Abstract
The rotational use of insecticides with different modes of action for indoor residual spraying (IRS) is recommended for improving malaria vector control and managing insecticide resistance. Insecticides with new chemistries are urgently needed. Broflanilide is a newly discovered insecticide under consideration. We investigated the efficacy of a wettable powder (WP) formulation of broflanilide (VECTRON T500) for IRS on mud and cement wall substrates in laboratory and experimental hut studies against pyrethroid-resistant malaria vectors in Benin, in comparison with pirimiphos-methyl CS (Actellic 300CS). There was no evidence of cross-resistance to pyrethroids and broflanilide in CDC bottle bioassays. In laboratory cone bioassays, broflanilide WP-treated substrates killed > 80% of susceptible and pyrethroid-resistant An. gambiae sl for 6–14 months. At application rates of 100 mg/m2 and 150 mg/m2, mortality of wild pyrethroid-resistant An. gambiae sl entering experimental huts in Covè, Benin treated with VECTRON T500 was similar to pirimiphos-methyl CS (57–66% vs. 56%, P > 0.05). Throughout the 6-month hut trial, monthly wall cone bioassay mortality on VECTRON T500 treated hut walls remained > 80%. IRS with broflanilide shows potential to significantly improve the control of malaria transmitted by pyrethroid-resistant mosquito vectors and could thus be a crucial addition to the current portfolio of IRS insecticides.
Similar content being viewed by others
Background
Indoor residual spraying (IRS) has historically been shown to be a powerful malaria control intervention1. When applied correctly, IRS can quickly reduce malaria transmission by reducing adult mosquito vector density and longevity. It involves the application of a residual insecticide formulation to potential resting surfaces for malaria vectors such as internal walls, eaves, and ceilings of houses, giving opportunity for vector mosquitoes to contact the insecticide and be killed in the process. IRS contributed substantially to the success of the malaria eradication campaign of the 1950s and 60s which resulted in the elimination of malaria from Europe and several countries in Asia and the Caribbean2. The recent reductions in malaria morbidity and mortality observed in endemic countries in Africa and Asia over the last two decades has also been partly attributed to a significant increase in coverage with IRS3,4,5.
The efficacy of IRS for malaria control is unfortunately threatened by widespread resistance in malaria vectors to the rather limited collection of insecticides approved for public health use6. Pyrethroid resistance is now established across Africa and is increasing substantially in intensity the more they are used, making this previously ideal class of vector control insecticides almost unusable for IRS. Resistance to carbamates and organophosphates, which were for many years the only alternative IRS insecticide classes to pyrethroids5,7 is also increasing rapidly in malaria vector populations in Africa6,8,9,10,11. To mitigate the impact of insecticide resistance on malaria control, vector control programmes are encouraged to implement a rotational application of insecticides for IRS, alternating between insecticides with different modes of action12. The use of rotations for insecticide resistance management relies on the concept that removing selection pressure for a given insecticide by switching between different modes of action, will result in resistance declining over time. An IRS rotation plan which will effectively reduce selection pressure for existing insecticide resistance genes and prevent the development of further resistance will, however, require a more diversified portfolio of IRS insecticides with more novel modes of action than what is currently available13. This is driving the development of a new generation of IRS insecticide formulations containing new chemistries which can provide improved and prolonged control of insecticide-resistant malaria vector populations14,15,16.
Broflanilide is a novel insecticide discovered by Mitsui Chemicals Agro, Inc17 which has been formulated as a wettable powder for IRS. It has a unique chemical structure characterized as a meta-diamide which acts as a non-competitive antagonist (NCA) of the γ-aminobutyric acid (GABA) receptor of chloride channels of the insect inhibitory nervous system18. Broflanilide was classified by the Insecticide Resistance Action Committee (IRAC) as a GABA-gated chloride channel allosteric modulator (IRAC Group 30), causing hyperexcitation and convulsion in insects19. Its mode of action is distinct from that of other NCAs of the GABA-gated chloride channel, such as picrotoxinin, dieldrin, fipronil, lindane and α-endosulfan20. There is no known cross-resistance between broflanilide and current public health insecticides. The active metabolite exhibits high selectivity for the insect RDL GABA receptor compared to the mammalian receptors18 but exhibits no cross-resistance to dieldrin. Broflanilide has demonstrated excellent insecticidal activity against many insect species including Lepidopteran and Coleopteran pests and Thysanopteran pests17 and has also shown low acute toxicity against non-target aquatic organisms21, demonstrating high potential for public health and agricultural use.
In this study, we investigated the potential of VECTRON T500, a wettable powder (WP) formulation of broflanilide (broflanilide WP), for indoor residual spraying against mosquito vectors of malaria. The insecticide was assessed for its efficacy and residual activity on local IRS wall substrates in a series of WHO phase I laboratory bioassays with susceptible Anopheles gambiae s.s. and resistant strains of An. gambiae sl and in WHO phase II experimental hut studies against wild free-flying pyrethroid-resistant An. gambiae sl in southern Benin, West Africa.
Results
WHO phase I laboratory bioassays
Following WHO guidelines22, laboratory bioassays were performed to investigate possible cross-resistance to broflanilide and pyrethroid-resistance mechanisms in CDC bottle bioassays using technical grade insecticide and to identify an effective dose of broflanilide WP (VECTRON T500), for IRS using WHO cone bioassays. Cone bioassays were also performed to investigate the residual efficacy of broflanilide WP on cement and mud block substrates. The bioassays were conducted using laboratory-maintained mosquitoes of the susceptible An. gambiae ss Kisumu strain and the pyrethroid-resistant An. gambiae sl Covè strain. The An. gambiae sl Covè strain is susceptible to carbamates and organophosphates but has shown over 200-fold resistance to pyrethroids mediated by a high frequency of the knockdown resistance L1014F allele (> 90%) and overexpression of the cytochrome P450 CYP6P3, associated with pyrethroid detoxification23.
No evidence of cross-resistant to broflanilide and pyrethroids in CDC bottle bioassays
Mosquito mortality following exposure in CDC bottles coated with alpha-cypermethrin 12.5 µg was 100% with the insecticide susceptible An. gambiae sensu stricto Kisumu strain and 45% with the pyrethroid-resistant An. gambiae sl Covè strain thus confirming the high levels of pyrethroid resistance in the Covè strain23. The mortality results of both strains exposed to broflanilide in CDC bottles treated with a range of doses between 5 µg and 200 µg per bottle are presented in Fig. 1 with more details in supplementary information (Table S1). Using log dosage-probit mortality analysis, the lethal concentration (LC) required to kill 50% (LC50) and 95% (LC95) of exposed mosquitoes were 8.5 µg and 70 µg respectively with the susceptible Kisumu strain and 18.1 µg and 73.6 µg with the pyrethroid-resistant Covè strain (Table 1). A small resistance ratio of 2.1 (95% limits: 1.7–2.7) for the Covè strain to the Kisumu strain at the LC50 was thus detected suggesting the absence of cross-resistance to broflanilide and pyrethroids.
Mortality of susceptible An. gambiae Kisumu (red) and pyrethroid-resistant An. gambiae sl Covè (blue) mosquitoes in CDC bottle bioassays treated with a technical grade of broflanilide insecticide. Mosquitoes (150/dose) were exposed for 1-h in cohorts of 25 per bottle. Based on preliminary findings of delayed mortality effect with broflanilide, mortality in bottle bioassays was recorded after 72 h. The red line represents the response of the susceptible Kisumu strain while the blue line represents the response of the pyrethroid-resistant Covè strain. PoloPlus 1.0, LeOra Software.
Dose–response cone bioassay studies
According to WHO guidelines22, the target dose of a new insecticide for IRS should be investigated from doses 2–4 times the minimum dose that will cause 100% mortality in a fully susceptible mosquito vector population. To identify a suitable target dose of broflanilide WP for IRS, WHO cone bioassays were conducted on cement and mud block substrates treated with a range of concentrations of broflanilide WP between 5 and 100 mg/m2, to detect the minimum dose that will cause 100% mortality. The cone bioassays were performed 1-week post block treatment using the insecticide-susceptible An. gambiae ss Kisumu strain. The mortality rates observed are presented in Fig. 2. No knockdown was recorded with broflanilide WP at any of the doses and with any of the substrates tested. Mortality reached 100% within 24 h at a dose of 100 mg/m2 on cement block substrates (Fig. 2a) and a dose of 12.5 mg/m2 on mud block substrates (Fig. 2b). Broflanilide WP performed better on mud than on cement. Data with cement blocks showed a delayed mortality effect with broflanilide WP doses below 100 mg/m2 with mortality increasing gradually from 24 h and reaching a peak at 72 h. At the doses tested, there was no measurable increase in mortality when holding time was extended beyond 72 h. This demonstrated a delayed mortality effect with broflanilide WP, as a result, for subsequent studies with the insecticide, mosquito mortality was recorded only up to 72 h post-exposure.
A dose of 200 mg/m2, which was two times the dose that induced 100% mosquito mortality within 24 h on both substrates, was identified as a suitable dose for further laboratory studies on the residual efficacy of broflanilide WP. Since the insecticide performed better on mud block substrates inducing optimal mortality at even much lower doses, residual efficacy on mud block substrates was also assessed at 100 mg/m2.
Broflanilide WP shows prolonged residual efficacy on block substrates in laboratory cone bioassays
The residual efficacy of broflanilide WP was investigated at application rates of 200 mg/m2 on cement block substrates and 100 mg/m2 and 200 mg/m2 on mud block substrates. Blocks of each substrate-type were treated at each selected dose and tested in WHO cone bioassays at 1-week post-treatment and monthly intervals subsequently using the insecticide-susceptible An. gambiae ss Kisumu and the pyrethroid-resistant An. gambiae sl from Covè.
Monthly cone bioassay mortality (72 h) on cement blocks treated at 200 mg/m2 was > 80% with the susceptible An. gambiae ss Kisumu strain for 6 months after which it ranged between 57 and 100% up to month 18 post-treatment (Fig. 3). With the pyrethroid-resistant An. gambiae sl Covè strain, mortality on cement blocks (200 mg/m2) remained > 80% for up to 14 months post-treatment (Fig. 3). Monthly cone bioassay mortality of both strains at both doses tested on mud blocks (100 mg/m2 and 200 mg/m2) remained > 80% for 16 months (Fig. 4).
Monthly cone bioassays mortality of insecticide-susceptible An. gambiae ss Kisumu and pyrethroid-resistant An. gambiae sl Covè strain mosquitoes on broflanilide WP-treated cement block substrates in the laboratory. At each time point, forty 2–5 days old female mosquitoes were exposed for 30-min in WHO cone bioassays and mortality recorded after 72 h.
Monthly cone bioassays mortality of insecticide-susceptible An. gambiae ss Kisumu and pyrethroid-resistant An. gambiae sl Covè strain mosquitoes on broflanilide WP-treated mud block substrates. At each monthly time point, forty 2–5 days old female mosquitoes were exposed for 30-min in WHO cone bioassays and mortality recorded after 72 h.
WHO phase II experimental hut evaluation of broflanilide WP in Covè, Benin
To investigate the efficacy of the broflanilide WP formulation for IRS against wild free-flying pyrethroid-resistant malaria vectors, we performed an experimental hut trial at the CREC/LSHTM experimental hut station in Covè, southern Benin (7°14′N 2°18′E). The local vector population in Covè is resistant to pyrethroids and DDT. Molecular analysis has revealed a kdr (L1014F) allele frequency of 89% and microarray studies have also found overexpression of CYP6P3, a P450 that is as an efficient metabolizer of pyrethroids23.
Experimental huts simulate household conditions and are thus used to assess the capacity of indoor vector control interventions to prevent mosquito entry, induce early exiting of vector mosquitoes, prevent mosquito feeding and induce mosquito mortality under carefully controlled household conditions22,24. Broflanilide WP was evaluated for IRS against wild pyrethroid-resistant An. gambiae sl in Covè in experimental huts treated at application rates of 100 mg/m2 and 150 mg/m2. To investigate efficacy on commonly used wall substrates in Benin, for each application rate, the inner walls and ceiling of the experimental huts were plastered with either cement or mud. Broflanilide WP was compared to the main IRS insecticide used in Benin at the time of the trial, pirimiphos-methyl CS (Actellic 300CS) applied at 1000 mg/m2 on cement walls, as a positive control. At the time of this study, the toxicity and potential risk of broflanilide WP for IRS was yet to be fully assessed, so it was not acceptable to use human volunteer sleepers as hosts to attract wild vector mosquitoes into the experimental huts until a human risk-assessment was performed and the product was found safe for IRS at the potential application rates. Preliminary studies revealed that the local wild An. gambiae sl at the Covè experimental hut station were also attracted to and blood-fed on cows, although less than to humans. Hence, cows were used as replacement hosts to attract mosquitoes into the experimental huts during the trial.
Mosquito exiting rates in experimental huts
A total of 745 female An. gambiae sl and 771 female An. ziemanni were collected in the experimental huts over the 6-month trial (Tables 2 and 3). Molecular species analysis (SINE PCR) performed using the protocol proposed by Santolamazza et al.25 on DNA extracted from a random selection of 100 live and dead An. gambiae sl collected in the experimental huts during the 6-month trial, revealed that the vector was composed of 81% An. coluzzii and 19% An. gambiae ss.
Exiting rates of An. gambiae sl were generally higher in broflanilide WP-treated huts (88–92%) compared to the control hut (69%; P < 0.05), but not significantly lower than in pirimiphos-methyl CS-treated huts (95%). Exiting rates with broflanilide WP did not also differ substantially between the two application rates tested and between the two substrates assessed (P > 0.05; Table 2).
A similar trend in mosquito exiting was observed with the An. ziemanni collected in the experimental huts except that exiting rates with broflanilide WP-treated huts did not differ significantly from that in the negative control hut (P < 0.05) (Table 2).
Broflanilide WP induces similar overall mortality of wild pyrethroid-resistant An. gambiae s.l. compared to pirimiphos-methyl CS in experimental huts
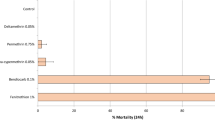
Mortality (at 72 h) of free-flying wild, pyrethroid-resistant An. gambiae sl entering the control untreated hut was 1% while mortality with broflanilide WP treated huts ranged between 57 and 66% (Table 2). The mortality rates observed with broflanilide WP were similar to mortality observed with pirimiphos-methyl CS (57–66% with broflanilide WP vs. 56% with pirimiphos-methyl CS; P > 0.05). For each substrate type, mortality with broflanilide WP did not differ significantly between the doses tested; cement: 57% with 100 mg/m2 vs 66% with 150 mg/m2 (P = 0.439) and mud: 63% with both application rates (P = 0.922). Broflanilide WP generally performed the same on mud and cement substrates at both application rates; at 100 mg/m2: 57% on cement vs. 63% on mud (P = 0.938) and at 150 mg/m2: 66% with cement vs. 63% with mud (P = 0.664). In addition, for each application rate, the difference in mortality between substrates was not significant (P > 0.05). As in the phase 1 cone bioassays, the free-flying mosquito experimental hut data also showed a delayed mortality effect with broflanilide WP on An. gambiae sl (Fig. 5). Mortality increased steadily from 35–41% at 24 h to 57–63% at 72 h with the 100 mg/m2 application rate and from 48–52% at 24 h to 63–66% at 72 h with the 150 mg/m2 application rate (P < 0.05).
Overall mortality of wild free-flying pyrethroid-resistant An. gambiae sl at 24, 48 and 72 h after collection from experimental huts in Covè, Benin. Bars bearing the same letter label are not significantly different at the 5% level (logistic regression). Error bars represent 95% confidence intervals. Broflanilide WP induced a delayed mortality effect on wild vector mosquitoes in experimental huts.
Mortality rates achieved with broflanilide WP against An. ziemanni (Table 3) were generally higher than what was observed with An. gambiae sl (74–88% vs. 50–61%). Mortality in cement huts did not differ between the two application rates tested (74% with 100 mg/m2 vs 77% with 150 mg/m2 (P > 0.05) but for mud-walled huts, mortality was significantly higher with the higher application rate of broflanilide WP (P < 0.05). Mortality rates achieved with broflanilide WP on cement walls were also similar to what was observed with pirimiphos-methyl CS (P > 0.05). Broflanilide WP also induced delayed mortality with An. ziemanni entering huts during the trial.
Blood-feeding rates of wild pyrethroid-resistant An. gambiae sl in experimental huts
As expected of IRS treatments, blood-feeding rates of both mosquito species were generally very high across all huts (> 90%). For pyrethroid-resistant An. gambiae sl, there was no significant difference in blood-feeding rates between the two application rates (100 mg/m2 and 150 mg/m2) of broflanilide WP for either substrate type (P > 0.05, Table 2). With An. ziemanni, while a significantly lower blood-feeding rate was observed with the higher dose of broflanilide WP in mud-walled huts, all other treatments tested gave similar blood-feeding rates irrespective of substrate type (Table 3).
Mosquito mortality in cone bioassays on experimental hut walls treated with broflanilide WP is high and prolonged, lasting over 6 months
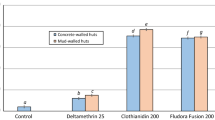
To assess the residual efficacy on the different hut wall substrates (mud and cement), monthly 30-min cone bioassays were performed using unfed pyrethroid-resistant An. gambiae sl Covè and insecticide-susceptible An. gambiae ss Kisumu strains. The results from the wall cone bioassays are presented in Figs. 6 and 7. Broflanilide WP performed better on cement and mud wall substrates compared to pirimiphos-methyl CS. For both strains, cone bioassay mortality with broflanilide WP remained over 80% with both doses and substrates throughout the 6-month trial while a drop below 80% was observed with pirimiphos-methyl CS within 2–4 months.
Cone bioassays mortality (72 h) with pyrethroid-resistant An. gambiae sl Covè on Broflanilide WP-treated experimental hut walls. At each monthly interval, ~ 50 2–5 days old female mosquitoes were exposed on treated walls of each hut in cohorts of 10 per cone. Error bars represent 95% confidence intervals.
Assessment of IRS application quality
To assess the quality of the IRS applications in the experimental huts, prior to spraying, filter papers measuring 5 cm × 5 cm were fixed on the hut walls to be sprayed as described in the WHO guidelines22. After spraying, they were left to dry for 1 h and then wrapped in aluminium foil and stored at 4° C (± 2 °C) after which they were shipped within 2 weeks after IRS application to the Liverpool School of Tropical Medicine for chemical analysis by HPLC. The summary results showed that the IRS treatment applications rates were generally within an acceptable deviation of < 50% from the target dose as recommended by WHO26 (Table 4).
Discussion
There is a critical need for new insecticides with novel modes of action for indoor residual spraying against malaria vectors. We investigated the bioefficacy of a newly discovered insecticide, broflanilide17, as an indoor residual treatment in WHO phase I laboratory bioassays and phase II experimental hut studies22. Broflanilide binds to the γ-aminobutyric acid (GABA) receptor of the chloride channel at a different site to the cyclodiene insecticide dieldrin and the phenyl pyrazole fipronil and thus presents a new mode of action for malaria vector control.
VECTRON T500, a wettable powder formulation of broflanilide was assessed for IRS against pyrethroid-susceptible and pyrethroid-resistant strains of An. gambiae sl on the principal wall substrates used in village-type housing in Benin. The results clearly demonstrate the potency of the insecticide, inducing high vector mosquito mortality at doses as low as 5 mg/m2 in laboratory cone bioassays. In experimental hut studies, VECTRON T500, when used for IRS in housing in southern Benin at application rates of 100 mg/m2 and 150 mg/m2, showed similar performance to Actellic 300CS, a WHO approved IRS insecticide, killing substantial proportions of wild free-flying pyrethroid-resistant malaria vector mosquitoes which entered the hut (57–66%), for 6-months. At both levels of evaluation, mosquito mortality with VECTRON T500 was high but slow acting compared to what is achievable with most neurotoxic public health insecticides, lasting up to 72 h post-exposure. This delayed activity could be attributed to its mechanism of action; broflanilide has to be metabolized to its active form, desmethyl-broflanilide, before binding to its site of action18. Delayed mosquito mortality effects has also been observed with clothianidin14,27 and chlorfenapyr15,28 which are the main active ingredients in newly approved IRS insecticide formulations and insecticide treated nets for malaria vector control13. While the relative impact of such slow acting insecticides is yet to be fully assessed, modelling studies have suggested that development of resistance to these insecticides may be slower compared to fast-acting neurotoxic insecticides29.
One of the main objectives of this study was to identify a suitable IRS application rate of VECTRON T500 for field use. The hut trial results showed no difference in wild vector mosquito mortality between the two application rates tested, hence the lower dose of 100 mg/m2 was chosen for operational use of the insecticide in communities. Recent human risk assessment studies have also shown acceptable tolerance of broflanilide for IRS at this application rate. Further studies to assess the efficacy of VECTRON T500 applied at 100 mg/m2 in human occupied experimental housing and in village communities are underway in Benin and Tanzania.
Longer lasting IRS insecticide formulations are ideal for most endemic areas in Africa characterised by stable and extended malaria transmission as they offer continuous protection without the need for multiple resource-demanding and labour-intensive IRS campaigns. Earlier experimental hut studies in Benin which demonstrated for the first time the potential of the micro-encapsulated formulation of pirimiphos-methyl (Actellic 300CS) to provide improved and prolonged control of pyrethroid-resistant vector populations lasting 6–9 months when applied on cement walls30, were followed by several reports of significantly improved malaria control with one annual IRS campaign with this insecticide in many epidemiological settings across Africa31,32,33. In laboratory cone bioassays, mortality of susceptible and pyrethroid-resistant vector mosquito strains with VECTRON T500 remained > 80% for 6–14 months on mud and cement block substrates. Likewise, throughout the 6-month experimental hut trial, vector mosquito mortality in in situ wall cone bioassays in huts treated with VECTRON T500, remained > 80% demonstrating the potential of the insecticide to provide prolonged vector control and significantly improved malaria control in many endemic areas in Africa. While the residual efficacy of the insecticide was assessed in the hut trial for only 6 months, the laboratory cone bioassay results indicate the potential for VECTRON T500 to last well beyond 6 months in village-type housing. Ongoing studies in Benin will assess the residual efficacy of VECTRON T500 for IRS in human occupied experimental huts for 12 months.
Compared to cement plastered walls, mud walls are often considered a more challenging substrate for IRS usually resulting in shorter residual vector mosquito control30,34. By contrast, our study demonstrated longer residual efficacy of VECTRON T500 in laboratory cone bioassays with the susceptible Kisumu strain on mud block substrates (> 80% mortality for 18 months) compared to cement block substrates (> 80% mortality for 6 months). This corroborates our previous findings in similar studies with another IRS insecticide27. The improved residual efficacy with the mud block substrates could be attributed to the addition of a small amount of cement to the mud paste during the preparation of the block substrates, in line with local practices in some areas in Benin, making the substrates more stable and less porous. Nevertheless, it is worth noting that contrary to the laboratory study, mortality of wild free flying vector mosquitoes and residual efficacy in wall cone bioassays in the VECTRON T500-treated experimental huts, did not differ between both substrate-types.
As new modes of action are introduced for vector control, it is essential to investigate the potential for cross-resistance to these insecticides and existing insecticide resistance mechanisms in vector populations as this could severely limit the usefulness of the new chemistry. The small resistance ratio observed in CDC bottle bioassays (2.1) and high cone bioassay mortality rates (> 80% for 6–14 months) achieved with the pyrethroid-resistant An gambiae sl strain from Covè—a strain which has shown > 200 fold resistance to pyrethroids mediated by high kdr frequencies and overexpressed detoxifying P450 enzymes23—would indicate the absence of cross-resistance to broflanilide and pyrethroids. This demonstrates the potential of broflanilide WP to effectively control malaria vector populations that have developed intense resistance to pyrethroids. Although mutations in the GABA receptor conferring resistance to some non-competitive antagonist agrochemicals such as cyclodienes and fipronil have been reported in malaria vectors across Africa35,36, the site of action of broflanilide within the GABA receptor has been demonstrated to be distinct from that of non-competitive antagonist20. This suggests a low probability of cross-resistance to broflanilide in malaria vectors. Indeed, recent studies in which broflanilide was tested against a strain of An. gambiae Kisumu containing mutations in the GABA receptor conferring resistance to dieldrin (RDL), found a very low resistance ratio (1.73) compared to the susceptible Kisumu strain which would indicate the absence of cross-resistance to broflanilide and dieldrin37. Mutations in the GABA receptor which confer resistance to meta-diamides have however been detected in Drosophila20. Studies to investigate the possible presence of this mutation and other mechanisms that could confer resistance to broflanilide in wild populations of malaria vectors will be advisable.
Despite its strong anthropophilic behaviour, a considerable number of An gambiae (81% An. coluzzii and 19% An. gambiae ss), were attracted to the cow hosts during the experimental hut trial, although the numbers were substantially lower than what would be expected in hut trials with human volunteer sleepers at the Covè experimental hut station14,15. The animal bait also attracted a more zoophilic Anopheline species into the experimental huts—An. ziemanni. Although this species has been much less studied compared to the An. gambiae complex, An. ziemanni has been previously implicated in malaria transmission in the North West region of Cameroon38 and suspected to transmit malaria in Western Kenya39. Such secondary vectors have been recognized for their importance in malaria transmission, as they may help to augment or extend the malaria transmission period. In our study, broflanilide killed 74–88% of wild An. ziemanni entering the experimental huts over the 6-month trial thus demonstrating the potential of the insecticide to control this Anopheline species which could be sustaining malaria transmission as a secondary vector in some parts of Africa.
Among the strategies proposed by the GPIRM for mitigating the impact of insecticide resistance in malaria vectors, the rotation of IRS formulations containing insecticides with different modes of action is currently considered the most promising tactic for insecticide resistance management12. The uptake of this strategy has been seriously limited by the very restricted number of safe and long-lasting IRS insecticides available to malaria control programmes40. Two new IRS formulations containing clothianidin (a neonicotinoid) have recently been added to the WHO’s list of pre-qualified vector control products, and are already being deployed for IRS in many endemic countries13. It is, however, crucial that vector control programmes do not become overly dependent on any one mode of action for IRS as this may lead to resistance evolving more rapidly. For the rotational strategy to work optimally, several modes of action need to be available at the same time to allow sub-national rotations and restrict selection of resistance to any single class of insecticide. Based on its novel mode of action and efficacy for IRS against pyrethroid-resistant malaria vectors as demonstrated in the present study, VECTRON T500 shows potential to effectively complement other IRS insecticide formulations in an IRS rotation plan that can manage insecticide resistance and extend the effective lives of these promising new insecticides.
Conclusion
In this study, we demonstrate the efficacy of a wettable powder formulation of broflanilide (VECTRON T500), a newly discovered insecticide with a novel mode of action, for IRS against wild malaria vectors. VECTRON T500 showed high activity against both pyrethroid-susceptible and resistant strains of An. gambiae sl which lasted 6 months or more on local cement and mud substrates in both laboratory bioassays and experimental hut studies. Indoor residual spraying with broflanilide WP shows potential to provide improved and prolonged control of pyrethroid-resistant malaria vector populations.
Materials and methods
WHO phase I laboratory bioassays
CDC bottle bioassays to investigate resistance to broflanilide
The CDC bottle bioassays were performed with unfed 2–5 days old insecticide-susceptible An. gambiae ss Kisumu and pyrethroid-resistant An. gambiae sl adult female mosquitoes from Covè, Benin. Eight doses were selected between 5 µg/bottle and 200 µg/bottle as follows: 5 µg, 10 µg, 16.5 µg, 27.1 µg, 44.7 µg, 73.7 µg, 121.4 µg and 200 µg based on results from preliminary studies. Stock solutions were prepared by serial dilutions of the technical grade insecticide in acetone. 1 ml of each stock solution was used to coat each 250 ml Wheaton bottle and 6 bottles were prepared per dose as described in the CDC bottle bioassay guideline41. The CDC bottle bioassay protocol was modified; approximately 100–150 female mosquitoes per insecticide dose were exposed for 1 h in cohorts of 25 mosquitoes per bottle. Mosquitoes were held at 27 °C ± 2 °C and 80 ± 10% RH and mortality recorded after 72 h based on preliminary evidence of a slower mode of action of broflanilide. Mosquitoes were also exposed to untreated control and alpha-cypermethrin 12.5 µg treated bottles for comparison. Estimates of the dose required to kill 50% (LD50) and 95% (LD95) of each strain and the resistance ratio of the wild Covè strain relative to the susceptible Kisumu strain were generated by log dosage-probit analysis (PoloPlus version 1.0).
Preparation and treatment of block substrates
Cement and mud block substrates used in cone bioassays were prepared and treated using similar methods described in previous studies by our group27. Blocks were moulded in Petri dishes (9 cm diameter and 1 cm thick) and dried at 30 °C ± 2 °C and 80 ± 10% RH for 30 days before insecticide application. Cement blocks were made by mixing cement with sand at a 1:1 ratio while mud blocks were made from local mud paste to which 10% cement was added to improve its durability and reduce cracking, in line with local practices. These substrates were treated using a Potter tower sprayer (Burkard Manufacturing Co Ltd) to achieve a homogeneous and accurate deposit of the target concentration of active ingredient (a.i) per unit area as described in WHO testing guidelines22. Blocks were weighed before and after treatments to ensure the target amount of insecticide was delivered. All treated blocks were stored, unsealed at 30 °C ± 2 °C and 80% ± 10% RH in between bioassays. Four replicate blocks of each substrate-type were prepared for each dose of insecticide tested.
Dose–response cone bioassays
The dose–response cone bioassays were performed on mud and cement blocks treated with broflanilide WP at application rates of 5 mg/m2, 12.5 mg/m2, 25 mg/m2, 50 mg/m2 and 100 mg/m2, 1-week post block treatment using the insecticide-susceptible An. gambiae ss Kisumu strain. For each dose and substrate-type, forty (40) unfed 2–5 days old mosquitoes were exposed for 30 min in cohorts of 10 mosquitoes per cone and per block. Mosquitoes were held at 27 °C ± 2 °C and 80% ± 10% RH post-exposure and knockdown was recorded after 1 h. To investigate delayed mosquito mortality with broflanilide WP, mortality was recorded every 24 h for up to 120 h.
Residual efficacy of broflanilide WP (VECTRON T500) in laboratory cone bioassays
For each insecticide dose and substrate-type used for assessment of residual efficacy, forty (40) unfed 2–5 days old female mosquitoes were exposed in cone bioassays for 30 min in cohorts of 10 mosquitoes per block as described in WHO guidelines22. Mosquitoes were held under the same conditions as described earlier and mortality recorded every 24 h for up to 72 h. Bioassays were performed monthly for up to 6 months post-treatment and subsequently every two months for up to 18 months post-treatment.
WHO phase II experimental hut trial
Experimental hut site
The experimental hut site is located in an irrigated valley producing rice almost year-round and providing suitable breeding habitats for mosquitoes. The rainy season extends from March to October and the dry season from November to February. The vector population is susceptible to carbamates and organophosphates but over 200-fold resistant to pyrethroids23,42. It consists of both An. coluzzii and An. gambiae sensu stricto (s.s.) with the latter occurring at lower frequencies (~ 23%) and mostly in the dry season23. The experimental huts used were of the West African design and are made from cement bricks with a corrugated iron roof. Each hut was built on a cement plinth surrounded by a water-filled moat to prevent the entry of scavenging ants and had a wooden framed veranda trap to capture exiting mosquitoes22. Mosquito entry occurred via four 1-cm window slits situated on three sides of the hut.
Application of IRS treatments
Six experimental huts were used for the study. Preliminary mosquito collections at the hut site revealed that the chosen huts were equally attractive to mosquitoes. The following 6 treatments were tested in the huts:
-
1.
Untreated hut (negative control)—cement-walled hut.
-
2.
Broflanilide WP (VECTRON T500) applied at 100 mg/m2—cement-walled hut.
-
3.
Broflanilide WP (VECTRON T500) applied at 100 mg/m2—mud-walled hut.
-
4.
Broflanilide WP (VECTRON T500) applied at 150 mg/m2—cement-walled hut.
-
5.
Broflanilide WP (VECTRON T500) applied at 150 mg/m2—mud-walled hut.
-
6.
Pirimiphos-methyl CS (Actellic 300CS) applied at 1000 mg/m2—cement-walled hut.
The IRS treatments were randomly allocated to experimental huts and were applied using a Hudson Xpert compression sprayer equipped with a 8002 flat-fan nozzle and calibrated. To improve spray accuracy, spray swaths were marked before spraying and sprayed from the top to the bottom using a predetermined lance speed. After spraying each hut, the volume remaining in the spray tank was measured to assess the overall volume sprayed. All spray volumes were within 30% of the target.
Hut trial procedure
Six (6) cows were brought to sleep in the huts from 21:00 to 06:00 each trial night and were rotated between huts on successive nights to adjust for variation in individual attractiveness to mosquitoes. The cows were maintained according to institutional and national guidelines for the protection of experimental animals. The trial ran for 6 months from September 2018 to March 2019 and followed WHO guidelines22. Data collection was performed for 6 nights each week. On the 7th day of each week, the huts were cleaned and aired in preparation for the next cycle. Each morning, mosquitoes were collected from the room and veranda and brought to the laboratory where they were identified using standard identification keys and scored as fed or unfed and dead or alive. Live mosquitoes were provided with 10% glucose solution and mortality scored every 24 h for up to 72 h.
Outcome measures in experimental huts
The efficacy of each experimental hut treatment was expressed in terms of the following outcome measures:
-
Exiting rates—the proportion of mosquitoes collected in the veranda.
-
Blood-feeding rates—the proportion of blood-fed mosquitoes.
-
Mortality—the proportion of mosquitoes found dead after a 72-h holding time.
Monthly residual cone bioassays on treated experimental hut walls
Five cones were fixed to each treated surface of each hut (1 per wall + 1 on the ceiling). Approximately fifty (50) unfed 2–5 days old mosquitoes of each strain (insecticide-susceptible An. gambiae ss Kisumu and pyrethroid-resistant An. gambiae sl Covè) were exposed for 30 min to each hut treatment in batches of 10 mosquitoes per cone. After exposure, mosquitoes were transferred into netted plastic cups and supplied with 10% sugar solution. Mortality was recorded every 24 h up to 72 h.
Data management and statistical analysis
Cone bioassay data were pooled for each substrate and dose and mean mortalities obtained using Microsoft Excel. CDC bottle bioassay data were analysed using log dosage-probit mortality analysis (Poloplus version 1.0) to determine the lethal concentration (LC) required to kill 50% (LC50) and 95% (LC95) of exposed mosquitoes. The resistance ratio of the pyrethroid-resistant An gambiae s.l. Covè strain was obtained by dividing its LC50 by that of the susceptible Kisumu strain. Proportional outcomes (blood-feeding, exophily and mortality) for each experimental hut treatment were analysed using blocked logistic regression in Stata version 15.1 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC) with adjustments for the attractiveness of the individual cow hosts.
Ethical considerations
Institutional ethical approval for the study was obtained from the Ethics Review Committee of the Ministry of Health Benin (Ethical decision n°39). The cows used in experimental huts to attract mosquitoes were maintained following institutional standard operating procedures (SOPs) designed to improve care and protect animals used for experimentation. During the day, the cows were allowed to graze freely in an open field not too far from the experimental hut site. A veterinarian was available throughout the study to examine them daily and any cows found unwell were replaced and treated appropriately. All studies were performed according to relevant national and international guidelines.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- IRS:
-
Indoor residual spraying
- WHO:
-
World Health Organization
- PQ:
-
Prequalification team
- GPIRM:
-
Global Plan for Insecticide Resistance Management
- GABA:
-
γ-Aminobutyric acid
- WHOPES:
-
WHO Pesticide Evaluation Scheme
- WP:
-
Wettable powder
- CDC:
-
Centres for Disease Control and Prevention, USA
- CREC:
-
Centre de Recherche Entomologique de Cotonou
- LSHTM:
-
London School of Hygiene & Tropical Medicine
- Kdr:
-
Knockdown resistance
- RDL:
-
Resistant to dieldrin
- LSTM:
-
Liverpool School of Tropical Medicine
References
WHO. Guidelines for Malaria Vector Control (World Health Organization, 2019).
WHO. Indoor residual spraying: use of indoor residual spraying for scaling up global malaria control and elimination. WHO position statement. https://apps.who.int/iris/handle/10665/69386 (World Health Organisation, 2006).
Pluess, B., Tanser, F. C., Lengeler, C. & Sharp, B. L. Indoor residual spraying for preventing malaria. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD006657.pub2 (2010).
Cook, J. et al. Trends in parasite prevalence following 13 years of malaria interventions on Bioko island, Equatorial Guinea: 2004–2016. Malar. J. 17, 62. https://doi.org/10.1186/s12936-018-2213-9 (2018).
Tangena, J.-A.A. et al. Indoor residual spraying for malaria control in sub-Saharan Africa 1997 to 2017: An adjusted retrospective analysis. Malar. J. 19, 150. https://doi.org/10.1186/s12936-020-03216-6 (2020).
WHO. Global Report on Insecticide Resistance in Malaria Vectors: 2010–2016 (World Health Organization, 2018).
Oxborough, R. M. Trends in US President’s Malaria Initiative-funded indoor residual spray coverage and insecticide choice in sub-Saharan Africa (2008–2015): Urgent need for affordable, long-lasting insecticides. Malar J 15, 146. https://doi.org/10.1186/s12936-016-1201-1 (2016).
Edi, C. V., Koudou, B. G., Jones, C. M., Weetman, D. & Ranson, H. Multiple-insecticide resistance in Anopheles gambiae mosquitoes, Southern Cote d’Ivoire. Emerg. Infect. Dis. 18, 1508–1511. https://doi.org/10.3201/eid1809.120262 (2012).
Ahoua Alou, L. P. et al. Distribution of ace-1Rand resistance to carbamates and organophosphates in Anopheles gambiae s.s. populations from Côte d’Ivoire. Malaria J. 9, 167. https://doi.org/10.1186/1475-2875-9-167 (2010).
Antonio-Nkondjio, C. et al. Review of the evolution of insecticide resistance in main malaria vectors in Cameroon from 1990 to 2017. Parasit. Vectors 10, 472. https://doi.org/10.1186/s13071-017-2417-9 (2017).
Elanga-Ndille, E. et al. The G119S acetylcholinesterase (Ace-1) target site mutation confers carbamate resistance in the major malaria vector Anopheles gambiae from cameroon: A challenge for the coming IRS implementation. Genes https://doi.org/10.3390/genes10100790 (2019).
WHO. Global Plan for Insecticide Resistance Management in Malaria Vectors (World Health Organization, 2012).
WHO. List of WHO prequalified vector control products. https://www.who.int/pq-vector-control/prequalified-lists/PrequalifiedProducts27January2020.pdf?ua=1 (World Health Organization, 2020).
Ngufor, C., Fongnikin, A., Rowland, M. & N’Guessan, R. Indoor residual spraying with a mixture of clothianidin (a neonicotinoid insecticide) and deltamethrin provides improved control and long residual activity against pyrethroid resistant Anopheles gambiae sl in Southern Benin. PLoS ONE 12, e0189575. https://doi.org/10.1371/journal.pone.0189575 (2017).
Ngufor, C. et al. Indoor spraying with chlorfenapyr (a pyrrole insecticide) provides residual control of pyrethroid-resistant malaria vectors in southern Benin. Malar. J. 19, 249. https://doi.org/10.1186/s12936-020-03325-2 (2020).
Agossa, F. R. et al. Efficacy of a novel mode of action of an indoor residual spraying product, SumiShield(R) 50WG against susceptible and resistant populations of Anopheles gambiae (s.l.) in Benin, West Africa. Parasit Vectors 11, 293. https://doi.org/10.1186/s13071-018-2869-6 (2018).
Katsuta, H. et al. Discovery of broflanilide, a novel insecticide. J. Pestic. Sci. 44, 120–128. https://doi.org/10.1584/jpestics.D18-088 (2019).
Nakao, T. & Banba, S. Broflanilide: A meta-diamide insecticide with a novel mode of action. Bioorg. Med. Chem. 24, 372–377. https://doi.org/10.1016/j.bmc.2015.08.008 (2016).
IRAC. The IRAC Mode of Action Classification Online. https://irac-online.org/modes-of-action/ Accessed 24 July 2020 (2020).
Nakao, T., Banba, S., Nomura, M. & Hirase, K. Meta-diamide insecticides acting on distinct sites of RDL GABA receptor from those for conventional noncompetitive antagonists. Insect Biochem. Mol. Biol. 43, 366–375. https://doi.org/10.1016/j.ibmb.2013.02.002 (2013).
Jia, Z. Q. et al. Acute toxicity, bioconcentration, elimination, action mode and detoxification metabolism of broflanilide in zebrafish, Danio rerio. J. Hazard Mater 394, 122521. https://doi.org/10.1016/j.jhazmat.2020.122521 (2020).
WHO. Guidelines for Testing Mosquito Adulticides for Indoor Residual Spraying and Treatment of Mosquito Nets (World Health Organisation, 2006).
Ngufor, C. et al. Insecticide resistance profile of Anopheles gambiae from a phase II field station in Cove, southern Benin: Implications for the evaluation of novel vector control products. Malar J. 14, 464. https://doi.org/10.1186/s12936-015-0981-z (2015).
WHO. Guidelines for Laboratory and Field Testing of Long-Lasting Insecticidal Nets (World Health Organization, 2013).
Santolamazza, F. et al. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar J. 7, 1–10 (2008).
WHO. Data requirements and protocol for determining non-inferiority of insecticide-treated net and indoor residual spraying products within an established WHO intervention class. https://apps.who.int/iris/bitstream/handle/10665/276039/WHO-CDS-GMP-2018.22-eng.pdf?ua=1 (2018).
Fongnikin, A. et al. Efficacy of Fludora Fusion (a mixture of deltamethrin and clothianidin) for indoor residual spraying against pyrethroid-resistant malaria vectors: Laboratory and experimental hut evaluation. Parasit. Vectors 13, 466. https://doi.org/10.1186/s13071-020-04341-6 (2020).
Ngufor, C. et al. Which intervention is better for malaria vector control: Insecticide mixture long-lasting insecticidal nets or standard pyrethroid nets combined with indoor residual spraying?. Malar J. 16, 340. https://doi.org/10.1186/s12936-017-1987-5 (2017).
Read, A. F., Lynch, P. A. & Thomas, M. B. How to make evolution-proof insecticides for malaria control. PLoS Biol. 7, e1000058. https://doi.org/10.1371/journal.pbio.1000058 (2009).
Rowland, M. et al. A new long-lasting indoor residual formulation of the organophosphate insecticide pirimiphos methyl for prolonged control of pyrethroid-resistant mosquitoes: An experimental hut trial in Benin. PLoS ONE 8, e69516. https://doi.org/10.1371/journal.pone.0069516 (2013).
Abong’o, B. et al. Impact of indoor residual spraying with pirimiphos-methyl (Actellic 300CS) on entomological indicators of transmission and malaria case burden in Migori County, western Kenya. Sci. Rep. 10, 4518. https://doi.org/10.1038/s41598-020-61350-2 (2020).
Salako, A. S. et al. Efficacy of Actellic 300 CS-based indoor residual spraying on key entomological indicators of malaria transmission in Alibori and Donga, two regions of northern Benin. Parasit. Vectors 12, 612. https://doi.org/10.1186/s13071-019-3865-1 (2019).
Tugume, A. et al. Effects and factors associated with indoor residual spraying with Actellic 300 CS on malaria morbidity in Lira District, Northern Uganda. Malar. J. 18, 44. https://doi.org/10.1186/s12936-019-2681-6 (2019).
Agossa, F. R. et al. Small-scale field evaluation of the efficacy and residual effect of Fludora((R)) Fusion (mixture of clothianidin and deltamethrin) against susceptible and resistant Anopheles gambiae populations from Benin, West Africa. Malar J. 17, 484. https://doi.org/10.1186/s12936-018-2633-6 (2018).
Wondji, C. S. et al. Identification and distribution of a GABA receptor mutation conferring dieldrin resistance in the malaria vector Anopheles funestus in Africa. Insect. Biochem. Mol. Biol. 41, 484–491. https://doi.org/10.1016/j.ibmb.2011.03.012 (2011).
Du, W. et al. Independent mutations in the Rdl locus confer dieldrin resistance to Anopheles gambiae and An. arabiensis. Insect. Mol. Biol. 14, 179–183. https://doi.org/10.1111/j.1365-2583.2005.00544.x (2005).
Lees, R. S. et al. Tenebenal: A meta-diamide with potential for use as a novel mode of action insecticide for public health. Malar. J. 19, 398. https://doi.org/10.1186/s12936-020-03466-4 (2020).
Tabue, R. N. et al. Anopheles ziemanni a locally important malaria vector in Ndop health district, north west region of Cameroon. Parasit. Vectors 7, 262. https://doi.org/10.1186/1756-3305-7-262 (2014).
Kamau, L., Mulaya, N. & Vulule, J. M. Evaluation of potential role of Anopheles ziemanni in malaria transmission in western Kenya. J. Med. Entomol. 43, 774–776. https://doi.org/10.1603/0022-2585(2006)43[774:eoproa]2.0.co;2 (2006).
Mnzava, A. P. et al. Implementation of the global plan for insecticide resistance management in malaria vectors: Progress, challenges and the way forward. Malar J. 14, 173. https://doi.org/10.1186/s12936-015-0693-4 (2015).
Brogdon, W. & Chan, A. Guideline for Evaluating Insecticide Resistance in Vectors Using the CDC Bottle Bioassay (CDC Atlanta, 2010).
Syme, T. et al. Which indoor residual spraying insecticide best complements standard pyrethroid long-lasting insecticidal nets for improved control of pyrethroid resistant malaria vectors?. PLoS ONE 16, e0245804. https://doi.org/10.1371/journal.pone.0245804 (2021).
Acknowledgements
We thank Dr. Kunizo Mori of Mitsui Chemicals Agro, Inc for supplying the insecticide. We also thank the technical staff of CREC (Abibath Odjo, Damien Todjinou, Josias Fagbohoun, Martial Gbegbo etc.) for their assistance. We appreciate Dr. Graham Small, Dr. Derric Nimmo and Dr. Sarah Rees of IVCC for coordination. We acknowledge Dr. Mark Paine of LSTM for performing the chemical analysis. We are grateful to the rice farmers at Cove for their support in the hut study.
Funding
The study was funded by the Innovative Vector Control Consortium (IVCC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
C.N. co-designed the study, supervised the project, analysed the data and prepared the final manuscript. R.G. supervised the hut trial and contributed to data analysis and manuscript preparation. E.V. performed the laboratory bioassays while A.F. performed the hut trial. T.S. contributed to data analysis. M.A. provided administrative and logistics support. M.R. co-designed the study and contributed to manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ngufor, C., Govoetchan, R., Fongnikin, A. et al. Efficacy of broflanilide (VECTRON T500), a new meta-diamide insecticide, for indoor residual spraying against pyrethroid-resistant malaria vectors. Sci Rep 11, 7976 (2021). https://doi.org/10.1038/s41598-021-86935-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86935-3
- Springer Nature Limited