Abstract
The aim of the present study was to establish the effect of management type and of environmental variables on the structure, abundance and species richness of soil mites (Acari: Mesostigmata) in twelve urban green areas in Bucharest-Romania. Three categories of ecosystem based upon management type were investigated: protected area, managed (metropolitan, municipal and district parks) and unmanaged urban areas. The environmental variables which were analysed were: soil and air temperature, soil moisture and atmospheric humidity, soil pH and soil penetration resistance. In June 2017, 480 soil samples were taken, using MacFadyen soil core. The same number of measures was made for quantification of environmental variables. Considering these, we observed that soil temperature, air temperature, air humidity and soil penetration resistance differed significantly between all three types of managed urban green area. All investigated environmental variables, especially soil pH, were significantly related to community assemblage. Analysing the entire Mesostigmata community, 68 species were identified, with 790 individuals and 49 immatures. In order to highlight the response of the soil mite communities to the urban conditions, Shannon, dominance, equitability and soil maturity indices were quantified. With one exception (numerical abundance), these indices recorded higher values in unmanaged green areas compared to managed ecosystems. The same trend was observed between different types of managed green areas, with metropolitan parks having a richer acarological fauna than the municipal or district parks.
Similar content being viewed by others
Introduction
Over the past century, the world urban community has increased exponentially, with about half the world population living and working in urban areas that occupy only 2.8% of the land surface1. Urban areas contribute to human well-being from both economic and ecological points of view. Even if we discussed about the urban green ecosystems, each of them represent a dynamic complex of microorganism, plant and animal communities, which are correlated with abiotic factors (as: temperature, humidity of air and soil, acidity of soil, type of soil, etc.). In terms of ecosystem services, each type of ecosystems from natural to anthropic ones (as urban green areas) provides some benefits to people. Benefits, from ecological point of view, mean any services or goods that are used by the humans, directly or indirectly from nature. In general, urban areas, if well managed, can contribute to (or at least influence the delivery of) the following ecosystem goods and services: fresh water, food, timber, fiber, fuel, new products and industries from biodiversity; nutrient cycling, climate and air quality; ecosystem regulation of infectious diseases, waste processing and detoxification, regulation of natural hazards (floods and fires); and cultural services. In almost all cases, urban areas depend on nature. Human well-being depends on the health of urban and adjacent ecosystems2,3,4.
Biological diversity is a key component of almost all ecosystem services essential to human life. According to the Millennium Ecosystem Assessment, biodiversity contributes to human security, resilience, health and freedom of choice and action5,6. In general, cities are characterised by the following features: high density of human population, increased numbers of industrial, business and residential areas, high anthropogenic impact on natural habitats, higher temperatures in comparison to adjacent ecosystems, extinction of native species and an increased impact and number of invasive and allochthonous plant and animal species6,7. All these factors, together with increased urbanisation, lead to high pressure on ecosystems and biodiversity within the urban environment.
Soil invertebrates are a valuable component of biodiversity. They influence nutrient cycling by feeding directly on plant materials and organic substrates. They have a direct or indirect influence on litter decomposition, having a consistent positive effect on this soil process at global and biome scales8,9. Some components of the soil fauna are ecosystem engineers, influencing soil structure, as well as mineral and organic matter composition10. Mites (Acari) are one of the most abundant groups of soil invertebrate. Up to the present day, approximately 55,000 species of mite have been recognised and described, although researchers have estimated that the actual total may be between 500,000 and 1,000,00011. Research has highlighted several important reasons for studying invertebrates in urban areas: (a) they represent a good assessment tool for biodiversity status; (b) they respond rapidly to any environmental disturbance (due to their short generation times); (c) they are easy to collect; (d) they are present at many trophic levels; and (e) are important in terms of ecosystem services correlated with anthropogenic changes12,13.
Predatory mites (Acari-Mesostigmata) are free-living species which occupy various niches that are primarily or secondarily related to soil and litter (e.g. litter-fermentation layers, moss, bark, dead wood, trunks, stumps, tree-hollows and nests) present within different types of ecosystems e.g. forest, scrub, dunes, grassland, agricultural land and urban areas14,15,16,17,18,19,20,21,22,23. Together with other small soil invertebrates (springtails, enchytraeids, insect larva, oribatids), they participate indirectly in the decomposition process and ultimately affect soil quality (fertility and productivity)4,10. At the same time, they are the main regulator of the other soil invertebrate populations11,24,25. Most species prefer habitats rich in organic matter, with high soil moisture, medium soil temperature and a low pH26,27,28. Because of these ecological requirements and their marked sensitivity to environmental/anthropogenic disturbance, soil mites are often used as soil bioindicators15,29,30,31,32,33.
Research studies on predatory soil mites (Acari-Mesostigmata) from cities around the world are relatively few, either in urban sites (forests, roadsides, greeneries, gardens, galleries, parks, housing estates, town centres, grasslands, cemeteries, botanical gardens) or in suburban areas and industrial areas. Such studies have been conducted in Europe (Latvia, Poland, Italy, Slovakia, Hungary) or in the USA, but they focussed on all soil-invertebrate communities, on Uropodina, as a suborder of Mesostigmata order or only on few urban habitats29,34,35,36,37,38,39,40,41,42,43,44,45. Some of these studies investigated phoretic species, especially those from the Uropodina suborder46,47,48. In Romania, there are few studies of the Mesostigmata fauna in urban areas, all located in three Bucharest parks and two forests close to the city21,22,23,32,49,50,51. All these European and national studies are mainly faunistic or taxonomic, almost without any information on the ecological aspect of soil mite communities in relation to urban environmental factors.
To address this research gap, we focussed on the communities of Mesostigmata soil mites in managed and unmanaged urban areas in Bucharest, through a more extensive study. The project also includes one of the few urban protected areas in Europe (Văcăreşti Natural Park). The main objectives of the study were investigated: (1) the effect of management regime in urban areas on the community structure, abundance and species richness of mesostigmatid mites; (2) some key urban environmental variables; and (3) the influence of the selected abiotic parameters on the structure of mesostigmatid communities in the ecosystems studied (as: soil and air temperature, soil moisture and atmospheric humidity, soil pH and soil penetration resistance).
Material and methods
Study area
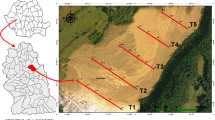
The study was conducted in June 2017, in twelve urban green areas in Bucharest (Fig. 1). Bucharest is situated in Central Eastern Europe, in the lower Danube region. The mean annual temperature is about 10–11 °C, and annual precipitation is 615 mm52. Of the total city area (22,800 ha), urban green space occupies 2275 ha (9.97%) while urban parks represent 29.9% of Bucharest and approximately 3% of the total city area but are unequally distributed. Urban parks were classified as (a) metropolitan: MtP (average area = 52.4 ha, over 5000 visitors/weekend day), (b) municipal: MnP (average area = 40.3 ha, 2000–5000 visitors/weekend day), and (c) district: DrP (average area = 6.4 ha, under 2000 visitors/weekend day)52,53.

Copyright Esri. All rights reserved. For more information about Esri software, please visit www.esri.com.
Geographical locations of the natural protected urban area (PA), managed (MGA) and unmanaged green areas (UGA) in Bucharest, investigated in 2017. Map created using ArcGIS software by Esri. ArcGIS and ArcMap are the intellectual property of Esri and are used herein under license.
The ecosystems studied were classified in three management scenarios (MS): (1) urban protected area (PA) i.e. Văcăreşti (V), (2) managed green urban areas (MGA) i.e. metropolitan parks [Tineretului (TN) and Titan (TT)], municipal parks (Plumbuita (PL) and Carol (C) and district parks (Floreasca (FL) and Crângaşi (CR), and (3) unmanaged green urban areas (UGA) i.e. Băneasa (B), Pantelimon (P), Griviţa (G), Lacul Morii (LM) and Fundeni (F). A detailed description of these areas is given in Supplementary Appendix 1. Văcăreşti Natural Park is the biggest (183 ha) and the newest park designated in Bucharest as well as the first urban nature park in Romania. It is located at 5 km distance from the city centre and it was classified in the category V (“protected landscape”) following the IUCN criteria54.
In all managed green areas (with one exception—Văcăreşti Natural Park), the local administrations together with the Plant Protection Centre subordinated to the General Council of Bucharest carries out treatments to combat plant pests (through biological and integrated methods): mites, aphids, thrips, moths, powdery mildew, rust, wasps, defoliating caterpillars, San Jose lice, woolly lice, cicadas, etc. Weed control is also applied in all managed green areas, using manual methods. These methods are governed by the Regulation of the public service for the administration of the public and private domain, regarding the activities of arrangement and maintenance of the parks and of the gardens in the municipality of Bucharest. All managed areas are irrigated.
Soil fauna
Soil samples were collected in June 2017, using a MacFadyen soil core (5 cm diameter) to 10 cm depth. In total, 480 soil samples were collected randomly (40 samples/urban area). In each urban ecosystem, the sampled area was 100 m2 Due to the small number of immature individuals, they were not considering separately in our statistical analysis. The mites were extracted with a Berlese-Tullgren funnel, in ethyl alcohol, clarified in lactic acid and identified to species level, using published identification keys55,56,57,58,59,60,61,62,63. The mites extraction lasted 10 days. Some specimens were mounted on permanent slides. All species were deposited in the collection of the Institute of Biology-Bucharest, Romanian Academy-Research Station Posada.
Environmental variables
In order to establish the relationship between soil mite communities and environmental variables, we measured air and soil temperature (T-air, T-soil as °C), air and soil moisture (H-air and H-soil as %), soil pH, and soil penetration resistance (RP as MPa). Air and soil moisture and temperature were measured with a digital thermo-hygrometer PCE-310. Air temperature and moisture were measure at 5 cm above the soil level. Penetration resistance was determined with a soil penetrometer, Step System GmbH, 41,010. The pH was measured with a C532 Jasco Consort pH-meter. The average values of environmental variables are presented in Tables 1 and 2.
Data analysis
The completeness of the species inventory was examined by visual inspection of Mao Tau species accumulation curves for each management scenario and for pooled data of all sites.
We examined differences across management scenarios: managed green areas, unmanaged green areas and sites (Băneasa, Pantelimon, Griviţa, Lacul Morii, Fundeni, Carol, Plumbuita, Tineretului, Titan, Floreasca and Crângaşi) using non-metric multidimensional scaling (NMDS) with the Bray–Curtis (B–C) distance index.
The NMDS was conducted using the function “metaMDS” in the R package “vegan”64. The function automatically applies a square root transformation to the data matrix of species abundance. The environmental variables were standardised to have mean zero and unit variance. We ran NMDS using 500 random starts and tested the goodness of fit of the data using the R2 value and examining the Shepard plot (i.e. the scatter around the regression of the distances between each pair of communities against their original dissimilarities). The significance of differences between communities from management scenarios and sites was assessed with an overall PERMANOVA based on B–C dissimilarities with the function “adonis” within the vegan package and pairwise using the function “pairwise.perm.manova” within the “RVAideMemoire” package65. The same analyses were repeated to examine the differences among different types of MGAs, i.e. metropolitan, municipal and district parks.
To determine whether physical characteristics influenced the species communities, we conducted a canonical correspondence analysis (CCA). Before performing the CCA, we selected the significant environmental variables using the ANOVA permutation test for CCA (999 random permutation) and only introduced the variables with P > 0.05 in the CCA.
We used linear mixed models (LMMs) to test whether the main community feature (i.e. the species richness SR) was related to: (i) management scenario, (ii) environmental variables and (iii) a combination of management scenarios and environmental variables. In the LMMs, the management scenario and the environmental variables were introduced as fixed effects and sites as random effects. We assessed the relative performance of the models using the selection technique based on Akaike’s information criterion corrected for sample size—AICc66,67. We ranked the models and the model with the lowest AICc was used as the reference for calculating the AIC difference (Δi) and the likelihood of a model given the data and model weights (wi). Models within two AIC units of the AICmin were considered competitive and more plausible than others66.
To identify any taxonomic group-specific pattern across management scenarios we examined the extent to which each management scenario was characterised by rare species, i.e. singletons and doubletons. All analyses were performed using R version 3.2.1.
Based on the reproduction strategy of soil mites (“k” or “R”)68, the index of maturity was calculated for each studied urban green area (Supplementary Appendix 2). Using PAST software, we quantified the following parameters: dominance (D), Shannon index of diversity (H) and equitability (J)69.
To identify mite species characteristic to the three MS we applied the indicator Value method using the “indicspecies” R package70,71. The method assesses the specificity (uniqueness to a particular MS) and fidelity (frequency of occurrence) of mite species. A high indicator value (IndVal, expressed as percentage) indicates that a mite species can be considered characteristic to a particular or combination of MS. The IndVal for each mite species was calculated based on the matrix of mite species abundance. We used a random reallocation procedure of sites among site groups to test for the significance of IndVal measures for each mite species70. According to Dufrêne and Legendre (1997), a mite species is considered characteristic to a MS or combination of MS if the species IndVal is > 25% and significant at p < 0.05.
Results
Analysing the entire Mesostigmata community, we discovered 68 species, with 790 individuals and 49 immatures. If we consider the protected area, 8 species were identified, with 37 individuals and 15 immatures. In managed urban areas 33 species were identified, with 435 individuals and 25 immatures while in unmanaged green ecosystems, we recorded 49 species, with 318 individuals and 9 immatures (Tables 3, 4). None of the species accumulation curves for each management scenario or for pooled data from all sites approached an asymptote, indicating that more samples are required to detect all the species theoretically expected (Fig. 2).
Examining the mean values for environmental variables, we observed that soil temperature, air temperature, air humidity and soil penetration resistance differed significantly between all three types of managed urban green area (p < 0.05). The MGA areas were characterised by the highest mean values of air and soil moisture, RP and by the lowest mean values of air temperature and pH. The PA had the highest mean values of air and soil temperature, pH and moderate mean values of the remaining abiotic factors. In UGA, the lowest mean values of soil temperature, RP air and soil moisture were recorded (Table 1).
Measuring the environmental variables from the three types of MGA, we recorded a significant difference between their mean values (p < 0.05). MtP was characterised by the highest mean values of air and soil temperatures, soil moisture and the lowest mean value of pH. MnP were characterised by the highest values of atmospheric humidity (RP) and lowest air temperature. In DrP, the lowest average values of soil temperature and moisture (RP) and the highest mean value of pH were observed (Table 2).
The Shepard plot showed that original dissimilarities were well preserved in the reduced number of dimensions and low stress was found for both NMDS analyses examining the differences across the MS (R2 = 1, stress = 0.001) and different types of MGA (R2 = 0.966, stress = 0.055) (Supplementary Appendix 3), respectively.
The NMDS analysis showed low clustering of data points by both management scenarios, types of MGA and sites (Supplementary Appendix 4).
PERMANOVA showed significant differences between MS (F[2,243] = 3.77, R2 = 0.03, p < 0.001), MGA (F[2,106] = 2.91, R2 = 0.050 = 3, p < 0.001) and sites (F[11,243] = 3.04, R2 = 0.126, p < 0.001). Pairwise comparisons of species communities identified marginally significant differences only between MGA and UGA (p = 0.05) and DrP and MtP (p = 0.05). Comparisons between species assemblages between all pairs of sites are shown in Table 5.
The CCA model including all the environmental variables explained a total of 52.00% of the overall variation in species composition, of which 26.01% was explained by the first axis and 25.9% by the second axis, respectively. The first canonical axis was highly correlated with pH (− 0.944) whereas the second canonical axis was correlated with T-soil (0.710), T-air (− 0.598) and H-air (0.712). The biplot classification on the two first axes showed species from the upper right quadrant were associated with H-soil, Rp, T-soil and H-air. The lower quadrant contains species associated with T-air. Species associated with pH were placed in the upper left quadrant (Fig. 3).
Biplots of the canonical correspondence analysis model of species abundance matrix in relation environmental variables. Length and direction of arrows indicate the relative importance and direction of change in the environmental variables. Variables are: T-soil and T-air, H-soil and H-air, pH, RP. Species names were abbreviated using the initials of the genus and species name (abbreviations are reported in Tables 3 and 4).
The ANOVA permutation test for CCA showed that all the environmental variables were significantly related to community assemblage (Supplementary Appendix 5).
Based on species richness, the model selection using AICc indicated one highly supported model that included the pH (Table 6). The second best supported model included the MS. Species richness significantly increased with pH (F[1,231] = 4.00, p = 0.04) for all management scenarios (Fig. 4). No significant differences in species richness were found among the UGA (F[2,106] = 0.9, p = 0.533).
Focussing on individual Bucharest parks, the highest number of identified species and number of individuals was recorded in TN park (17 species with 157 individuals), in comparison with V, CL or FL, PL areas, where the values were lowest (number of species between 8 and 10 and number of individuals between 25 and 50 individuals). These results are confirmed by the Shannon index of diversity, which had the highest value in TN (H = 2.085) and lowest in V or TT urban parks (H = 1.696 or H = 1.572). The maturity index of predatory mite communities had its highest values in C and V (0.66 and 0.61). In contrast, communities from CL, TN and FL had a maturity index of only 11 or 10 (Table 3).
Turning to the dominance index, the mite communities from TT, V and PL are represented by a few dominant species: Pergamasus quisquiliarum and Arctoseius cetratus in TT, Rhodacarellus silesiacus in V, and Hypoaspis aculeifer in all three ecosystems. In the remaining green areas studied, representation of remaining species was almost equitable. This fact was indicated by the equitability index, characteristic for each urban area (Table 3).
Comparing the taxonomic structure of the mite communities, we observed that each managed urban green area was defined by some characteristic species i.e. (a) for the natural park: Pergamasus crassipes and Parasitus fimetorum, (b) for metropolitan parks: Cherosieus bryophilus and Leioseius minusculus, (c) for municipal parks: Paragamasus similis, Protogamasellus singularis and Olopachys vysotskajae, and (d) for district parks: Pergamasus laetus, Parasitus beta, Pseudolaelaps doderoi and Laelaps astronomica.
In the different types of managed parks in Bucharest, we observed that in MtP, the number of species as well as numerical densities is higher than in DtP. In MtP the average value of soil moisture is higher than in DtP (15.63 in comparison with 10.84). The mean value of soil temperature is higher in MtP (18.06 °C) that in DtP (13.41 °C). These environmental variables could influence the mite communities from the two types of managed urban areas.
Making an ecological analysis of the mite communities from UGA areas, the highest number of identified species was recorded in B park (22 species), as well as the Shannon index of diversity (H = 2.791) and equitability (J = 0.903). The highest value for numerical abundance was obtained in park F (110 individuals). In contrast, park P has lower values for all these parameters, with the exception of dominance, whose value was D = 0.294 and of maturity index (M = 0.71) (Table 4).
In UGA, the most common species were Veigaia planicola, Hypoaspis aculeifer, Rhodacarellus silesiacus (present in all parks), Pergamasus crassipes, Parasitus fimetorum, Veigaia exigua, Veigaia nemorensis, Macrocheles recki, Onchodellus karawaiewi and Alliphis siculus (identified in three of the parks investigated) (Table 4).
Indicator species analysis identified two characteristic mite species to UGA, i.e., Veigaia planicola (Ind.Val = 25.8%, p = 0.006) and Macrocheles recki (Ind.Val = 22.2%, p = 0.015) and one species to MGA and PA combination, i.e., Pergamasus quisquiliarum (Ind.Val = 30.1%, p = 0.002).
Discussion
Comparing the present research with data obtained from other studies in Europe on the Mesostigmata of urban soils, the number of species identified in Bucharest is comparable with totals obtained in Latvia and Poland, in such different habitats as roadsides, greenery, parks and urban forests (25–51 species), but is higher than that for mite communities in a botanical garden in Hungary (12 species)40,42,45,47. However, the number of recorded species is lower than suburban and urban green areas from Poland (62–68 species), urban grasslands from Latvia or cemeteries and botanical garden in Slovakia (123 species, in three years of study)34,35,38,39,43.
Analysing the above presented results, we observed that management intensity in urban green areas, from Bucharest-Romania, impacts mesostigmatid mite diversity and community composition. The effect of management intensity on mesostigmatid mite diversity was highlighted by the results of pairwise comparison of species communities that revealed significant differences between MGA and UGA areas. The number of recorded species in UGA is higher than that for MGA. The Romanian results agree with those obtained in Latvia for Riga city, where there was a high diversity of soil invertebrates in the disturbed urban forest habitats, but undisturbed soils harbour a greater species richness of mites than disturbed soils40.
The management type of the urban green areas influenced the species composition as well. Species composition differs in the three management scenarios for urban green areas. About 27.54% of the total number of species were characteristic of MGA, with Pergamasus quisquiliarum, Dendrolealaps foveolatus and Gamasellus bicolor having the highest recorded abundance. Other research has indicated that Pergamasus quisquiliarum is a species with affinity for parks and street green areas in Warsaw, Poland. Dendrolealaps foveolatus and Gamasellus bicolor have been identified in urban forest in Riga city and in suburban green areas in Warsaw35,45.
In UGA, 47.83% are species identified only in these parks. In these mite communities, species from the Macrochelidae and Pachylaelapidae families were best represented, with Macrocheles recki, Aliphis halleri and Parasitus fimetorum most numerous. These species were also associated with industrial and post-industrial areas in Poland38.
Comparing the mite communities of PA with those from the remaining MGA, we observed not only the lowest number of species and numerical densities in PA but also the lowest value of the Shannon index of diversity (H = 1.696). The same situation is found if we compare PA mite communities with all communities from unmanaged urban green areas. These results are supported by the MANOVA pairwise comparisons of species communities among sites (Table 5). Analysing abiotic factors, we observed that in the PA, the highest mean values of soil and air temperature were recorded (16.87 °C and 30.02 °C), potentially affecting the structure of mite communities. Despite the fact that this PA (Văcăreşti) is wetland, higher temperatures during summer and autumn cause high evapotranspiration and water depletion at depth in the soil72.
Comparison between species communities from MGA revealed that those from DrP and MtP were significantly different (according to PERMANOVA test). In managed types of district park green areas the number of species as well as numerical densities are higher than in metropolitan parks. Some environmental variables are significantly different between parks e.g. soil moisture and temperature. There are also differences in community composition structure. All these differences are correlated with specific environmental conditions. Higher soil humidity and temperature provided more favourable environmental conditions for predatory soil mites in metropolitan parks. Drought or lower soil humidity has a negative impact on soil microarthropod fauna, both in general and on mites specifically73.
Using the presented information, based on the obtained results from this research, we could propose that the future new created urban parks should be designed taking into account the natural/unmanaged model. This means that, from acarological point of view, the autochthone vegetation and native soils constitute a proper habitat for Mesostigmata species. In unmanaged urban green areas, the ecological process are natural and undisturbed (not anthropized as in managed parks) and these ecosystems will follow the natural ecological succession. If we put into discussion the relation between ecological characteristics of the managed urban green areas and Mesostigmata fauna, we consider that their management could be reconsidered. This reconsideration means to use native species of plant, trees and soil, reach in organic matter (not indigenous brought from Asia or Central America, usually accompanied by the alochtonous soil and other invasive species).
Research studies from all over the world revealed that the Mesostigmata communities correlated with environmental variables could constitute a very useful biological tool to characterized the ecological quality of terrestrial ecosystems and the quality of soils9,10,18,21,25,26,27,29,30,31,33. In the case of urban green areas from Bucharest, under different management scenarios, their ecological characterization was made taking into account the variation of the environmental parameters, in correlation with spatial dynamics of the communities’ parameters of Mesostigmata fauna (species diversity and numerical abundance).
Analysing the environmental parameters, the highest average value of soil moisture (13.16%) was recorded in MGA areas where the soil penetration resistance is higher (RP = 1.8). However, the differences between mean values of soil moisture for all the green areas are not significant (p = 0.334), due to the fact the soils throughout Bucharest are mainly clay (Table 1). Because of their very small particle size and consequent large surface area, clays are able to retain greater amounts of water than sandy or most anthropogenic soils74. Other research studies revealed that there was a strong correlation between soil penetration resistance, pore space, soil moisture and quantity of organic matter75,76.
In general, an increased value of RP means a decreased quantity of organic matter, a lower value of soil moisture and reduced pore space. Although soil moisture in our study is higher in MGA than in UGA or PA, it is possible that the lack of organic matter is the main limiting factor for species diversity. However, clay particles from MGA soils had a much greater tendency to stick together than sands (which were present mainly in UGA), and not only did soil moisture have recorded high values but also the RP is higher74.
In the investigated plots, especially those in UGA, the dominant species of Mesostigmata are predatory mites. Their presence is linked to organic matter (correlated with pH), which represents a trophic reservoir and favourable habitat for other invertebrate groups—a relationship supported by the higher values of the soil maturity index15,28,77,78. The relationship between clay soil and pH is well established. Clays have a large specific surface and are predominantly negatively charged, retaining nutrients against leaching and buffering the soil against extreme pH changes79. It has also been discovered that on basic soil some species of bacteria develop, with these two variables being linearly correlated80. Bacterial community development constitutes a favourable factor for the soil invertebrates that represent the food source for predatory mites9,77. This may also explain the linear relation between pH value and species richness obtained in our research.
Soil structure is undisturbed in UGA compared with MGA; in MGA there is some modification every year due to the establishing of different ornamental plants. According to specialists81, some soils in Bucharest were identified that, although less modified, nonetheless received (and were still receiving) mainly negative impacts caused by daily household or industrial activities. Soils of this type were found in those green spaces less modified by urbanism, and soils from peripheral and suburban areas.
If we consider the influence of the environmental variables on all the mite species identified, we observed that species such as Dendrolealaps foveolatus, Pergamasus quisquiliarum, Laelaps astronomica, Hypoaspis praesternalis and Onchodellus karawaiewi were influenced by soil moisture and air humidity, soil temperature and RP. In Europe, these species have been identified mostly in grasslands, grassy arable fallows or urban habitats, their communities being correlated with soil moisture (Onchodellus karawaiewi being a euryhygrophilous species) or organic matter content. In our study, this explains the relationship between such mites and RP20,25,44,60,79.
Three species (Rhodacarus denticulatus, Olopachys suecicus and Glyptholaspis americana) were influenced by soil pH. Soil pH itself is influenced by different vegetation types, including those from urban green areas20,82. Olopachys suecicus is eurytopic, with wide ecological plasticity, able to colonise poorly shaded habitats (urban parks, scrub, grasslands). Glyptholaspis americana is cosmopolitan and mostly found in acid habitats such as dung or leaf litter58.
Rather surprisingly, in these urban ecosystems air temperature influenced the composition of mite communities indirectly. In such ecosystems, the most striking difference from surface habitats was that the moisture/humidity and temperature extremes were much less pronounced in the soil. Similar patterns have been identified in other habitat types, such as screes83.
Spatial dynamics of the investigated parameters of soil mite communities related to environmental factors could constitute a response of this invertebrate group to the specific conditions of urban green areas under different types of management scenario.
Conclusions
The presented study established the effect of management type and of environmental variables on the structure, abundance and species richness of soil mites (Acari: Mesostigmata). For the first time in Romania, an intensive study on urban Mesostigmata fauna has been made. Twelve urban areas were characterised by specific environment conditions and mite communities. Six environmental factors were investigated (soil and air temperature, soil moisture and atmospheric humidity, soil pH, soil penetration resistance). In total 480 soil samples were analysed for the soil fauna, as well for the abiotic factors.
Examining the mean values of environmental variables, we observed that soil temperature, air temperature, air humidity and soil penetration resistance showed significant differences between all three types of managed urban green area. All environmental variables, especially soil pH, significantly influenced the mite communities. The MGA areas were characterised by the highest mean values of air and soil moisture, the PA had the highest mean values of air and soil temperature, pH and the UGA by the lowest values of these parameters. From all collected soil samples, 68 Mesostigmata species were identified, with 790 individuals and 49 immatures. In terms of the number of species and numerical abundance in relation to management regimes and to the specific investigated environmental conditions, differences in mesostigmatid mite communities were recorded between managed green areas (MGA) and unmanaged green areas (UMG). In comparison with MGA, UGA were characterised by higher values of the community parameters (Shannon diversity, dominance and equitability), as well as by the highest values of the soil maturity index. Comparative analysis between the three types of MGA revealed that the species communities from metropolitan parks were richer than those from district parks.
The investigated indices that showed different values in relation to environmental variables demonstrate important links between mite communities in specifically urban ecosystems that are under anthropogenic pressure. On the other hand, we consider that unmanaged urban green areas as “hotspots” of Mesostigmata diversity. This will constitute an argument of the usage of native vegetation and soil, for creation of new urban parks or for management of those existing ones.
Data availability
The data sets analysed during the current study are available from the corresponding author on reasonable request.
References
Bremner, J. et al. World population highlights: Key findings from PRB’s 2010 world population data sheet. Popul. Bull. 65(2), 1–12 (2010).
McGranahan, G., Marcotullio, P. Urban systems. In Ecosystems and Human Well-being: Current State and Trends. Volume I (eds. Hassan, R., Scholes, R., Ash, N.) 795–825 (Island Press, Washington, DC, 2005).
Robrecht, H. & Lorena, L. Ecosystem services in cities and public management. In The Economics of Ecosystems and Biodiversity for Local and Regional Policy (ed Wittmer, H.) 60–80 (Progress Press, 2010).
Adhikari, K. & Hartemink, A. E. Linking soils to ecosystem services—A global review. Geoderma 262, 101–111 (2016).
Millennium Ecosystem Assessment Panel. Ecosystems and Human Well-being: Synthesis. Washington, DC: Island Press. https://www.millenniumassessment.org/en/index.html (2005).
McDonald, R.I., Marcotullio, P.J. & Güneralp, B. Urbanization and Global Trends in Biodiversity and Ecosystem Services. In Urbanization, Biodiversity and Ecosystem Services: Challenges and Opportunities. A Global Assessment (eds. Elmqvist, T. et al.) 31–52 (Springer, Dordrecht, 2013).
Anthrop, M. Changing patterns in the urbanized countryside of Western Europe. Landsc. Ecol. 15, 257–270 (2000).
Coleman, D.C. & Wall, D.H. Soil fauna: Occurrence, biodiversity, and roles in ecosystem function. In Soil Microbiology, Ecology and Biochemistry (ed Paul, E.) 111–149 (Academic Press, Waltham, 2015).
Dirilgen, T. et al. Mite community composition across a European transect and its relationships to variation in other components of soil biodiversity. Appl. Soil Ecol. 97, 86–97 (2016).
Culliney, T. W. Role of arthropods in maintaining soil fertility. Agriculture. 3, 629–659 (2013).
Krantz, G. W. & Walter, D. E. A manual of Acarology. (ed. Texas Tech University Press, USA) 98–100 (Krantz & Walter, 2009).
McIntyre, N. E. Ecology of urban arthropods: A review and a call to action. Ann. Entomol. Soc. Am. 93, 825–835 (2000).
Jones, E. L. & Leather, S. R. Invertebrates in urban areas: A review. Eur. J. Entomol. 109, 463–478 (2012).
Koehler, H. H. Predatory mites (Gamasina, Mesostigmata). Agric. Ecosyst. Environ. 74, 395–410 (1999).
Gulvik, M. E. Mites (Acari) as indicators of soil biodiversity and land use monitoring: A review. Pol. J. Ecol. 55, 415–450 (2007).
Salmane, I. & Brumelis, G. Species list and habitat preference of Mesostigmata mites (Acari, Parasitiformes) in Latvia. Acarologia. 50, 373–394 (2010).
Kaczmarek, S., Marquardt, T. & Falenczyk-Kozirog, K. Diversity of the Mesostigmata (Acari) in tree-hollows of selected deciduous tree species. Biol. Lett. 48, 29–37 (2011).
Madej, G., Barczyk, G. & Gawenda, J. Importance of microhabitats for preservation of species diversity, on the basis of mesostigmatid mites (Mesostigmata, Arachnida, Acari). Pol. J. Environ. Stud. 20(4), 961–968 (2011).
Huhta, V., Pietikäinen, A. S. & Penttinen, R. Importance of dead wood for soil mite (Acarina) communities in boreal old-growth forests. Soil Organ. 84(3), 499–512 (2012).
Wissuwa, J., Salamon, J. A. & Frank, T. Effects of habitat age and plant species on predatory mites (Acari, Mesostigmata) in grassy arable fallows in Eastern Austria. Soil Biol. Biochem. 50, 96–107 (2012).
Manu, M. Structure and dynamics of the predatory mites (Acari: Mesostigmata- Gamasina) from the central parks and forest ecosystems from/near Bucharest. In Species Monitoring in the Central Parks of Bucharest (ed. Onete, M.) 68–78 (Ars Docendi, Universitatea Bucureşti, 2008).
Manu, M., Szekely, L., Vasiliu, Oromulu, L., Bărbuceanu, D., Honciuc, V. et al. Bucharest. In Vertebrates and Invertebrates of European Cities: Selected Non-Avian Fauna (ed. Kelcey, J.G.) 257–322 (Springer Science+Business Media LLC, New York, 2015).
Manu, M., Băncilă, R. I. & Onete, M. Importance of moss habitats for mesostigmatid mites (Acari: Mesostigmata) in Romania. Turk. J. Zool. 42(6), 673–683 (2018).
Klarner, B., Maraun, M. & Scheu, S. Trophic diversity and niche partitioning in a species rich predator guild—natural variations in stable isotope ratios (13C/12C, 15N/14N) of mesostigmatid mites (Acari, Mesostigmata) from Central European beech forests. Soil Biol. Biochem. 57, 327–333 (2013).
da Groot, A. G., Jagers op Akkerhuis, G. J. A. M., Dimmers, W. J., Charrier, X. & Faber, J. H. Biomass and diversity of soil mite functional groups respond to extensification of land management, potentially affecting soil ecosystem services. Front. Environ. Sci. 4, 1–15 (2016).
Manu, M., Iordache, V., Băncilă, R. I., Bodescu, F. & Onete, M. The influence of environmental variables on soil mite communities (Acari: Mesostigmata) from overgrazed grassland ecosystems—Romania. Ital. J. Zool. 83, 89–97 (2016).
Meehan, M. L., Zhuoyan Song, Z. & Proctor, H. Roles of environmental and spatial factors in structuring assemblages of forest-floor Mesostigmata in the boreal region of Northern Alberta, Canada. Int. J. Acarol. 44, 300–309 (2018).
Kamczyc, J. et al. Response of soil mites (Acari, Mesostigmata) to long-term Norway spruce plantation along a mountain stream. Exp. Appl. Acarol. 76(1–3), 1–18 (2018).
Santorufo, L., Van Gestel, C. M., Rocco, A. & Maisto, G. Soil invertebrates as bioindicators of urban soil quality. Environ. Pollut. 161, 57–63 (2015).
N’Dri, J. K., Hance, T., Andr,é, H. M., Lagerlöf, J. & Tondoh, J. E. Microarthropod use as bioindicators of the environmental state: Case of soil mites (Acari) from Côte d’Ivoire. J. Anim. Plant Sci. 29(2), 4622–4637 (2016).
George, P. B. L. et al. Evaluation of mesofauna communities as soil quality indicators in a national-level monitoring programme. Soil Biol. Biochem. 115, 537–546 (2017).
Manu, M., Onete, M. & Băncilă, R. I. The effect of heavy metals on mite communities (Acari: Gamasina) from urban parks—Bucharest, Romania. Environ. Eng. Manag. J. 17(9), 2071–2081 (2018).
Spiller, M. S., Spiller, C. & Garle, J. Arthropod bioindicators of environmental quality. Revista Agroambiente. 12(1), 41–57 (2018).
Niedbała, W., Błaszak, C., Błoszyk, J., Kaliszewski, M. & Kazmierski, A. Soils mites (Acari) of Warsaw and Mazovia. Memorabilia Zool. 36, 235–252 (1982).
Niedbała, W., Błoszyk, J., Kaliszewski, M., Kazmierski, A. & Olszanowski, Z. Structure of soil mite (Acari) communities in urban green of Warsaw. Fragmenta Faunistica. 33, 21–44 (1990).
Pouyat, R. V., Parmelee, R. W. & Carreiro, M. M. Environmental effects of forest soil-invertebrate and fungal densities in oak stands along an urban-rural land use gradient. Pedobiologia 38, 385–399 (1994).
Minor, M. A. & Cianciolo, J. M. Diversity of soil mites (Acari: Oribatida, Mesostigmata) along a gradient of use types in New York. Appl. Soil Ecol. 35, 140–153 (2007).
Skorupski, M., Horodecki, P. & Jagodziński, A. M. Roztocze z rzędu Mesostigmata (Arachnida, Acari) na terenach przemysłowych i poprzemysłowych w Polsce. (Mite species of Mesostigmata order (Arachnida, Acari) in industrial and post-industrial areas of Poland). Nauka Przyr. Technol. 11, 1–23 (2013).
Minova, S., Jankevica, L., Salmane, I. & Èekstere, G. Preliminary studies on microbial biomass and the microarthropod community as soil health and quality indicators in urban grasslands, Rîga as an example. Proc. Latvian Acad. Sci. Sect. B. 69(3), 140–144 (2015).
Telnov, D. & Salmane, I. Ecology and diversity of urban pine forest soil invertebrates in Rîga, Latvia. Proc. Latvian Acad. Sci. Sect. B Nat. 69(3), 120–131 (2015).
Napierała, A., Skwierczyñski, F. & Jankowiak, A. Materials to knowledge of Uropodina (Acari: Mesostigmata) of Poznań District. Badania Fizjograficzne R. I Seria C Zoologia. C51, 7–19 (2010).
Kontschán, J., Ács, A., Wang, G. Q. & Neményi, A. New data to the mite fauna of Hungarian bamboo plantations. Acta Phytopathol. Entomol. Hung. 50(1), 77–83 (2015).
Fend’a, P. & Hruzova, K. Mites (Acari, Mesostigmata) in urban green of Bratislava (Slovakia) In 8th Symposium of the European Association of Acarologist (ed Universitat Politecnica de Valencia) 41 (Book of Abstract, 2016).
Hrúzová, K. & Fend’a, P. First record of Parasitus americanus (Berlese, 1905) and Cornigamasus ocliferius Skorupski and Witaliński, 1997 (Acari: Mesostigmata: Parasitidae) from Slovakia. Check List. 13(4), 239–243 (2017).
Salmane, I. Soil microarthropods (Acari, Collembola) in the Rīga city habitats. Environ. Exp. Biol. 16, 73–74 (2018).
Błoszyk, J., Klimczak, I. & Leśniewska, M. Phoretic relationships between Uropodina (Acari: Mesostigmata) and centipedes (Chilopoda) as an example of evolutionary adaptation of mites to temporary microhabitats. Eur. J. Entomol. 103, 699–707 (2006).
Napierała, A. et al. Phoretic relationships between uropodid mites (Acari: Mesostigmata) and centipedes (Chilopoda) in urban agglomeration areas. Int. J. Acarol. 41(4), 250–258 (2015).
Mizser, S., Nagy, L. & Tóthmérész, B. Mite infection of Carabus violaceus in rural forest patches and urban parks. Period. Biol. 118(3), 307–309 (2016).
Honciuc, V. & Manu, M. Ecological study on the edaphically mite’s populations (Acari: Mesostigmata—Gamasina: Oribatida) in urban areas from Romania. Rom. J. Biol. Zool. 55(1), 19–30 (2010).
Manu, M. & Honciuc, V. Rang correlations at the level of the predator and the decomposer populations soil mites (Acari: Mesostigmata-Gamasina, Oribatida) from central parks of Bucharest city, Romania. Acta Entomol. Serb. 5(1), 129–140 (2010).
Manu, M. & Honciuc, V. Ecological research on the soil mite’s populations (Acari: Mesostigmata-Gamasina, Oribatida) from forest ecosystems near Bucharest city. Rom. J. Biol. Zool. 55(1), 19–30 (2010).
Iojă, C. I., Rozylowicz, L., Pătroescu, M., Niţă, M. R. & Vânau, G. O. Dog walkers’ vs other park visitors’ perceptions: The importance of planning sustainable urban parks in Bucharest, Romania. Landsc. Urban. Plan. 103, 74–82 (2011).
Pătroescu, M., Ioja, C., Necsuliu, R. & Brailescu, C. The quality of oxygenating surfaces. The green areas of Bucharest. A case studies. Rev. Roum. Geogr. 47–48, 205–216 (2004).
Trzyna, T. Urban Protected Areas: Profiles and best practice guidelines. Best Practice Protected Area Guidelines Series No. 22, Gland, Switzerland: IUCN (2014).
Ghiliarov, M.S. & Bregetova, N.G. Opredeliteli obitayushchikh v pochve kleshcheĭ Mesostigmata. (Akademia Nauk USSR, Zoologicheskiĭ Institut Evolyucionoĭ Morfologii i Ekologii zhivotnikh im A.H. Savertova, Izd. Nauka, Leningrad, 1977).
Hyatt, K. H. Mites of the subfamily Parasitinae (Mesostigmata: Parasitidae) in the British Isles. Bull. Br. Mus. Nat. Hist. Zool. 38, 237–378 (1980).
Karg, W. Acari (Acarina), Milben Parasitiformes (Anactinochaeta) Cohors Gamasina Leach. 59, 1–513 (1993).
Mašán, P. Macrochelid Mites of Slovakia (Acari, Mesostigmata, Macrochelidae) (Institute of Zoology, Slovak Academy of Science, Bratislava, 2003).
Mašán, P. Identification key to Central European species of Trachytes (Acari: Uropodina) with redescription, ecology and distribution of Slovak species. Eur. J. Entomol. 100, 435–448 (2003).
Mašán, P. & Fenďa, P. Zerconid Mites of Slovakia (Acari, Mesostigmata, Zerconidae (Institute of Zoology, Slovakia Academy of Science, Bratislava, 2004).
Mašán, P. A Review of the Family Pachylaelapidae in Slovakia with Systematics and Ecology of European Species (Acari: Mesostigmata: Eviphidoidea) (Institute of Zoology, Slovak Academy of Science, Bratislava, 2007).
Mašán, P., Fenďa, P. & Mihál, I. New edaphic mites of the genus Veigaia from Slovakia and Bulgaria, with a key to the European species (Acari, Mesostigmata, Veigaiidae). Zootaxa. 1897, 1–19 (2008).
Mášan, P. & Halliday, B. Review of the European genera of Eviphididae (Acari: Mesostigmata) and the species occurring in Slovakia. Zootaxa. 2585, 1–122 (2010).
Oksanen, J., Blanchet, G.F., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., Minchi, P.R., O'Hara, R.B., Simpson, G.L., Solymos, P., Stevens, M.H., Szoecs, E., Wagner, H. Vegan: Community Ecology Package. R package version 2.4–0. https://cran.r-project.org/package=RVAideMemoire (2019).
Herve, ́ M. RVAideMemoire: Testing and Plotting Procedures for Biostatistics. R package version 0.9-66. https://CRAN.R-project.org/package=RVAideMemoire (2017).
Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference: A Practical Information—Theoretic Approach (Springer, New York, 2002).
Johnson, J. B. & Omland, K. S. Model selection in ecology and evolution. Trends Ecol. Evol. 19, 101–108 (2004).
Hammer, Ø., Harper, D. A. T. & Ryan, P. D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4, 1–9 (2001).
Ruf, A. A maturity index for predatory soil mites (Mesostigmata, Gamasina) as an indicator of environmental impacts of pollution of forest soils. Appl. Soil Ecol. 9, 447–452 (1998).
De Caceres, M., Legendre, P. Associations Between Species and Groups of Sites: Indices an Statistical Inference. Ecology. http://sites.google.com/site/miqueldecaceres (2009).
Dufrêne, M. & Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monog. 67(3), 345–366 (1997).
Zaharia, V. & Găitănaru, D. Aspects of water budget in Văcăreşti wetland. Math. Model. Civ. Eng. 14(1), 12–23 (2018).
Xu, G.-L., Kuster, T. M., Günthardt-Goerg, M. S., Dobbertin, M. & Li, M.-H. Seasonal exposure to drought and air warming affects soil collembola and mites. PLoS ONE 7(8), e43102 (2012).
Gülser, C. & Candemir, F. Changes in penetration resistance of a clay field with organic waste applications. Eurasian J. Soil Sci. 1, 16–21 (2012).
Bergamin, A. C. et al. Relationship of soil physical quality parameters and maize yield in a Brazilian Oxisol. Chil. J. Agric. Res. 75(3), 357–365 (2015).
Jones, M. F. & Arp, P. A. Relating cone penetration and rutting resistance to variations in forest soil properties and daily moisture fluctuations. Open J. Soil Sci. 7, 149–171 (2017).
Ekschmitta, K., Liub, M., Vettera, S., Foxa, O. & Wolters, V. Strategies used by soil biota to overcome soil organic matter stability—why is dead organic matter left over in the soil?. Geoderma 128, 167–176 (2005).
Gulvik, M. E., Błoszyk, J., Austad, I., Bajaczyk, R. & Piwczyński, D. Abundance and diversity of soil microarthropod communities related to different land use regime in a traditional farm in Western Norway. Pol. J. Ecol. 56(2), 273–288 (2008).
Newman, A. C. D. The significance of clays in agriculture and soils. Philos. Trans. R. Soc. Lond A. 311, 375–389 (1984).
Shen, C. et al. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol. Biochem. 57, 204–211 (2013).
Lăcătuşu, R., Lăcătuşu, A. R., Lungu, M. & Breaban, I. G. Macro- and microelements abundance in some urban soils from Romania. Carpath. J. Earth Environ. Sci. 3(1), 75–83 (2008).
Chikoski, J. M., Ferguson, S. H. & Meyer, L. Effects of water addition on soil arthropods and soil characteristics in a precipitation-limited environment. Acta Oecol. 30, 203–211 (2006).
Nitzu, E. et al. Scree habitats: Ecological function, species conservation and spatial-temporal variation in the arthropod community. Syst. Biodivers. 12(1), 65–75 (2014).
Acknowledgements
This study was carried out in the framework of the project RO1567-IBB01/2020 of the Institute of Biology Bucharest of Romanian Academy and the data processing in the project RESET number 453/2020 PED, code PN-III-CERC-CO-PED-2-2019. We thank Simona Plumb, Rodica Iosif and Lazăr Dumitru for their assistance in the lab and the field.
Author information
Authors and Affiliations
Contributions
M.M. and R.I.B. designed the study, analysed and interpreting the data. M.M. and R.I.B. wrote the manuscript. R.I.B. provided the statistical analysis. M.M. provided the taxonomical data for Mesostigmata. M.O. provided the acquisition of the financial support for the project leading to this publication. M.O. has the management and coordination responsibility for the research activity planning and execution. C.C.B. was responsible for the investigation of the environmental parameters; O.M. and M.O. analysed and interpreted the data of vegetation. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Manu, M., Băncilă, R.I., Bîrsan, C.C. et al. Soil mite communities (Acari: Mesostigmata) as indicators of urban ecosystems in Bucharest, Romania. Sci Rep 11, 3794 (2021). https://doi.org/10.1038/s41598-021-83417-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-83417-4
- Springer Nature Limited
This article is cited by
-
Assemblages of Acari in shallow burials: mites as markers of the burial environment, of the stage of decay and of body-cadaver regions
Experimental and Applied Acarology (2021)