effects85. Novel safe and promising antimicrobial agents are urgently required to fight against drug-resistant bacterial and fungal strains. Flavonoids are known to inhibit microbial growth by inhibition of nucleic acid synthesis, cytoplasmic membrane function and energy metabolism pathways88. The antimicrobial properties of phenolic compounds have been reported due to the alteration of cell membrane permeability leading to the uncoupling of oxidative phosphorylation, inhibition of active transport and cytoplasmic membrane damage that leads to the loss of pool metabolites89. Tannins have been shown to possess inhibitory activities against Methicillin-resistant Staphylococcus aureus (MRSA)90 or bacteria, yeast and fungi91. In the current study all the extracts of M. paniculata displayed some level of inhibition of bacterial growth, but no antifungal activity was observed displaying its potency for the development novel antibiotics. Here, both the Gram negative and Gram positive bacteria were inhibited by the extracts. However, the hexane extract exhibited growth inhibition against all tested bacterial strains with a minimum observed MIC of 50 µg/mL against B. subtilis or MIC 250 µg/mL against P. aeruginosa. Fractionization of hexane extract yielded six fractions that revealed promising antibacterial potency. The P. aeruginosa was found to be very sensitive to the fractions of hexane extract displaying an inhibition zone of 9.6 mm against PC2 fraction, whereas the PC4 fraction exhibited considerable antibacterial activity against all tested strains. P. aeruginosa, an opportunistic pathogen, is the leading cause of morbidity and mortality in immune-compromised individuals or cystic fibrosis patients92 and displays remarkable antibiotic resistance capacities. It has been listed by WHO as one of the three bacterial species against which there is critical need for the development of new antibiotics93. Similarly, methicillin-resistant S. aureus have been associated with hospitals settings and has emerged as a widespread cause of community infections. The inhibition of both these strains by PC4 predicts it to be a source of novel antibacterial compound/s specifically against P. aeruginosa and S. aureus.
The GC–MS/MS analysis of PC4 fraction revealed a presence of cyclohexane and 3-(6-Methoxy-3-methyl-2- benzofuranyl) cyclohexanone that might be a reason for the observed antibacterial activity. The cyclohexane and derivates are lipophilic weak acids and possess exceptional nature of mode of action as antibacterial agents. Unlike other cationic antimicrobial compounds, these cyclohexane and derivatives will not interrupt the cytoplasmic membrane instead they block the transport of low molecular weight hydrophobic substances of bacterial cell94. The heterocyclic ring compounds, such as benzofuran, is a significant class of compounds obtained. This class of compounds is vital in numerous pharmacological areas and could be a one such reasons for the active biological properties of natural products95. The derivatives of benzofuran exhibited many noteworthy activities against viruses, fungi96,97 protozoan, tuberculosis98 and so on. Therefore, the wide range of biological importance coupled with this scaffold has resulted in the cyclohexane and benzofuran ring systems being considered as a privileged structure. Much attention is warranted for cyclohexane derivatives and benzofuran-based medicinal agents, hence it is important that research and development on these compounds be increased across a wide range of medicinal areas. Efforts have been invested in the past several decades to develop more effective and less toxic agents to treat many infectious and resistant strains of microbes. Cyclohexane and benzofuran based compounds as microbial agents could be a promising, as they possess structural diversity and excellent therapeutic potency. Our antibacterial activity results coupled with previous studies emphasizes the importance of using plants as critical therapeutic agents.
Conclusion
The current study illustrates that the M. paniculata leaves extracts possess potent antioxidant activity and could stabilize the human RBC membranes in a dose-dependent manner, thus providing novel anti-inflammatory compounds. A preliminary chemical examination of different extracts reveals the presence of flavonoids, alkaloids, tannins, and polyphenols that might be responsible for the antimicrobial, antioxidant, RBC membrane stabilization, and anti inflammatory properties. The PC4 is the most potent hexane fraction that was bactericidal against E. coli, B. subtilis, P. aeruginosa, S. aureus, and further characterization revealed the presence of cyclohexane and banzofuran derivatives that might be responsible for the observed antimicrobial activity. The overall results of the present study demonstrate that traditionally used plants provide extremely promising prospects compounds for the development of novel anti-microbial drugs ; especially as treatments against multi-drug resistant bacterial strains.
Materials and method
All the methods were carried out in compliance with relevant guidelines.
Preparation of extracts
The leaves of M. paniculata L. growing in wild were collected from the Amarkantak region of Madhya Pradesh, India, and were identified by Dr. Ravindra Shukla, Department of Botany, Indira Gandhi National Tribal University, Amarkantak, MP (India). A voucher specimen of the plant (SS/DOB/MP13) has been deposited in the Department of Botany for future reference. The leaves were washed under tap water, shade dried and finally grinded it into fine powder form. The extraction was carried out using different solvents (hexane, acetone, chloroform, methanol and water) for approximately 7–8 h using Soxhlet extractor and concentrated using rotary evaporator. The extract was dried and stored in vials at 40C till future use.
Phytochemical screening
The phytochemical screening of all extracts was carried out according to standard methods as mentioned elsewhere with minor modifications99,100,101.
Total phenolic content
The total phenolic content was determined for different M. paniculata extracts using spectrophotometric methods102. Different extracts were taken at concentration 1 mg/mL for analysis. The reaction mixture was prepared (in triplicate) by mixing 0.5 mL of individual extracts, 2.5 mL of 10% Folin-Ciocalteu’s reagent dissolved in water and 2.5 mL 7.5% NaHCO3. The samples were incubated for 45mins at 450C and the absorbance was measured at 765 nm using spectrophotometer (Shimadzu UV–Vis spectrophotometer-1800). Based on the measured absorbance, the total phenolic content in the test samples was calculated using the calibration plot (Y = 0.00024x + 0.03510, R2 = 0.9963) and expressed in terms of Gallic acid equivalent (mg of GA/g of extract).
Total flavonoids content
The total flavonoid content was performed as per Dowd method with some modifications for the different extracts103,104,105. Different extracts were taken at 1 mg/mL concentration for analysis and reaction mixture prepared by mixing 0.5 mL of extracts, 10% aluminium chloride (0.1 mL), 1 M potassium acetate (0.1 mL) and distilled water (4.3 mL). Reaction mixture was incubated at room temperature for 30 min and absorbance was measured at 510 nm .Quercetin (1 mg/mL) was used for preparing the standard calibration curve. The total flavonoid content in the test samples was calculated using the calibration plot (Y = 0.002x + 0.158, R2 = 0.997) and expressed as mg quercetin equivalent (QE)/g of dried plant material.
Anti oxidant assays
ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) assay
ABTS is a synthetic radical widely used for both the polar and non-polar samples106. Mixture of ABTS (14 mM) and potassium per sulfate (4.95 mM) (1:1 v/v) was prepared and allowed to stand overnight at room temperature (RT) in dark. ABTS+ was diluted with water to obtain equilibration of absorbance 0.70 (± 0.02) at 734 nm and suitable blank was used without adding test samples. 50 mg/mL stock solutions of plant extracts were prepared and different concentrations used for assay. The following formula was used to calculate percentage of inhibition.
A0 –– Absorbance of blank, A1 –– Absorbance of Sample.
Hydrogen peroxide (H2O2) radical scavenging assay
Hydrogen peroxide radical scavenging activity was determined according to previously utilized method107. 40 mM H2O2 solution was prepared in 50 mM phosphate buffer (pH 7.4). 3.4 mL of different concentrations of extracts (1000, 2000, 3000, 4000, and 5000 μg/mL) were added to 0.6 mL of H2O2 solution. The absorbance was read at 230 nm after 10 min of incubation at RT against a blank solution containing only phosphate buffer without H2O2.
A0 –– Absorbance of blank, A1 –– Absorbance of Sample.
Ferric reducing antioxidant power (FRAP) assay
The total antioxidant potency of extracts of M. paniculata was estimated using a ferric reducing antioxidant power (FRAP) assay108. FRAP reagent (straw yellow color) was prepared by mixing 30 mM acetate buffer, 10 mM TPTZ, 20 mM FeCl3 and distilled water. The standard curve of ferrous sulfate (FeSO4.7H2O) was prepared with concentrations ranging from 0.1, 0.2, 0.4, 0.4, 0.6, 0.8 and 1.0 mM/L. Different concentrations of ascorbic acid and sample (1000, 2000, 3000, 4000, and 5000 μg/mL) were prepared from 1 mg/mL stock solution and 3 mL of FRAP reagent added to each and the tubes were incubated for 30 min in the dark, read absorbance at 593 nm.
Reducing power assay (RPA)
The total reducing power of extracts was estimated by the method of Oyaizu et al. (1986)109. The different concentrations of plant extracts (1 mL of 1000, 2000, 3000, 4000, and 5000 μg/mL) were mixed with 5 mL of 0.2 M phosphate buffer (pH-6.6), and 5 mL of 1% ferricyanide was added and incubated for 20 min at 500C. After incubation the 10% trichloro acetic acid (TCA) added and centrifuged at 3000 rpm for 10 min. Equal amounts of water and 1 mL of 1% ferric chloride were added to the supernatant, absorbance read at 700 nm. Higher the absorbance value of the reaction mixture indicated better reducing power.
Membrane stabilizing property
The HRBC membrane stabilization method is mainly used to estimate the anti-inflammatory activity of plant extracts110. The fresh blood was collected and mixed with an equal volume of sterilized Alsever’s solution (2% dextrose, 0.8% sodium citrate, 0.5% citric acid, and 0.42% sodium chloride in water). The administration of NSAIDS for 2 weeks before collecting the blood was avoided in sampled participants. The collected blood was further centrifuged at 3000 rpm for 10 min, the pellet (packed cells) was washed three times with isosaline (0.85%, pH 7.2), and finally 10% (v/v) suspension was made with isosaline. To the different concentrations of plant extracts, 1 mL phosphate buffer (0.15 M, pH 7.4), 2 mL hyposaline (0.36%), and 0.5 mL HRBC suspension was added. Standard and control were prepared without addition of the extracts. Indomethacin at different concentrations (1000, 2000, 3000, 4000, and 5000 μg/mL) was used as the standard drug and compared with respective concentrations of plant extract. The reaction mixtures were incubated at 370C for 30 min and centrifuged at 3000 rpm for 10 min. The hemoglobin content in the supernatant was estimated at 560 nm111.
The percentage hemolysis was calculated using the following equation:
The percentage of HRBC membrane stabilization was calculated using the following equation:
Antimicrobial assay
Crude extracts were evaluated for their antimicrobial properties against selected bacterial strains (Escherichia coli MTCC 1575, Staphylococcus aureus MTCC 1144, Bacillus subtilis MTCC 2413, Pseudomonas aeruginosa MTCC 1688) and fungal strains (Candida albicans MTCC 854, Penicillin chrysogenum MTCC 1996, Aspergillus niger MTCC 872 and A. flavus MTCC 1883).Standard drugs Amphotericin and Ciprofloxacin were used as positive controls.
Agar diffusion method
The stock cultures of bacteria were revived by inoculating in broth media and grown at 37ºC for 18 h. The agar plates were prepared, after solidification the 100 μl (10–4 cfu) 18 h old culture inoculated and evenly spread. After 20 min, the wells were filled with test compounds at different concentrations (1000, 500, 250, 100, 50, 25 µg/mL) kept for incubation at 37ºC for of 24 h. The diameter of inhibition zones measured and noted as described earlier112,113. Minimum inhibitory concentration (MIC) was calculated using the same plate.
Column chromatography of hexane extract
The most active crude extract (hexane extract) was allowed to separate using gravitation column chromatography. The slurry was prepared by mixing 500 g of absorbent (silica gel 60–120 mesh size) in n-hexane and stirred well to remove bubbles then poured in to glass column. The sample to be loaded on column was prepared by dissolving 5 g of extracting 25 ml of hexane and 20 g of silica. In Table S3, we review the ratio of solvents and fractions obtained in the column chromatography. Each fraction was spotted on activated TLC plate along with extract spot and mobile phase [Hexane: acetone (3:1)] used. The fractions showing more than one spot were concentrated and allowed for further purification using only different solvent mixtures (Hexane and Acetone). The column chromatography was repeated as the eluent system to obtain a single spots on TLC.
Antibacterial activity
The different fractions collected from the hexane extract were further evaluated for antibacterial activity as mentioned earlier and the zones of inhibition were recorded.
GC–MS/MS analysis
The GC–MS/MS analysis of PC4 fraction of M. paniculata hexane extract was carried out on Agilent technologies model 7890A GC coupled with a mass detector 5975C MS system. The Analytic column was Agilent J&W non polar column DB-5MS ((5%-Phenyl)-methylpolysiloxane, 30 m × 0.25 mm, ID 1.8 micron thickness). Flow rate of helium gas is 1.3 ml/min, used to separate components. The different GC conditions were standardized as follows: injector parameters were injection volume 1 μL under split of 3:1, while injector temperature was set at 280 °C (mass analyzer). During GC extraction the program of oven temperature was 1 min at 75 °C, increased to a temperature of 300 °C at a rate of 30 °C/min for 2 min; inlet and transfer line temperature was 250 °C and 290° C respectively. Mass spectra were taken at an ionization mode with an electron impact at 70 eV. Interpretation of mass spectrum GC–MS/MS analysis was done by matching list of known compound’s spectrum with Agilent’s GC/MS Chemstation, NIST MS Library and NIST’s Automated Mass Spectral Deconvolution and Identification software114.
Statistical analysis
All data were expressed as mean ± SE (n = 3) . Comparison of mean values between various extracts of M. paniculata was performed by one way-ANOVA, correlation coefficient (r), and coefficient of determination (r2) calculated using prism 8.0.1(244). The criterion of evaluating statistical significance was as follows: P value < 0.033 was considered significant and marked as *, P < 0.002 as highly significant and marked as **, P < 0.001 was very highly significant and marked as ***.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Ethics Committee of IGNTU (Approval no. IGNTU/IEC/03/2019) and with the 1964 Helsinki declaration and its later amendments. Researcher’s blood was used for the membrane stabilizing assay and no external participant was enrolled for the study.
Data availability
The data can be accessed/shared to the public.
Abbreviations
- ABTS:
-
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- ANOVA:
-
Analysis of variance
- FRAP:
-
Ferric reducing antioxidant power
- GC–MS/MS:
-
Gas chromatography mass spectroscopy
- HRBC:
-
Human red blood cell
- H2O2 :
-
Hydrogen peroxide
- MIC:
-
Minimum inhibitory concentration
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- NIST:
-
National Institute of Standards and Technology
- PC fraction:
-
Pooled concentrated fraction
- RPA:
-
Reducing power assay
- TCA:
-
Trichloro acetic acid
- TLC:
-
Thin layer chromatography
- WHO:
-
World health organization
References
Munita, J.M. & Arias, C.A., Mechanisms of Antibiotic Resistance. Microbiol Spectr. 4 (2). VMBF-0016–2015, (2016).
O’Neill, J., Antibiotic Resistance Threats in the United States. Centers for Disease Control and Prevention. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (2016).
Conly, J. & Johnston, B. Where are all the new antibiotics? The new antibiotic paradox. Can J Infect Dis Med Microbiol. 16(3), 159–160 (2005).
Gautam, M.K., Gangwar, M., Gopal, N., Rao, C.V. & Goel, R.K., In-vitro antibacterial activity on human pathogens and total phenolic, flavonoid contents of Murraya paniculata Linn. Leaves. Asian Pac J Trop Biomed. S1660-S1663 (2012).
Khameneh, B., Iranshahy,M., Soheili, & Bazzaz B. S.F., Review on plant antimicrobials: a mechanistic viewpoint. Antimicrob Resist Infect Control. 8, 118 (2019).
WHO traditional medicine strategy: 2014–2023. https://www.who.int/publications/i/item/9789241506096
Liguori, I. et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 13, 757–772 (2018).
SAT, C. S. Sati, N. Rawat, U and Sati, O.P., Medicinal Plants as a Source of Antioxidant. Res J Phytochem. 4(4),2123–224 (2010).
Dontha, S. A review on antioxidant methods. Asian J Pharm Clin ReS. 9(2), 14–32 (2016).
Noah, T. A., Zachary, M. W. & Randy, J. N. Inflammation: Mechanisms, Costs, and Natural Variation. Ann Review Eco, Evolu, and Systema. 43(1), 385–406 (2012).
Wongrakpanich, S., Wongrakpanich, A., Melhado, K. & Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 9(1), 143–150 (2018).
Ghasemian, M., Owlia, S. & Owlia, M. B. Review of Anti-Inflammatory Herbal Medicines. Adv Pharmacol Sci. 2016, 9130979. https://doi.org/10.1155/2016/9130979 (2016).
Yatoo, M. I. et al. Anti-Inflammatory Drugs and Herbs with Special Emphasis on Herbal Medicines for Countering Inflammatory Diseases and Disorders - A Review. Recent Pat Inflamm Allergy Drug Discov. 12(1), 39–58 (2018).
Palanisamy, A. et al. Role of Antioxidants and Natural Products in Inflammation. Oxid Med Cell Longe 2016, 15. https://doi.org/10.1155/2016/5276130 (2016).
Nimse, S. B. & Pal, D. K. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 5, 27986–28006 (2015).
Francenia, S-S. N. Salas-Coronado, R. Villanueva-Canongo, C. & Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. Antioxidants. (2019). https://doi.org/10.5772/intechopen.85270.
Singh, N., Kaur,N. & Arora, R., Isolation and Characterization of Murraya paniculata ethanol seed extract for their Antioxidant component. Inter J Pharm. 5(9), (2014).
Kinoshita, T. & Firman, K. Myricetin 5,7,3′,4′,5′-pentamethyl ether and other methylated flavonoids from Murraya paniculata. Phytochemistry 45(1), 179–181 (1997).
Zhang, Y. Jun Li, Shepo S. Zan,K. Pengfei, T, Glycosides of flavone methyl ethers from Murraya paniculata. Biochem Systema and Eco. 43,10–13(2012).
Ito, C. Furulawa, H. Ishiii, H. Ishikawa, T. Haginiwa, J., The chemical composition of Murraya paniculata. The structure of five new coumarins and one new alkaloid and the stereochemistry of murrangatin and related coumarins. J. Chem. Soc. Perkin Trans. 1, 2047–2055(1990).
Wang, X. Liang, H. Zeng,K. Zhao, M. Pengfei, T. Jun Li, Yong, J., Panitins A-G: Coumarin derivatives from Murraya paniculata from Guangxi Province, China show variable NO inhibitory activity. Phytochemistry. 162, 224–231(2019).
Kong, Y.C. But, P. PH. Kam-Hung, N. Cheng, KF. Chang, KL. Wonga, K.M. Gray, A. I. Waterman, P. G. The biochemical systematics of Merrillia; in relation to Murraya, the clauseneae and the aurantioideae. Biochem Systema and Eco. 16(1), 47–50 (1988).
Kinoshita,T. Tatara,S. Feng-Chi H., Sankawa,U., 3-Prenylindoles from Murraya paniculata and their biogenetic significance. Phytochemistry. 28(1), 147-151(1989).
Wang, X. Yang,B. Zhang,A. Sun,H. Dong,W., Chapter 14 - Rapid Analysis of Multiple Constituents of Suanzaoren Decoction by UPLC-MS, Editor(s): Wang,X. Serum Pharmacochemistry of Traditional Chinese Medicine, Academic Press. 201–208 (2017).
Lv, H.N. Guo, X.Y. Tu, P.F. Jiang, Y., Comparative analysis of the essential oil composition of Murraya paniculata and M. exotica. Nat Prod Commun. 8(10), 1473–5 (2013).
Arya, N. Kaur, J. Verma, A. Dhanik, J. Vivekanand., Chemical composition of leaf essential oil of wild and domestic genotypes of Murraya paniculata L. J Essen Oil Bear Pl. 20(2), 468–73 (2017).
Silva, F. F. Alves da, et al. Chemical constituents of essential oil from Murraya paniculata leaves and its application to in vitro biological control of the fungus Sclerotinia sclerotiorum. Food Sci. Technol. 39(2), 413–417 (2019.
Sharma, P., Batra, S., Kumar, A. & Sharma, A. In vivo antianxiety and antidepressant activity of Murraya paniculata leaf extracts. J Integr Med. 15(4), 320–325 (2017).
Yang, J.S. Du, M.H. Studies on the constituents of Murraya paniculata (L.) Jack grown in Yunnan. Acta Botanica Sinica. 26, 184–188(1984).
Selestino Neta, M.C. Vittorazzi, C. Guimaraes, A.C. Martins, J.D. Fronza, M. Endringer, D.C., Scherer R. Effects of β-caryophyllene and Murraya paniculata essential oil in the murine hepatoma cells and in the bacteria and fungi 24-h time-kill curve studies. Pharm Biol. 55(1), 190–197(2017).
Gautam, M. K. Gupta,A. Rao, C. V. and Goel, R. K., Antihyperglycemic and antioxidant potential of Murraya paniculata linn. Leaves: a preclinical study. J. Pharm Res. 5(3), 1334–1337(2012).
Zhu, C-h. Lei, Z-l . Luo, Y-p., Studies on antioxidative activities of methanol extract from Murraya paniculata. Food Science and Human Wellness. 4, 108–114(2015)
Shrivastava, V., Chauhan, P. S. & Tomar, R. S. A Biomimetic Approach for Synthesis of Silver Nanoparticles using Murraya paniculata Leaf Extract with Reference to Antimicrobial Activity. J. Pharml Sci & Res. 8(4), 247–250 (2016).
Ibarra, M. Moreno, L. Vera, R. Cogolludo, A. Duarte, J. Tamargo, J. Perez- Vizcaino, F., Effects of the flavonoid quercetin and its methylated metabolite isorhamnetin in isolated arteries from spontaneously hypertensive rats. Planta Med. 69(11), 995–1000 (2003).
Menezes, I. R. et al. Chemical composition and evaluation of acute toxicological, antimicrobial and modulatory resistance of the extract of Murraya paniculata. Pharm Biol. 53(2), 185–191 (2015).
Wang, X. et al. Panitins A-G: Coumarin derivatives from Murraya paniculata from Guangxi Province, China show variable NO inhibitory activity. Phytochemistry 162, 224–231 (2019).
Wu, J., Liu, K. & Shi, X. The anti-inflammatory activity of several flavonoids isolated from Murraya paniculata on murine macrophage cell line and gastric epithelial cell (GES-1). Phar m Biol. 54(5), 868–881 (2016).
Phongpaichit, S. Subhadhirasakul, S. and Wattanapiromsakul, C., Antifungal activities of extracts from Thai medicinal plants against opportunistic fungal pathogens associated with AIDS patients, Blackwell Publishing Ltd • Mycoses. 48, 333–338 (2005).
Faisal, Md. Sarker, Md. M.H. .Rahman,A. Hossain, A. I. Rahman, S. Anwarul, B. ABM, Jahan, R. and Rahmatullah, M., Murraya paniculata (L.) Jack: A Potential Plant for Treatment of Toothache. J. Dent Oral Dis & Ther. 2(3), 1–3(2014).
Saqib, F. Ahmed, M. G. Janbaz, K.H. Dewanjee, S. Jaafar, H.ZE. and Zia-Ul-Haq, M., Validation of ethnopharmacological uses of Murraya paniculata in disorders of diarrhea, asthma and hypertension. BMC Comp. Alte Med. 15, 9319. https://doi.org/10.1186/s12906-015-0837-7. (2015).
Noura, S. Dosoky, P. S. Gautam, T. P. and William, N. S., Composition and Biological Activities of Murraya paniculata (L.) Jack Essential Oil from Nepal. Medicines. 3, 7. 10.3390(2016).
Gautam, M.K. A. Singh, C.V. Rao and R.K. Goel, Toxicological Evaluation of Murraya Paniculata (L.) Leaves Extract on Rodents. American J.Pharm and Toxic. 7 (2) 62–67( 2012).
Zou, J. Yu, X. Qu, S. Li, X. Jin, Y. & Sui, D., Protective effect of total flavonoids extracted from the leaves of Murraya paniculata (L.) Jack on diabetic nephropathy in rats. Food Chem Toxicol. 64, 231–237 (2014).
Zou, J. Sui, D. Fu, W. Li, Y. Ping Yu, Yu, X. Xu, H., Total flavonoids extracted from the leaves of Murraya paniculata (L.) Jack alleviate oxidative stress, inflammation and apoptosis in a rat model of diabetic cardiomyopathy. J. Fun Foods. 76 104319. https://doi.org/10.1016/j.jff.2020.104319 (2021).
Ukpo, G. E., Owolabi, M. A., Imaga, N. O., Oribayo, O. O. & Ejiroghene, A. J. Effect of Carica papaya (Linn) aqueous leaf extract on pharmacokinetic profile of ciprofloxacin in rabbits. Trop J Pharm Res. 16(1), 127–134 (2017).
Sayar, K. Paydar, M. J. and Pingguan-Murphy, B., Pharmacological Properties and Chemical Constituents of Murraya paniculata (L.) Jack. Med. Aroma plants. 3 1–6(2014).
Adamski, Z., Blythe, L. L., Milella, L. & Bufo, S. A. Biological Activities of Alkaloids: From Toxicology to Pharmacology. Toxins (Basel). 12(4), 210 (2020).
Clinton, C. Plant tannins: a novel approach to the treatment of ulcerative colitis. Nat Med J 1, 13 (2009).
Cushnie, Tim & Cushnie, Benjamart & Lamb, Andrew, Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Inter J. Antimicrobe Agents. 44. https://doi.org/10.1016/j.ijantimicag.2014.06.001 (2014).
Udoh, P., Essien, I. & Udoh, F. Effect of Carica papaya (paw paw) seeds extract on the morphology of pituitary-gonadal axis of male Wistar rats. Phytother Res. 19, 1065–1068 (2005).
Sofowora, A., Research on medicinal plants and traditional medicine in Africa. J Altern Complement Med. 2(3), 365–372, http://www.liebertonline. https://doi.org/10.1089/acm.1996.2.365, (1996).
González-Paramás, A. M., Ayuda-Durán, B., Martínez, S., González-Manzano, S. & Santos-Buelga, C. The Mechanisms Behind the Biological Activity of Flavonoids. Curr Med Chem. 26(39), 6976–6990 (2019).
Rosakutty, P. J. & Roslin, A. S. Isolation and characterization of an antimicrobial compound from the traditional medicinal plant Pittosporum tetraspermum Wight & Arn. Int J Med Arom Plants. 2, 141–150 (2012).
Nagmoti, D. M., Khatri, D. K., Juvekar, P. R. & Juvekar, A. R. Antioxidant activity free radical-scavenging potential of Pithecellobium dulce Benth seed extracts. Free Radicals Antioxid. 2(2), 37–43 (2012).
Bele, A. A. & Khale, A. An overview on thin layer chromatography. J Pharmal Sci. 2(2), 256–267 (2011).
Brunetti, C., Ferdinando, M. D., Fini, A., Pollastri, S. & Tattini, M. Flavonoids as antioxidants and developmental regulators: relative significance in plants and humans. Int J Mol Sc. 14(2), 3540–3555 (2013).
Chirinos, R., Rogez, H., Campos, D., Pedreschi, R. & Larondelle, Y. Optimization of extraction conditions of antioxidant phenolic compounds from mashua (Tropaeolum tuberosum Ruiz and Pavon) tubers. Sep Purif Technol. 55, 217–225 (2007).
Sushmitha, H. S., Rajesh, V., Madappa, M. B. & Sathyamurthy, B. A comparative study on characterization of various extracts of a medicinal plants. Eur J Pharm Med Res. 5(6), 401–407 (2019).
Chen, Y. F., Yang, C. H., Chang, M. S., Ciou, Y. P. & Huang, Y. C. Foam properties and detergent abilities of the saponins from Camellia oleifera. Int J Mol Sci. 11(11), 4417–4425 (2010).
Brinda, P., Sasikala, B. & Purushothaman. K. Pharmacognostic studies on Merugan kilzhangu. BMEBR. 3, 84–96 (1981).
Elekofehinti, O. O. Saponins: anti-diabetic principles from medicinal plants - a review. Pathophysiology 22(2), 95–103 (2015).
Villano, D., Fernandez-Pachon, M. S., Moya, M. L., Troncoso, A. M. & Garcıa-Parrilla, M. C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 71, 230 (2007).
Ghasemzadeh, A. Flavonoids and phenolic acids: role and biochemical activity in plants and human. Journal of Med Plants Res. 5(31), 6697–6703 (2011).
Kumar, S. & Pandey, A.K. Chemistry and biological activities of flavonoids: an overview. Hindawi Publishing Corporation The Sci World J. 2013. https://doi.org/10.1155/2013/162750 (2013).
Chahar, M.K. Sharma, N. Dobhal, M.P. & Sharma, Y.C. Flavonoids: a versatile source of anticancer drugs. Pharmacognosy Rev. 5(9), http://www.phcogrev.com/text.asp?2011/5/9/1/79093(2011).
Couraud, S., Dell’Aniello, S., Bouganim, N. & Azoulay, L. Cardiac glycosides and the risk of breast cancer in women with chronic heart failure and supraventricular arrhythmia. Breast Cancer Res Treat. 146(3), 619–626 (2014).
Robbins, C. T., Mole, S., Hagerman, A. E. & Hanley, T. A. Role of Tannins in Defending Plants Against Ruminants: Reduction in Dry Matter Digestion?. Ecology 68(6), 1606–1615 (1987).
Zakia, K. Kong, H.S. Nur Hazerra, B.M.Z. Chua, H.C. & Irshad, U.H.B., Determination of polyphenolic content, HPLC analyses and DNA cleavage activity of Malaysian Averrhoa carambola L. fruit extracts. J King Saud Univ Sci. 27, 331–337 (2015).
Tan. S.S. Papaya (Carica papaya L.) seed oil. In: Ramadan M (ed) Fruit Oils: Chemistry and Functionality. Springer, Cham. (2019).
Vij, T. & Prashar. Y. A review on medicinal properties of Carica papaya Linn. Asian Pacific J Trop Dis. 5(1),1–6 (2015).
Halliwell, B., Aeschbach, R., Loliger, J. & Aruoma. O.I. The characterization of antioxidants. Food Chem Toxicol. 33(7), 601–617 (1995).
Cao, G., Sofic, E. & Prior. R.L. Antioxidant capacity of tea and common vegetables. J Agricul Food Chem. 44(11), 3426–3431 (1996).
Cushnie, T. & Lamb, A. J. Antimicrobial activity of flavonoids. Int J Antimicrobial Agents. 26(5), 343–356 (2005).
Zhang, J. Y. et al. Characterization of seventy polymethoxylated flavonoids (PMFs) in the leaves of Murraya paniculata by on-line high-performance liquid chromatography coupled to photodiode array detection and electrospray tandem mass spectrometry. J Pharm Biomed Anal. 56(5), 950–961 (2011).
Xu, G. H. et al. Minerals, phenolic compounds, and antioxidant capacity of citrus peel extract by hot water. J Food Sci. 73(1), C11–C18 (2008).
Yu, J. et al. Antioxidant activity of citrus limonoids, flavonoids, and coumarins. J Agric Food Chem. 53(6), 2009–2014 (2005).
Río, J. A. D., Gómez, P., Baidez, A.G., Arcas, M.C., Botía, J.M. & Ortuño, A. Changes in the Levels of Polymethoxyflavones and Flavanones as Part of the Defense Mechanism of Citrus sinensis (Cv. Valencia Late) Fruits against Phytophthora citrophthora. J. Agric. Food Chem. 52,1913–1917 (2004).
Río, J. A. D., Arcas, M. C., Benavente-García, O. & Ortuño, A. Citrus Polymethoxylated Flavones Can Confer Resistance against Phytophora citrophthora, Penicillium digitatum, and Geotrichum Species. J. Agric. Food Chem. 46, 4423–4428 (1998).
Piluzza, G. & Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharma Bio. 49(3), 240–247 (2011).
Coté, J., Caillet, S., Doyon, G., Sylvain, J. F. & Lacroix, M. Bioactive compounds in cranberries and their biological properties. Crit. Rev. Food Sci. Nutr. 50, 666–679 (2010).
Benslama, A. & Harrar, A. Free radicals scavenging activity and reducing power of two Algerian Sahara medicinal plants extracts. Inte. J of Herbal Medicine. 4(6), 158–161 (2016).
Benzie, I.F.F.& Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: concepts, procedures, limitations and applications. (ed. by Apak, R., Capanoglu, E. & Shahidi, F. Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications) (John Wiley & Sons Ltd., 2018) First published:15 December 2017. https://doi.org/10.1002/9781119135388.
Rice-Evans, C. A., Miller, N. J. & Paganga, G. Antioxidant properties of phenolic compounds. Trends in Plant Sci. 4, 304–309 (1997).
Benzie, F. F. & Szeto, Y. T. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agri and Food Chem. 47(2), 633–636 (1999).
Nunes, CDR. Barreto, A. M. Menezes de, FP. S, Leandro da, C. L. de Souza, P. M, Pereira de, M. L. Vieira, IJC, Barros de, O. D. Plants as Sources of Anti-Inflammatory Agents. Molecules. 25(16), 3726 (2020).
Oyedapo, O. O., Akinpelu, B. A. & Orefuwa, S. O. Anti-inflammatory effect of Theobroma cacao root extract. J Trop Med Plants 5(2), 161–166 (2004).
Jun, W., Kang, L. & Xinhong, S. The anti-inflammatory activity of several flavonoids isolated from Murraya paniculata on murine macrophage cell line and gastric epithelial cell (GES-1). Pharma Biol. 54(5), 868–881 (2016).
Hendra, R., Ahmad, S., Sukari, A., Shukor, M. Y. & Oskoueian, E. Flavonoid analyses and antimicrobial activity of various parts of Phaleria macrocarpa (Scheff.) Boerl fruit. Int J Mol Sci. 12, 3422–3431 (2011).
Salawu, S. O., Ogundare, A. O., Ola-Salawu, B. B. & Akindahunsi, A. A. Antimicrobial activities of phenolic containing extracts of some tropical vegetables. Afric J Pharm Pharmacol. 5(4), 486–492 (2011).
Adnan, S. N., Ibrahim, N. & Yaacob, W. A. Disruption of methicillin-resistant Staphylococcus aureus protein synthesis by tannins. Germs. 7(4), 186–192 (2017).
Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 30, 3875–3883 (1991).
Zheng, P., Renee, R., Bernard, R. G., Tong-Jun, L. Z. & C., ,. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotech Adv. 37(1), 177–192 (2019).
Tacconelli, E.Magrini, N. Carmeli, Y. Harbarth, S. Kahlmeter,G. Kluytmans, J. Mendelson, M. Pulcini, C. Singh, N. Theuretzbacher, U., Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization. 1–7, (2017).
Lloyd, W. J. et al. Cyclohexane Triones, Novel Membrane –Active Antibacterial Agents. Antimicrobe agents. Chemotherap. 32(6), 814–818 (1988).
Perkin, W.H. & F.R.S, XXIX. On some new bromine derivatives of coumarin. J. Chem. Soc. 23, 368–371 (1870).
Ryu, C.-K. et al. Synthesis and antifungal activity of benzofuran-5-ols. Bioorg. Med. Chem. Lett. 20, 6777–6780 (2010).
Abdel-Wahab, B. F., Abdel-Aziz, H. A. & Ahmed, E. M. Synthesis and antimicrobial evaluation of 1-(benzofuran-2-yl)-4-nitro-3-arylbutan-1-ones and 3-(benzofuran-2-yl)-4, 5-dihydro-5-aryl-1-[4-(aryl)-1, 3-thiazol-2-yl]-1H-pyrazoles. Eur. J. Med. Chem. 44, 2632–2635 (2009).
Manna, K. & Agrawal, Y. K. Design, synthesis, and antitubercular evaluation of novel series of 3-benzofuran-5-aryl-1-pyrazolyl-pyridylmethanone and 3-benzofuran-5-aryl-1-pyrazolylcarbonyl-4-oxo. Eur. J. Med. Chem. 45, 3831–3839 (2010).
Roopashree, T. S., Dang, R., Rani, S. R. H. & Narendra, C. Antibacterial activity of anti-psoriatic herbs: Cassia tora, Momordica charantia and Calendula officinalis. Int J. Applied Res Nat Products. 1(3), 20–28 (2008).
Audu, S. A., Mohammed, I. & Kaita, H. A. Phytochemical screening of the leaves of Lophira lanceolata (Ochanaceae). Life Sci J. 4(4), 75–79 (2007).
Obasi, N. L., Egbuonu, A. C. C., Ukoha, P. O. & Ejikeme, P. M. Comparative phytochemical and antimicrobial screening of some solvent extracts of Samanea saman pods. African J. Pure. Applied chem. 4(9), 206–212 (2010).
Singleton, V. L. & Rossi, J. A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. The American J. Enol. Viticul. 16, 144–158 (1965).
Arvouet-Grand, A., Vennat, B., Pourrat, A. & Legret, P. Standardization d’uneextrait de propolis et identification desprincipaux constituents. J. Pharm. Belgique. 49, 462–468 (1994).
Quettier, D. C. et al. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 72, 35–42 (2000).
Marinova, D., Ribarova, F. & Atanassova, M. Total phenolic and total flavonoids in Bulgarian fruits and vegetables. J. Uni of Chem Tech. Metallurgy. 40(3), 255–260 (2005).
Re, R. et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. J. Free Radi Bio. Med. 26, 1231–1237 (1999).
Ruch, R. J., Cheng, S. J. & Klaunig, J. E. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 10, 1003–1008 (1989).
Benzie, I. F. & Strain, J. J. The ferric reducing ability of plasma (FRAP) as measurement of antioxidant power: The Frap assay. Analytical Biochem. 239, 70–76 (1996).
Oyaizu, M. Studies on products of browning reactions: antioxidant activities of products of browning reaction prepared from glucose amine. Japanese J. Nutri. 44, 307–315 (1986).
Gandhisan, R., Thamaraichelvan, A. & Baburaj, K. Anti-inflammatory action of Lannea coromandelica HRBC membrane stabilization. Fitotherapia. 62, 82–83 (1991).
Chamlagai, D. & Singh, B. Study of in vitro anti-inflammatory activity of ethnomedicinal plants of sikkim Viscum articulatum and Acorus calamus. Asian J. pharm. clinical res. 9, 119–122 (2016).
Threlfall, E. J., Fisher, I. S. T., Ward, L., Tschape, H. & Gerner-Smidt, P. Harmonization of antibiotic susceptibility testing for salmonella: results of a study by 18 national reference laboratories within the european union-funded enter-net group. Microb. drug resist. 5, 195–199 (1999).
Prescott J.F., Baggot J.D., Walker R.D., Antimicrobial susceptibility testing and interpretation of results. In: Antimicrobial Therapy in Veterinary Medicine (ed. Ames, I.A.) 12–26 (Iowa State University Press, 2000).
George W Jr Latimer of referencing in official methods of analysis of AOAC International, 20th edition, (AOAC International, 2016).
Acknowledgements
This research work is funded by the European Union’s Horizon 2020 Innovative Training Network (ITN) under the Marie Skłodowska-Curie Actions [Integrated Training in Dry Eye Disease Drug Development (IT-DED3), Grant Number 765608].
Funding
The funding in the form of departmental grant was provided by Indira Gandhi National Tribal University, Amarkantak, Madhya Pradesh, India. No external funding was received for the research work. The funders had no role in the study design, experimental work or manuscript preparation.
Author information
Authors and Affiliations
Contributions
S.S., S.M., and M.K.D. collected the plant materials, performed the experiments, analyzed the data, and wrote the manuscript. P.K.S.: Designed the study, analyzed the data, and wrote the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Figure S1
: ABTS radical scavenging activity of different concentrations of M. paniculata leaf extracts. Figure S2: H2O2 radical scavenging activity of different concentrations of M. paniculata leaf extracts. Figure S3. Mass spectrum of major components identified through GC-MSMS analysis.Table S1: Phytoconstituents screening of M. paniculata leaf extracts. Table S2: Pearson’s correlation coefficient (r) of dose dependent correlation between concentrations versus various extract with inhibition of hemolysis. Table S3: Gradient solvent system used in the column-chromatography for the isolation of bioactive molecules from Hexane extract of M. paniculata
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sonter, S., Mishra, S., Dwivedi, M.K. et al. Chemical profiling, in vitro antioxidant, membrane stabilizing and antimicrobial properties of wild growing Murraya paniculata from Amarkantak (M.P.). Sci Rep 11, 9691 (2021). https://doi.org/10.1038/s41598-021-87404-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-87404-7
- Springer Nature Limited
This article is cited by
-
Evaluation and efficacy of plant extracts in eradicating medically important mosquitoes: a review
Toxicology and Environmental Health Sciences (2024)
-
First report on the presence of huanglongbing vectors (Diaphorina citri and Trioza erytreae) in Ghana
Scientific Reports (2023)
-
Antioxidant Activity of Pot Marigold (Calendula officinalis L.) in Response to Metal(loid) Induced Oxidative Stress from Fly Ash Amended Soil
Journal of Plant Growth Regulation (2023)
-
Sustainable phyto-fabrication of silver nanoparticles using Gmelina arborea exhibit antimicrobial and biofilm inhibition activity
Scientific Reports (2022)
-
GC–MS Based Metabolomics Strategy for Cost-Effective Valorization of Agricultural Waste: Groundnut Shell Extracts and Their Biological Inhibitory Potential
Waste and Biomass Valorization (2022)