Abstract
Current real-time high-throughput Polymerase Chain Reaction (qPCR) methods do not distinguish serotypes 6A from 6B, 18C from 18A/B and 22F from 22A. We established a nanofluidic real-time PCR (Fluidigm) for serotyping that included Dual-Priming-Oligonucleotides (DPO), a Locked-Nucleic-Acid (LNA) probe and TaqMan assay-sets for high-throughput serotyping. The designed assay-sets target capsular gene wciP in serogroup 6, wciX and wxcM in serogroup 18, and wcwA in serogroup 22. An algorithm combining results from published assay-sets (6A/B/C/D; 6C/D; 18A/B/C; 22A/F) and designed assay-sets for 6A/C; 18B/C/F; 18C/F, 18F and 22F was validated through blind analysis of 1973 archived clinical samples collected from South African children ≤ 5-years-old (2009–2011), previously serotyped with the culture-based Quellung method. All assay-sets were efficient (92–101%), had low variation between replicates (R2 > 0.98), and were able to detect targets at a limit of detection (LOD) of < 100 Colony-Forming-Units (CFU)/mL of sample. There was high concordance (Kappa = 0.73–0.92); sensitivity (85–100%) and specificity (96–100%) for Fluidigm compared with Quellung for serotyping 6A; 6B; 6C; 18C and 22F. Fluidigm distinguishes vaccine-serotypes 6A, 6B, 18C, next-generation PCV-serotype 22F and non-vaccine-serotypes 6C, 6D, 18A, 18B, 18F and 22A. Discriminating single serotypes is important for assessing serotype replacement and the impact of PCVs on vaccine- and non-vaccine serotypes.
Similar content being viewed by others
Introduction
Streptococcus pneumoniae (pneumococcus) remains a leading cause of morbidity and mortality globally, causing an estimated 294 000 deaths (uncertainty range [UR] 192 000–366 000) in children 0–59 months without HIV in 20151. Current PCV formulations include vaccine-serotypes (VT) 6A (PCV13), 6B and 18C (PCV13, PCV10). Serotype 22F is included in next-generation PCV15 and PCV202,3. Before the introduction of PCVs, serogroup 6 was associated with 14–18% of invasive pneumococcal disease (IPD) episodes worldwide, whereas serotype 18C was the fifth most common serotype identified in IPD cases in developed nations4,5. Since the introduction of PCV, IPD due to serotypes 6A, 6B, and 18C have declined, however, 6A is still a common VT identified in carriage studies6,7. Serotype 22F has a high invasive disease potential and is increasing globally8.
The referent standard for pneumococcus detection and serotype identification has been the culture-based Quellung method which is not very sensitive and is costly9,10. Real-time PCR provides faster diagnosis and higher sensitivity11; however, due to genetic similarity some serotypes have been difficult to distinguish with PCR. Serogroups 6A/B; 6C/D; 18A/B/C and 22A/F were detected using real-time PCR11 and further distinguished with sequencing-based methods12,13,14 or conventional PCR combined with gel electrophoresis15. Sequential multiplex PCR has now been expanded to differentiate serogroups 6 and 22 but not 1816. Distinguishing serogroups 6, 18, and 22 into single serotypes entirely in a high-throughput comprehensive molecular reaction-set has not been described.
Serotypes within serogroups 6, 18 and 22 are antigenically, biochemically, and genetically distinct13,17,18,19,20,21. The genetic basis of capsule variation in 6A and 6C relative to 6B and 6D involves a single nucleotide polymorphism (SNP) in the rhamnosyl-transferase gene (wciPα in 6A/C, wciPβ in 6B/D)18,22,23. Serotype 6B ‘sub-class II’ (6E) is a genetic variant expressing a 6B capsule that is neither biochemically nor antigenically distinct from 6B24. Serotypes 6F, 6G, and 6H express hybrid capsules, serologically and biochemically distinct within serogroup 625,26. Serotype 18A does not contain the acetyl-transferase gene (wciX) present in 18B/C/F. Relative to 18C/F, serotype 18B contains a SNP in wciX. Serotype 18F contains an additional acetyl-transferase gene (wcxM), also present in 16F and 28AF21. Within serogroup 22 the genetic locus from wcwA (glycosyl-transferase gene) to wcwC (acetyl-transferase gene) is heterogeneous13.
In this study, nanofluidic real-time PCR (Fluidigm®) was used to distinguish individual serogroup 6 and serogroup 18 serotypes by including Dual Priming Oligonucleotide (DPO) forward primers to target the SNPs for 6A/C and 18C/F. Further, a thermodynamically modified Locked Nucleic Acid (LNA) probe was designed to detect 18C/F. Modified primer and probe strategies have not previously been utilised within the Fluidigm system.
Results
Performance of Fluidigm for identification of individual serotypes
When assessed using the standard culture control strains, all assays and applied algorithms amplified their respective targets effectively. The efficiency (90–110%) and slope (− 3.6 ≥ slope ≥ − 3.3) of each of the serogroup 6, 18 and 22 assay-sets were within the prescribed ranges in amplifying control strains of known CFU/mL, with low variation between replicates (R2 > 0.98) (Table 1; Fig. 1).
Standard curves constructed from duplicate serial dilutions to derive the linear equation, efficiency, and reproducibility for each serogroup 6, 18 and 22 assay-set. Panel A shows the culture standard for serotype 6B assessed with the assay-set to detect 6A/B/C/D; panel B is 6A assessed with the 6A/C assay-set; panel C is 6C assessed with the 6C/D assay-set; panel D is 6D with the 6C/D assay-set; panel E is 18C with the 18A/B/C/F assay-set; panel F is 18C with the 18B/C/F assay-set; panel G is 18C with the 18C/F assay-set; panel H is 18F with the 16F/18F/28A/F assay-set; panel I is 22A with the 22A/F assay-set and panel J is 22F with the 22F assay-set.
The modified oligonucleotide strategies, including the DPO assays targeting 6A/C and 18C/F, combined with an LNA probe (18C/F), designed as part of this study were efficient (95% and 98% respectively), had low variation between replicates (R2 = 0.99), and were able to detect 6A/C and 18C/F at a LOD of 102 CFU/mL (Table 1). Further, there was no cross-reactivity between serogroup 6 assay-sets (four serotypes) or serogroup 18 assay-sets (four serotypes) and the other 82 control strains for pneumococcal serotypes and eight nasopharyngeal colonisers included but not targeted by these assays.
Detection of individual serotypes by Fluidigm® and culture
In the 1973 archived clinical samples, the serogroup 6 assay-sets and the applied algorithms were able to distinguish serogroup 6 to individual serotypes with 96–99% specificity, 85–100% sensitivity and excellent concordance compared to Quellung for 6A,6B and 6C (Kappa = 0.86, 0.73 and 0.91 respectively). Serotype 18C was distinguished within serogroup 18 with 99.9% specificity, and 88.9% sensitivity and with excellent concordance compared to uellung (Kappa = 0.86). Finally, serotype 22F was detected with 100% specificity, 85.7% sensitivity and excellent concordance (Kappa = 0.92) compared with Quellung. There was no significant difference between Quellung and Fluidigm for detection of individual serotypes 6A, 6C, 18C and 22F (McNemar’s test: p > 0.05). There was a significant difference in detection of serotype 6B (McNemar’s test: p < 0.01), where 6B was detected in 73 additional samples with Fluidigm. Fluidigm detected the same serotypes as Quellung for most of these samples (83%; n = 43/52) in addition to serotype 6B. In the remaining samples no pneumococcus was detected by Quellung (n = 12) or were allocated as 6A (n = 9) due to the bacterial load for these samples approaching the limit of detection and not detected by the assay-set targeted at 6A/C.
Specificity, sensitivity, and concordance could not be assessed for serotypes 6D, 18A, 18B, 18F and 22A as these are minor serotypes and no positive samples were detected by Quellung for comparison with Fluidigm.
Discussion
The novel DPO, LNA, and other assay-sets designed here, combined with previously published serogroup 6, 18, and 22 assay-sets effectively discriminated between current PCV-formulation (PCV10, PCV13) VT (6A and 6B, 18C), next-generation PCV-formulation (PCV15, PCV20) VT 22F and NVT (6C, 6D, 18A, 18B, 18F and 22A) within a nanofluidic molecular serotyping reaction-set. Serogroups 6, 18, and 22 have previously been discriminated using conventional PCR15,27,28, or sequencing-based methods12,13,29. Sequencing-based methods are expensive, not all laboratories have access to sequencing platforms, bioinformatics software and expertise. Further, some methods were unable to fully distinguish 6C/D or 18B/C29. Microarray assays have been developed, but these are also costly and require initial time-consuming culture steps30. Recently the CDC sequential multiplex real-time serotyping PCR was updated with two and three additional assay-sets respectively to detect and discriminate serogroup 6 (6B/D, 6A/B) and 22 (22A/F, 22A, 22F) respectively16. The CDC’s unique 6B/D assay-sets utilized patented ‘BHQ-plus’ probes that include modified C and T nucleotides as duplex stabilizing chemistry to target the SNP that discriminates these serotypes. Our reaction-set was designed for comprehensive and high-throughput serotyping within a single nanofluidic real-time PCR, hence we used a compact strategy of assay-sets by designing just one additional assay-set each to further serotype serogroups 6 and 22. A benefit of our DPO primers is that they are not patented and may be purchased from any manufacturer globally to be incorporated with other probe chemistries if required.
No previous high-throughput real-time PCR assay has been reported to fully distinguish serogroup 6, 18, and 2210,31,32,33 within a single reaction-set or made use of modified primer or probe strategies such as LNA-probes or DPO primers as utilised in this study. This is the first study to validate the use of thermodynamically modified assay-sets within the Fluidigm platform.
Comprehensive accurate serotyping is essential where VTs may still be circulating and to assess the relative benefit of next-generation PCVs that will include serotype 22F. Current published molecular assays, including the serogroup 6 and 18 described here, now enable detection of PCV13-VT 1, 3, 4, 5, 6A, 6B, 14, 18C, 19A, 19F, and 23F individually10,11. Our reaction-set is the first to distinguish all PCV13-serotypes except serogroup 9A/V and 7A/F to single serotypes. Relative to 9 V, 9A has a frameshift deletion in a G-polymer region surrounded by an AT-rich region21. Similarly, 7A lacks a side chain due to a frameshift mutation in the glycosyl transferase (wcwD) gene21 located in a poly-T region within a GC region. These molecular features make the SNPs in 9 V and 7F difficult to target and thermodynamically improbable to distinguish with molecular methods other than sequencing-based approaches.
The performance (sensitivity, specificity, and limit of detection) of the serogroup 6, 18, and 22 reaction-sets in the Fluidigm platform is comparable with other high-throughput strategies including the TaqMan Array Cards and the same Fluidigm platform including using different real-time PCR chemistry10,33,34. The performance of this reaction-set demonstrates that modified primers (DPO) or probes (LNA) are easily adapted to high-throughput real-time PCR, including where specific target pre-amplification is undertaken in multiplex (up to 34 assay-sets per tube). The successful combination of a DPO primer with a LNA probe to target 18C/F is a novel strategy. There is capacity for 96 samples and controls to be tested against 96 targets within a single run for the Fluidigm 96.96 Dynamic Array IFC (integrated fluidic circuit) that we utilized in the study, however the reaction-sets described here can be easily adapted to other real-time PCR platforms for lower through-put applications.
This study was limited in that non-pneumococcal Streptococcus species were not included in the validation of the assay-sets. Previous studies have validated the assay-sets for 6A/B/C/D, 6C/D, 18A/B/C, and 22AF against non-pneumococcal Streptococcus10 and our algorithm included that samples must be detected with the serogroup 6 assay-set (6A/B/C/D), 18A/B/C, and 22AF to be assigned a serogroup 6, 18 or 22 type respectively, hence the designed assays are unlikely to detect other non-pneumococcal species. The methods described here are not currently aimed at detecting the hybrid serotypes 6F/G/H, as no reference specimens or clinical samples were available to validate the detection of these additional serotypes. Serotype 6B ‘sub-class II’ (6E) is a genetic variant of 6B, and the genetic regions targeted here are ubiquitous in 6B sub-classes so would be correctly assigned serotype 6B24. Prospective studies should include surveillance for hybrid serotypes and conduct confirmatory sequencing for isolates typed as serogroup 6 where PCVs are in use and residual serogroup 6 carriage is observed.
The Fluidigm could not be fully evaluated against the Quellung-method for detection of serotypes 6D, 18A/B/F or 22A in clinical isolates as these serotypes were not detected in the archived clinical samples by Quellung, most likely due to their low prevalence in Africa. Nevertheless, our algorithm correctly identified cultured control strains and would be able to correctly discern serotype 6D, 18A, 18F, 22A and 22F in clinical isolates. While the culture-based Quellung method is sensitive and specific, PCR is more sensitive and capable of detecting multiple co-colonising or culture non-typeable serotypes, even at a low density35,36. Genetic heterogeneity or the presence of a target of interest detected with molecular methods are not always accurate predictors of phenotypic capsule type and capsular expression in isolates24. Since the positive predictive value of Quellung is not perfect in cases of multiple serotype carriage or at low density, discrepant serotype positive designations by PCR are resolved as putative by confirming the presence of pneumococcal reference genes37. As Fluidigm PCR is compared to Quellung in this study, this may affect the measured concordance, for example serotype 6B where this serotype was detected in an additional 73 samples with Fluidigm. The benefit of real-time PCR is the enhanced detection of multiple serotypes carried concurrently compared with the Quellung method. Indeed, the serotypes assigned by the Quellung method were detected correctly in addition to serotype 6B in samples with multiple colonizing serotypes detected by our Fluidigm method. Further, the clinical samples that were re-analysed with Fluidigm as part of this study, had undergone multiple freeze–thaw cycles. This may have affected the sensitivity of Fluidigm compared with Quellung.
The DPO assay-sets targeting wciPα (6A/C) and wciX (18C/F) in conjunction with the designed wciX (18B/C/F), wcxM (18F), wcwA (22F), and published serogroup 6, 18 and 22 (6A/B/C/D; 18A/B/C; 22AF)11 and 6C/D38 assay-sets using the applied algorithms, can be used to correctly serotype circulating serogroup 6, 18 and 22 to individual serotypes using Fluidigm® real-time PCR in clinical samples.
Methods
DPO strategies overcome the limitations of primer design by combining the thermodynamic advantages of two isolated priming regions to enable sensitive and specific detection of SNPs in low GC regions39,40. LNA-based probes contain conformation-modified bases with a methylene bridge connecting the 2′-oxygen and 4′-carbon of the ribose ring in a nucleic acid base thereby increasing the melting temperature (Tm) for sensitive detection of low GC target regions41.
Primer design
We included oligonucleotide primers and dye-labelled Minor Grove Binding (MGB) probes targeting serogroup 6 (6A/B/C/D), sub-group 6C/D, subgroup 18A/B/C, and serogroup 22 (22A/F) from published sequences11,38 that have been optimized previously on the Biomark™ HD platform real-time PCR (Fluidigm)33 in the reaction-set.
Genbank FASTA sequences of representative serogroup 6, 18, and 22F capsular genes (Table 2) were aligned separately for each serogroup in BioEdit using ClustalW Multiple alignment42,43. Standard primers and dye-labelled MGB probes were manually designed to detect the genes for wciX in 18B/C/F, wcxM in 18F, and wcwA unique to 22F, based on the Genbank accession numbers in Table 2. Sequences for serotypes 6B ‘sub-class II’ (6E), 6F, 6G, and 6H were included only for in silico analysis of their detection pattern. All oligonucleotide sequences that are listed in Table 2 were analysed to ensure specificity in silico using the Basic Local Alignment Search Tool (BLAST; http://www.ncbi.nlm.nih.gov/BLAST/) prior to inclusion.
To further distinguish individual serogroup 6 and 18 serotypes, DPO primers and a LNA probe were newly designed (Table 3). The forward DPO for serogroup 6A/C and 18C/F were designed based on the Genbank sequence accession numbers in Table 2. The highly specific and shorter 3’ ‘foot’ sequence to target the SNPs in 6A/C and 18C/F were designed based on the capsule gene locus for wciPα, and wciXCF respectively with the ‘interrogating nucleotide’ (to detect the target SNP) located at the penultimate 3’ position. The location of the longer and thermodynamically stable 5’ ‘anchor’ sequence was predicated on the length of the non-complimentary ‘bridge’ sequence (that separates the two priming regions) and the location of the upstream SNP specific ‘foot’ sequence (example for 6A/C in Fig. 2). A standard tagged Integrated DNA Technologies (IDT) PrimeTime® fluorescent probe (6A/C) and a PrimeTime LNA® real-time PCR probe (18C/F) and reverse primers were designed manually for both DPOs.
Schematic representation of the Dual Priming Oligonucleotide (DPO) forward primer (A) for the detection of wciPα and the target 6A/C sequence (B) as an example of the DPO designs used in this study. DPO primers combine the thermodynamic advantages of two priming regions, a short highly specific ‘foot’ sequence terminating at the 3’ end, and a longer stable and sensitive 5’ ‘anchor’ sequence with their action separated by a non-complimentary ‘bridge’ sequence that forms a bubble. The ‘bridge’ keeps the highly specific foot sequence close to the target sequence by connecting it to the anchor, increasing the frequency of target-primer hybrid formation to enable highly specific primer extension. Due to the dual priming nature of these oligonucleotides, the extension will not proceed if the 3’ short foot sequence is mismatched. A1 shows the ‘anchor’ sequence which is the first priming region, A2 is the ‘bridge’ sequence which is non-complimentary to the intervening sequence (B1) which is not a priming region and the second priming region with the ‘foot’ sequence (A3) containing the interrogating nucleotide (A4) which targets the SNP on the wciPα target strand (B2).
The final reaction-set included primer–probe sets which amplified target regions of serogroup 6 types with wciP (6A/B/C/D), wciNβ (6C/D), wciPα (6A/C); serogroup 18 with wciW (18A/B/C), wciX (18B/C/F), wciX (18C/F), wcxM (18F) and serogroup 22 with wcwV (22A/F) and wcwA (22F) (Table 3).
Since each serotype was not targeted individually, an algorithm combining results from published primer–probe sets (6A/B/C/D, 6C/D, 18A/B/C, and 22A/F) and newly designed sets (6A/C, 18BCF, 18C/F, 18F and 22F) was applied to distinguish individual serogroup 6, 18 and 22 serotypes (Table 4 and further described in supplementary).
To confirm the presence of S. pneumoniae, pan-pneumococcal reference genes LytA (pneumococcal autolysin gene)47 and PiaB (permease gene of the pia ABC transporter)48 were also included in the reaction-set. Samples in which a serotype-specific target gene was detected, also needed to be positive for the two pneumococcal reference genes to be confirmed as S. pneumoniae.
Bacterial and pneumococcal reference isolates
Control strains for S. pneumoniae serotypes (1; 2; 3; 4; 5; 6A; 6B; 6C; 6D; 7A; 7B; 7F; 7C; 8; 9A; 9L; 9 N; 9 V; 10A; 10B; 10C; 11A; 11B; 11C; 11D; 11F; 12B; 12F; 13; 14; 15A; 15B; 15C; 15F; 16A; 16F; 17A; 17F; 18A; 18B; 18C; 18F; 19A; 19B; 19C; 19F; 20; 21; 22A; 22F; 23A; 23B; 23F; 24A; 24B; 24F; 25A; 25F; 27; 28A; 28F; 29; 31; 32A; 32F; 33A; 33B; 33C; 33D; 33F; 34; 35A; 35C; 35F; 35B; 36; 37; 38; 39; 40; 41A; 42; 43; 44; 45; 46; 47A; 47F; 48) and other nasopharyngeal species (Acinetobacter baumannii, Haemophilus influenzae, Klebsiella pneumoniae, Moraxella catarrhalis, Neisseria lactamica, Neisseria meningitidis, Staphylococcus aureus and Streptococcus pyogenes) were obtained from the National Institute for Communicable Diseases, South Africa, Murdoch Children’s Research Institute, Australia and Vaccines and Infectious Diseases Analytics Research Unit, Soweto, South Africa. DNA from these strains were used to optimize the PCR assay-sets.
Bacterial culture and DNA extraction
Control isolates were grown according to standard microtiter culture methods to quantify their relative density (colony forming units, CFU/mL) for use as reference standards and quantification calibrators. The density of viable cells (CFU/mL) was calculated using a serial dilution method (further detailed in Supplementary). Total DNA was extracted from 1 ml THB or BHI into 100 μL of elution buffer using the BioMérieux NucliSens easyMAG® automated benchtop nucleic acid extraction system (BioMérieux, Marcy l′Etoile, France) with standard reagents and protocols. Extracted DNA from reference strains were stored at – 20 °C until assayed. Where required, bacterial isolates of known CFU/mL were used as positive template controls, in addition to gBlocks™ synthetic external calibrators (Supplementary Tables S1 and S2).
Clinical samples
Two cross-sectional surveys were undertaken in Agincourt (May–October 2009) and Soweto (May 2010–February 2011) to assess the prevalence of serotype-specific pneumococcal colonisation during the early introduction of PCV7 in South Africa49,50. Nasopharyngeal swabs (NPS) in STGG (Skim-milk-tryptone-glucose-glycerine transport media)51 were collected from households (Agincourt) and children 0–12 years old (Soweto) and serotyped using the Quellung method, as previously described49,50.
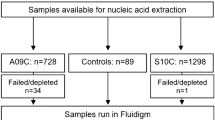
In this study, NPS in STGG from children aged 0 to 5-years-old were re-tested by Fluidigm to validate the assay-set for accurate serotyping in clinical samples compared with the Quellung method. Serotype data for 1973 samples were therefore available for comparison between Fluidigm PCR and Quellung method. Nucleic acids were extracted from 400 µL of STGG and eluted into 100 µL of elution buffer using the BioMérieux NucliSens easyMAG® according to standard manufacturer protocols. No-template-control (NTC) that was STGG alone was included in each batch of clinical samples (n = 23) that were extracted. Nucleic acids were stored at – 20 °C until assayed.
Fluidigm Real-time qPCR
The serogroup 6, serogroup 18 and serogroup 22 assay-sets designed in this study were included in a high-throughput nanofluidic real-time PCR serotyping panel on the Fluidigm platform that was optimised as described previously33 and here was expanded to include other published assay-sets to increase the number of individual serotypes detected. The Fluidigm workflow is summarized in Fig. 3. Primers for all assay-sets were separated into three specific target amplification (STA) multiplex pools (Table S3) to prevent any cross-reactivity of primers.
Flow diagram of the Specific Target Amplification (STA) within single 0.6 mL PCR tubes in the Bio-Rad T100 Thermal Cycler and the nano-fluidic high throughput real-time PCR within the 96.96 Gene Expression (GE) Dynamic Array Integrated Fluidic Circuit (IFC) carried out in the IFC Controller HX and the BioMark HD (Fluidigm).
Specific target amplification/pre-amplification pools
The process of preparing each STA mix is detailed in Fig. 3 and further described in supplementary. For each pool, each included assay-set (n = 30–32 assay-sets per pool; Table S1) were combined to make up 200 µL of a multiplex STA pool. The final STA mix (5 µL) per sample contained 1.25 µL pooled assays at a final concentration of 45 nM for each primer, 1 µL Fluidigm Gene Expression (GE) Pre-Amp reagent, 1.50 µL ddH2O and 1.25 µL of sample. Reactions were amplified in a T100 Thermal Cycler (Bio-Rad, Inc, CA, USA) and cycling conditions were: 95 °C for 2 min, 14 cycles of 95 °C for 15 s and 60 °C for 4 min, and a hold of 4 °C. STA products (5 µL) from the three PCR reactions were then combined and diluted with 10 µL H2O to further dilute any remaining primers to less than 9 nM each in a final volume of 25 µL and tested in the Fluidigm.
The Fluidigm 96.96 GE Dynamic Array IFC™ was primed and loaded according to manufacturer specifications and placed in the Biomark™ HD for Fluidigm PCR, using the following thermal cycling conditions: 50 °C for 2 min, 70 °C for 30 min, 25 °C for 10 min, 50 °C for 2 min, 96.5 °C for 10 min followed by 40 cycles of 96 °C for 15 s, 60 °C for 60 s. Data were analysed with the supplied Real-Time PCR Analysis Software for the BioMark™ HD instrument (Fluidigm® Corporation, CA, USA) using manually defined thresholds and a Cycle of quantification (Cq) cut off value of 35.
Primer optimization
Secondary structures, self-annealing sites, and melting temperature (Tm) of each assay-set were predicted using the online tool OligoCalc52. Duplicate serial dilutions of total DNA from culture controls with CFU/mL of 100 to 106 were used to develop standard curves for all assay-sets to assess efficiency and reproducibility (R2). Triplicate dilutions at 100 to 103 CFU/mL or copy number equivalents (for Synthetic gBlock external calibrators) were included to assess the limit of detection (LOD), that was defined as the lowest concentration that was detected in triplicate. Further, all assays were tested for cross-reactivity against 90 pneumococcal positive culture controls, including individual serotype 6,18, 22 serotypes and 8 other common nasopharyngeal bacterial colonisers.
Assay-set validation for use with clinical samples
To validate the serogroup 6,18 and 22 serotyping reaction-sets for use in clinical isolates, a blind analysis was conducted on 1973 clinical samples described above. Synthetic gBlock external calibrators (positive controls), and no-template controls (NTC) were included within each Fluidigm assay.
Statistical analysis
The slope (m) of the linear equation (y = mx + c) generated from the standard curves of tenfold serial dilutions of calibrators (bacterial culture controls or gBlock) were used to calculate the percent efficiency (E) for each assay-set (E = [10(−1/m)−1] × 100). Analyses were done in Stata, version 13.0, including applying relevant algorithms for serotype assignment. The McNemar’s test was used to determine the sensitivity of the Fluidigm® assay-sets for serotyping serogroups 6, 18 and 22 to individual types compared to the gold standard culture-based Quellung. Cohen’s kappa coefficient was used to concordance for each of the archived clinical samples serotyped using Fluidigm and the described algorithm compared with the Quellung results. Results were considered significant when p-values were ≤ 0.05.
Ethial approval
Ethical consent was obtained from the Medical Human Research Ethics Committee (HREC) of the University of Witwatersrand for the initial collection of the included samples (Soweto Cohort HREC: M090115; Agincourt Cohort HREC: M090114). Informed consent was obtained from all participants and their legal guardians as part of the original study in which the samples were obtained. All experimental protocols were approved by Medical Human Research Ethics Committee (HREC) of the University of Witwatersrand (HREC: M170314). All methods were performed in accordance with the relevant local and international guidelines and regulations for Good Clinical Practice.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding authors on reasonable request.
References
Wahl, B. et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: Global, regional, and national estimates for 2000–15. Lancet Glob. Health 6, e744–e757. https://doi.org/10.1016/S2214-109X(18)30247-X (2018).
Platt, H. L. et al. A phase II trial of safety, tolerability and immunogenicity of V114, a 15-valent pneumococcal conjugate vaccine, compared with 13-valent pneumococcal conjugate vaccine in healthy infants. Pediatr. Infect. Dis. J. 39, 763–770. https://doi.org/10.1097/inf.0000000000002765 (2020).
Hurley, D. et al. Safety, tolerability, and immunogenicity of a 20-valent pneumococcal conjugate vaccine (PCV20) in adults 60 to 64 years of age. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciaa1045 (2020).
Johnson, H. L. et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: The pneumococcal global serotype project. PLoS Med. 7, e1000348. https://doi.org/10.1371/journal.pmed.1000348 (2010).
Pilishvili, T. et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J. Infect. Dis. 201, 32–41. https://doi.org/10.1086/648593 (2010).
Adegbola, R. A. et al. Carriage of Streptococcus pneumoniae and other respiratory bacterial pathogens in low and lower-middle income countries: A systematic review and meta-analysis. PLoS ONE 9, e103293. https://doi.org/10.1371/journal.pone.0103293 (2014).
Balsells, E., Guillot, L., Nair, H. & Kyaw, M. H. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: A systematic review and meta-analysis. PLoS ONE 12, e0177113. https://doi.org/10.1371/journal.pone.0177113 (2017).
Lindstrand, A. et al. Unaltered pneumococcal carriage prevalence due to expansion of non-vaccine types of low invasive potential 8years after vaccine introduction in Stockholm, Sweden. Vaccine 34, 4565–4571. https://doi.org/10.1016/j.vaccine.2016.07.031 (2016).
Habib, M., Porter, B. D. & Satzke, C. Capsular serotyping of Streptococcus pneumoniae using the Quellung reaction. J. Vis. Exp. https://doi.org/10.3791/51208 (2014).
Pholwat, S., Sakai, F., Turner, P., Vidal, J. E. & Houpt, E. R. Development of a TaqMan array card for pneumococcal serotyping on isolates and nasopharyngeal samples. J. Clin. Microbiol. 54, 1842–1850. https://doi.org/10.1128/JCM.00613-16 (2016).
Azzari, C. et al. Realtime PCR is more sensitive than multiplex PCR for diagnosis and serotyping in children with culture negative pneumococcal invasive disease. PLoS ONE 5, e9282. https://doi.org/10.1371/journal.pone.0009282 (2010).
Pai, R., Limor, J. & Beall, B. Use of pyrosequencing to differentiate Streptococcus pneumoniae serotypes 6A and 6B. J. Clin. Microbiol. 43, 4820–4822. https://doi.org/10.1128/JCM.43.9.4820-4822.2005 (2005).
Kapatai, G. et al. Whole genome sequencing of Streptococcus pneumoniae: Development, evaluation and verification of targets for serogroup and serotype prediction using an automated pipeline. PeerJ 4, e2477–e2477. https://doi.org/10.7717/peerj.2477 (2016).
Wyllie, A. L. et al. Sequencing of the variable region of rpsB to discriminate between Streptococcus pneumoniae and other streptococcal species. Open Biol. https://doi.org/10.1098/rsob.170074 (2017).
Jin, P. et al. Simple, accurate, serotype-specific PCR assay to differentiate Streptococcus pneumoniae serotypes 6A, 6B, and 6C. J. Clin. Microbiol. 47, 2470–2474. https://doi.org/10.1128/JCM.00484-09 (2009).
Velusamy, S. et al. Expanded sequential quadriplex real-time polymerase chain reaction (PCR) for identifying pneumococcal serotypes, penicillin susceptibility, and resistance markers. Diagn Microbiol Infect Dis 97, 115037. https://doi.org/10.1016/j.diagmicrobio.2020.115037 (2020).
Park, I. H. et al. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J. Clin. Microbiol. 45, 1225–1233. https://doi.org/10.1128/JCM.02199-06 (2007).
Park, I. H., Park, S., Hollingshead, S. K. & Nahm, M. H. Genetic basis for the new pneumococcal serotype, 6C. Infect. Immun. 75, 4482–4489. https://doi.org/10.1128/IAI.00510-07 (2007).
Bratcher, P. E., Kim, K.-H., Kang, J. H., Hong, J. Y. & Nahm, M. H. Identification of natural pneumococcal isolates expressing serotype 6D by genetic, biochemical and serological characterization. Microbiology 156, 555–560. https://doi.org/10.1099/mic.0.034116-0 (2010).
Song, J.-H., Baek, J. Y. & Ko, K. S. Comparison of capsular genes of Streptococcus pneumoniae serotype 6A, 6B, 6C, and 6D isolates. J. Clin. Microbiol. 49, 1758–1764. https://doi.org/10.1128/JCM.02628-10 (2011).
Mavroidi, A. et al. Genetic relatedness of the Streptococcus pneumoniae capsular biosynthetic loci. J. Bacteriol. 189, 7841–7855. https://doi.org/10.1128/JB.00836-07 (2007).
Mavroidi, A. et al. Evolutionary genetics of the capsular locus of serogroup 6 pneumococci. J. Bacteriol. 186, 8181–8192. https://doi.org/10.1128/JB.186.24.8181-8192.2004 (2004).
da Gloria Carvalho, M. et al. PCR-based quantitation and clonal diversity of the current prevalent invasive serogroup 6 pneumococcal serotype, 6C, in the United States in 1999 and 2006 to 2007. J. Clin. Microbiol. 47, 554–559. https://doi.org/10.1128/JCM.01919-08 (2009).
Burton, R. L., Geno, K. A., Saad, J. S. & Nahm, M. H. Pneumococcus with the “6E” cps locus produces serotype 6B capsular polysaccharide. J. Clin. Microbiol. 54, 967–971. https://doi.org/10.1128/jcm.03194-15 (2016).
Oliver, M. B., van der Linden, M. P., Kuntzel, S. A., Saad, J. S. & Nahm, M. H. Discovery of Streptococcus pneumoniae serotype 6 variants with glycosyltransferases synthesizing two differing repeating units. J. Biol. Chem. 288, 25976–25985. https://doi.org/10.1074/jbc.M113.480152 (2013).
Park, I. H. et al. Genetic, biochemical, and serological characterization of a new pneumococcal serotype, 6H, and generation of a pneumococcal strain producing three different capsular repeat units. Clin. Vaccine Immunol. 22, 313–318. https://doi.org/10.1128/CVI.00647-14 (2015).
Tanmoy, A. M., Saha, S., Darmstadt, G. L., Whitney, C. G. & Saha, S. K. PCR-based serotyping of Streptococcus pneumoniae from culture-negative specimens: Novel primers for detection of serotypes within serogroup 18. J. Clin. Microbiol. 54, 2178–2181. https://doi.org/10.1128/JCM.00419-16 (2016).
Gillis, H. D. et al. PCR-based discrimination of emerging Streptococcus pneumoniae serotypes 22F and 33F. J. Microbiol. Methods 144, 99–106. https://doi.org/10.1016/j.mimet.2017.11.017 (2018).
Gonzales-Siles, L. et al. Identification and capsular serotype sequetyping of Streptococcus pneumoniae strains. J. Med. Microbiol. 68, 1173–1188. https://doi.org/10.1099/jmm.0.001022 (2019).
Turner, P. et al. Improved detection of nasopharyngeal cocolonization by multiple pneumococcal serotypes by use of latex agglutination or molecular serotyping by microarray. J. Clin. Microbiol. 49, 1784. https://doi.org/10.1128/JCM.00157-11 (2011).
Dhoubhadel, B. Y. et al. Bacterial load of pneumococcal serotypes correlates with their prevalence and multiple serotypes is associated with acute respiratory infections among children less than 5 years of age. PLoS ONE 9, e110777. https://doi.org/10.1371/journal (2014).
Sakai, F., Sonaty, G., Watson, D., Klugman, K. P. & Vidal, J. E. Development and characterization of a synthetic DNA, NUversa, to be used as a standard in quantitative polymerase chain reactions for molecular pneumococcal serotyping. FEMS Microbiol. Lett. https://doi.org/10.1093/femsle/fnx173 (2017).
Olwagen, C. P., Adrian, P. V. & Madhi, S. A. Performance of the Biomark HD real-time qPCR System (Fluidigm) for the detection of nasopharyngeal bacterial pathogens and Streptococcus pneumoniae typing. Sci. Rep. 9, 6494. https://doi.org/10.1038/s41598-019-42846-y (2019).
Dhoubhadel, B. G. et al. A novel high-throughput method for molecular serotyping and serotype-specific quantification of Streptococcus pneumoniae using a nanofluidic real-time PCR system. J. Med. Microbiol. 63, 528–539. https://doi.org/10.1099/jmm.0.071464-0 (2014).
Azzari, C. et al. Molecular detection methods and serotyping performed directly on clinical samples improve diagnostic sensitivity and reveal increased incidence of invasive disease by Streptococcus pneumoniae in Italian children. J. Med. Microbiol. 57, 1205–1212. https://doi.org/10.1099/jmm.0.2008/000935-0 (2008).
Olwagen, C. P., Adrian, P. V. & Madhi, S. A. Comparison of traditional culture and molecular qPCR for detection of simultaneous carriage of multiple pneumococcal serotypes in African children. Sci. Rep. 7, 4628. https://doi.org/10.1038/s41598-017-04915-y (2017).
Arguedas, A. et al. Upper respiratory tract colonization with Streptococcus pneumoniae in adults. Expert Rev. Vaccines 19, 353–366. https://doi.org/10.1080/14760584.2020.1750378 (2020).
Sakai, F. et al. Single-plex quantitative assays for the detection and quantification of most pneumococcal serotypes. PLoS ONE 10, e0121064. https://doi.org/10.1371/journal.pone.0121064 (2015).
Chun, J.-Y. et al. Dual priming oligonucleotide system for the multiplex detection of respiratory viruses and SNP genotyping of CYP2C19 gene. Nucleic Acids Res. 35, e40–e40. https://doi.org/10.1093/nar/gkm051 (2007).
Vargas, D. Y., Kramer, F. R., Tyagi, S. & Marras, S. A. E. Multiplex real-time PCR assays that measure the abundance of extremely rare mutations associated with cancer. PLoS ONE 11, e0156546. https://doi.org/10.1371/journal.pone.0156546 (2016).
Braasch, D. A. & Corey, D. R. Locked nucleic acid (LNA): Fine-tuning the recognition of DNA and RNA. Chem. Biol. 8, 1–7. https://doi.org/10.1016/S1074-5521(00)00058-2 (2001).
Hall, T. A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98 (1999).
Thompson, J. D., Higgins, D. G. & Gibson, T. J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680. https://doi.org/10.1093/nar/22.22.4673 (1994).
Bentley, S. D. et al. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2, e31. https://doi.org/10.1371/journal.pgen.0020031 (2006).
Elberse, K. et al. Sequence diversity within the capsular genes of Streptococcus pneumoniae serogroup 6 and 19. PLoS ONE 6, e25018. https://doi.org/10.1371/journal.pone.0025018 (2011).
Jin, P. et al. First report of putative Streptococcus pneumoniae serotype 6D among nasopharyngeal isolates from Fijian children. J. Infect. Dis. 200, 1375–1380. https://doi.org/10.1086/606118 (2009).
da Gloria Carvalho, M. et al. Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA. J. Clin. Microbiol. 45, 2460–2466. https://doi.org/10.1128/JCM.02498-06 (2007).
Brown, J. S., Gilliland, S. M. & Holden, D. W. A Streptococcus pneumoniae pathogenicity island encoding an ABC transporter involved in iron uptake and virulence. Mol. Microbiol. 40, 572–585. https://doi.org/10.1046/j.1365-2958.2001.02414.x (2001).
Nzenze, S. V. G. et al. Temporal changes in pneumococcal colonization in HIV-infected and HIV-uninfected mother-child pairs following transitioning From 7-valent to 13-valent pneumococcal conjugate vaccine Soweto, South Africa. J. Infect. Dis. 212, 1082–1092. https://doi.org/10.1093/infdis/jiv167 (2015).
Nzenze, S. A. et al. Temporal changes in pneumococcal colonization in a rural African community with high HIV prevalence following routine infant pneumococcal immunization. Pediatr. Infect. Dis. J. 32, 1270–1278. https://doi.org/10.1097/01.inf.0000435805.25366.64 (2013).
O’Brien, K. L. et al. Evaluation of a medium (STGG) for transport and optimal recovery of Streptococcus pneumoniae from nasopharyngeal secretions collected during field studies. J. Clin. Microbiol. 39, 1021–1024. https://doi.org/10.1128/JCM.39.3.1021-1024.2001 (2001).
Kibbe, W. A. OligoCalc: An online oligonucleotide properties calculator. Nucleic Acids Res. 35, W43–W46. https://doi.org/10.1093/nar/gkm234 (2007).
Acknowledgements
This work was supported by the Bill & Melinda Gates Foundation [OPP1189378]. There was also partial support from the Department of Science and Technology and National Research Foundation: South African Research Chair Initiative in Vaccine Preventable Diseases; and the South African Medical Research Council. We would like to acknowledge the South African Medical Research Council/Wits Rural Public Health and Health Transitions Research Unit (Agincourt) that facilitated the original sample collections and the National Institute of Communicable Diseases, South Africa where the culture-based Quellung serotyping was originally undertaken. We would also like to acknowledge all the participants from the Agincourt and Soweto enrolments that contributed samples.
Author information
Authors and Affiliations
Contributions
S.L.D., S.A.M., M.C.N and C.P.O. conceived and designed the study. S.L.D. was responsible for molecular assay design, data curation and analysis. C.P.O and S.L.D were responsible for gBlock design. S.L.D. and L.v.d.M. were responsible for lab analysis. Funding acquisition was undertaken by S.L.D., S.A.M., M.C.N and C.P.O. S.L.D drafted the manuscript and all authors reviewed and contributed to editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Downs, S.L., Madhi, S.A., Van der Merwe, L. et al. High-throughput nanofluidic real-time PCR to discriminate Pneumococcal Conjugate Vaccine (PCV)-associated serogroups 6, 18, and 22 to serotypes using modified oligonucleotides. Sci Rep 11, 23728 (2021). https://doi.org/10.1038/s41598-021-03127-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-03127-9
- Springer Nature Limited