Abstract
To verify whether omitting radiotherapy from breast cancer treatment for patients ≥ 70 years old following breast-conserving surgery (BCS) without axillary lymph node dissection is safe. Previous studies have shown that omitting breast radiotherapy after BCS and axillary lymph node dissection is safe for elderly breast cancer patients. We aimed to evaluate the safety of BCS without axillary surgery or breast radiotherapy (BCSNR) in elderly patients with breast cancer and clinically negative axillary lymph nodes. We performed a retrospective analysis of 481 patients with breast cancer, aged ≥ 70 years, between 2010 and 2016. Of these, 302 patients underwent BCSNR and 179 underwent other, larger scope operations. Local recurrence rate, ipsilateral breast tumor recurrence (IBTR) rate, distant metastasis rate, breast-related death, disease-free survival (DFS), and overall survival (OS) were compared between the two groups. After a median follow-up of 60 months, no significant differences in local recurrence, distant metastasis rate, breast-related death, and DFS were noted. The OS was similar (P = 0.56) between the BCSNR group (91.7%) and other operations group (93.0%). The IBTR rate was considered low in both groups, however resulted greater (P = 0.005) in the BCSNR group (5.3%) than in other operations group (1.6%). BCSNR did not affect the survival of elderly patients with breast cancer with clinically negative axillary lymph nodes. IBTR was infrequent in both groups; however, there was a significant difference between the two groups. BCSNR is a feasible treatment modality for patients with breast cancer ≥ 70 years old with clinically negative axillary lymph nodes.
Similar content being viewed by others
Introduction
In women, breast cancer incidence rates far exceed other cancers worldwide, accounting for nearly 1 in 4 cancer cases1. Furthermore, breast cancer is the leading cause of cancer death among women1. The incidence of breast cancer increases with age2,3; in China, the second most frequent age group diagnosed with breast cancer is 70–74 years4. At the time of diagnosis, more than 35% of patients are > 65 years old5. Elderly patients with breast cancer form a large, heterogeneous group, and many patients have concomitant diseases and senility6. Studies have found that the overall survival (OS) rate of patients with complications after surgery is lower than that of patients without complications7. Furthermore, postoperative complications in elderly patients with breast cancer also increase with age.
Breast cancer is classified into five intrinsic subtypes by gene expression microarray: luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)-enriched, basal-like, and normal-like8. Favorable subtypes (luminal tumors) increase with age, whereas subtypes with poor outcomes (basal-like tumors) decrease with age8. The prognosis of elderly patients with breast cancer is good and most of them die from non-breast cancer diseases9. The mortality of patients with breast cancer older than 50 years gradually decreases with age10; therefore, some studies recommend that surgical scope or the use of adjuvant treatments for elderly patients with breast cancer should be reduced. In elderly patients with breast cancer and clinically node-negative disease do not perform axillary clearance yields similar efficacy than perform axillary clearance11. Although radiotherapy is a standard recommendation in patients with breast cancer12, in low- and medium-risk patients, omitting radiotherapy did not cause an increase in mortality13. In the CALGB 9343 trial14, low-risk and elderly patients with breast cancer who underwent systemic therapy were randomly divided into radiotherapy and non-radiotherapy groups. The disease-free survival (DFS) and OS were not significantly different between the two groups.
Therefore, we aimed to evaluate whether the survival of elderly patients with breast cancer is affected when solely local enlarged tumors are excised without axillary lymph node dissection nor radiotherapy.
Materials and methods
The Peking Union Medical College Hospital (PUMCH) database includes the data of patients with breast cancer who have underwent treatment in this hospital since 1975. The data includes age, operation methods, radiotherapy, chemotherapy, endocrine therapy, concomitant diseases, recurrence, metastasis, and other information. Any hard copies of patient records were electronically filed by scanning. The necessary data was extracted and inputted into a new database15.
Since 1975, more than 2000 patients with breast cancer over 70 years old had operations and other treatments at our hospital. This study included 481 patients with breast cancer aged over 70 years. All patients underwent surgery between 2010 and 2016 and underwent other treatments and clinical visits at PUMCH. All patients provided written informed consent.
The study was approved by the Institutional Review Board of Peking Union Medical College Hospital (S-K971). All study participants provided informed consent and all the patients’ data were anonymous. The present research was performed in accordance with relevant regulations.
Patient data
Among 481 patients who underwent operations at our hospital, 302 underwent breast-conserving surgery without axillary lymph node dissection, sentinel lymph node biopsy, or radiotherapy (BCSNR). Patients with a life expectancy of less than 3 months were not included in our study. All patients were informed of the surgical options, including BCSNR, mastectomy plus axillary lymph nodes dissection, mastectomy plus sentinel lymph node biopsy, breast-conserving surgery plus axillary lymph nodes dissection, and breast-conserving surgery plus sentinel lymph node biopsy. Surgeons communicated the operation risks, advantages, and disadvantages to the patients before treatment. The operation method chosen depended on patients’ preferences and doctors’ opinions.
All patients had a preoperative clinical evaluation that included physical examination (PE), ultrasonography, and mammography. If the patients had underwent BCSNR, PE for axillary had been negative with no suspicious calcification on the mammograph or other suspicious tumors in multiple quadrants on the ultrasound. Patients with clinically suspected lymph nodes underwent axillary lymph nodes dissection or sentinel lymph node biopsy. Axillary dissection was performed in patients with enlarged suspicious lymph nodes by PE. Sentinel lymph node biopsy was performed in patients with no enlarged lymph nodes by PE but with suspicious lymph nodes in ultrasonography or mammography. The clinical and radiological free lymph nodes include clear boundary between cortex and medulla, no suspicious blood flow, no abnormal morphology, no cortical thickening and without enlarged lymph nodes by PE.
Follow-up
The median follow-up period was 60 months from the date of operation. The shortest follow-up period was 5 months and the longest was 111 months.
Tumor characteristics
Pathological information from all patients was obtained from the Department of Pathology at PUMCH. Estrogen receptor (ER)- and progestogen receptor (PR)-positive disease were defined by immunohistochemical (IHC) staining of > 1% of cells. HER2-positive was defined by HER2 protein expression IHC 3+ positive and if HER2 was 2+ positive on IHC, we performed immunofluorescence hybridization (FISH) for HER2. FISH was positive if the average HER2 gene copy number ≥ 6.0 signals/cell or HER2/CEP17 ratio ≥ 2.016. T stage was categorized as T1 (< 5 cm), T2 (> 2 cm, ≤ 5 cm), T3 (> 5 cm) or T4 (tumor of any size with direct extension to the chest wall or to the skin). Pathological axillary lymph node stage was categorized as N0 (0 positive axillary lymph nodes), N1 (metastases to 1–3 axillary lymph nodes), N2 (metastases to 4–9 axillary lymph nodes), N3 (metastases to ≥ 10 axillary lymph nodes). Histology type was determined according to the World Health Organization classification17.
Outcome variables
Regional recurrence included local breast recurrence, chest wall recurrence, or axillary lymph node recurrence. Breast cancer metastasis included bone, liver, lung, and brain metastases or distant skin metastasis. DFS was defined as survival without recurrence or metastasis. OS was defined as survival without death. Breast cancer-related death was defined as death caused by the recurrence or metastasis of breast cancer. Non-breast cancer-related death was defined as death caused by a concomitant non-breast cancer disease.
Statistical analysis
All statistical analyses were performed with SPSS 22.0 software. All statistical tests were two-sided, and significance was defined as P < 0.05. Baseline characteristics of patients were analyzed by χ2 and Mann–Whitney U-tests. OS and DFS were calculated using Kaplan–Meier analysis. The associations between recurrence, metastasis, and operation method were evaluated by Mann–Whitney U-tests. Tests for interaction between age group, T stage, histology type, HR status, molecular type, and operation method and DFS and OS were analyzed by univariate Cox regression analysis and a multivariate Cox regression model.
Results
The median age of 481 patients was 76 (range 70–91) years. Of 481 patients, 286 (38.7%) patients were 70–74 years old, 171 (35.6%) patients were 75–79 years old and 124 (25.8%) patients were > 80 years old. Regarding operations, 302 (62.8%) patients underwent BCSNR, 148 (30.8%) underwent mastectomy plus axillary lymph nodes dissection, 18 (3.7%) underwent breast-conserving surgery plus axillary lymph nodes dissection, seven (1.5%) underwent mastectomy plus sentinel lymph node biopsy, and six (1.2%) underwent breast-conserving surgery plus sentinel lymph node biopsy. Thirty patients underwent chemotherapy with capecitabine. Regarding HR status, 382 (79.4%) patients had HR-positive disease and underwent aromatase inhibitor treatment. Regarding breast cancer subtype, 176 (36.6%) patients had the luminal A subtype, 208 (43.2%) had the luminal B subtype, 37 (7.7%) had the HER2-enriched subtype, and 60 (12.5%) had triple-negative breast cancer. Most patients had T1 and T2 stage tumors; 284 (59.0%) patients had T1 stage tumors and 182 (37.8%) patients had T2 tumors. Regarding histological grade, 105 (21.8%) patients had grade 1 and 231 (48.0%) had grade 2 (Table 1).
Recurrence
During follow-up, 31 regional recurrences, of which 17 were ipsilateral breast tumor recurrences (IBTR), and 13 distant recurrences were observed. In patients who underwent BCSNR, 21 regional recurrences, including 16 IBTR and seven distant recurrences, were observed. In patients who underwent other operations, 10 regional recurrences, including one IBTR and six distant recurrences were observed. The IBTR rates between the two groups were significantly different, which was higher in BCSNR group (P = 0.005). However, regional and distant recurrences were not significantly different between the two groups (Table 2).
Survival
There was a total of 48 deaths among the patients. Of these, 11(2.3%) were breast cancer-related and 37 (7.7%) were non-breast cancer-related. In the BCSNR group, there were 6 (2.0%) breast cancer-related deaths and 23 (7.6%) non-breast cancer-related deaths. In the other operations group, there were 5 (2.8%) breast cancer-related deaths and 14 (7.8%) non-breast cancer-related deaths (Table 3). The breast cancer-related and non-breast cancer-related deaths were not significantly different between the two groups.
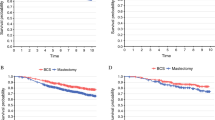
Kaplan–Meier analysis showed that the 5-year DFS was 91.6% and 92.9% in the BCSNR and other operations groups, respectively, and was not significantly different (P = 0.53; Fig. 1). Furthermore, the 5-year OS was 91.7% and 93.0% in the BCSNR and other operations groups, respectively, and was not significantly different (P = 0.56; Fig. 2).
Univariate and multivariate Cox regression analyses showed that patients who underwent BCSNR had a similar DFS to that of patients who underwent other operations. According to the multivariate analysis, using patients who underwent BCSNR as a reference, the hazard ratio for patients who underwent other operations was 0.76 [95% confidence interval (CI) (0.39–1.47); Table 4].
Univariate and multivariate Cox regression analyses showed that patients who underwent BCSNR had a similar OS to that of patients who underwent other operations. According to the multivariate analysis, using patients who underwent BCSNR as a reference, the hazard ratio for patients who underwent other operations was 1.16 [95% CI (0.62–2.17); Table 5].
Associations between DFS and OS and age, T stage, histology type, HR status, and molecular subtype were evaluated. Univariate Cox regression analysis demonstrated that HR status and molecular subtype was significantly associated with DFS. Multivariate analysis demonstrated that molecular subtype was significantly associated with DFS (P = 0.004; Table 4). Univariate Cox regression analysis demonstrated that the T stage, HR status, and molecular subtype were significantly associated with OS. Multivariate analysis demonstrated that age and T stage were significantly associated with OS (P = 0.035 and P = 0.039 respectively; Table 5).
Discussion
With the aging population, the incidence of breast cancer in elderly women is increasing worldwide18. In elderly patients with breast cancer, there is a greater incidence of HR-positive and HER2-negative patients, with a lower incidence of triple-negative breast cancer19. As age increases, breast cancer-related mortality decreases, whereas non-breast cancer-related mortality increases14. The incidence of postoperative complications also increases with age in these patients7. A study on patients with breast cancer ≥ 70 years of age randomly divided patients into two groups, tamoxifen vs. tamoxifen + mastectomy20. There was no significant difference in breast cancer-related deaths after the 10-year follow-up. However, local control in the tamoxifen group was poor at 1.9% compared to 43.0% in the tamoxifen + mastectomy group. Therefore, local operation cannot be omitted for elderly patients with breast cancer. However, some studies suggest that the operation scope and treatment measures for breast cancer can be reduced in elderly patients21.
Martelli et al.21 assessed the long-term safety of forgoing axillary clearance in elderly patients with breast cancer. After a median follow-up of 15 years, there was no significant difference in breast cancer-related mortality between the axillary clearance and no axillary clearance groups. The crude cumulative 15-year incidence of axillary disease in the no axillary clearance group was low at 5.8% for all patients and 3.7% for the pT1 (tumor size < 2 cm) subgroup. The IBCSG Trial 10–9311 also investigated primary surgery plus axillary clearance versus surgery without axillary clearance and found that omitting axillary clearance for elderly women with clinically node-negative disease yielded similar efficacy with better early quality of life. Both studies compared the survival benefits of patients with axillary dissection to those without axillary dissection, however in the former study, 229 of 691 patients underwent breast irradiation, and in the latter study, more than half of the patients underwent breast-conserving surgery with radiotherapy. It has also been reported that sentinel lymph node biopsy can be omitted in elderly patients22. In this study, low-risk patients or those with T1 tumors were recommended not to receive sentinel lymph node biopsy. Additionally, Several studies have also found that the local recurrence rate is relatively low for elderly patients with breast cancer treated with breast-conserving surgery without radiotherapy23,24. Therefore, we aimed to evaluate whether breast-conserving surgery without axillary lymph nodes dissection, sentinel lymph node biopsy, nor radiotherapy affects the survival outcomes of elderly patients.
Our study showed that there was no significant difference in DFS between the BCSNR and other operations groups. Similarly, there was no significant difference between the two groups in OS. Our results are similar to those of two previous studies11,21 reporting axillary lymph node dissection did not affect the survival of elderly patients with breast cancer. In Trial 10–93, results for surgery plus axillary clearance and surgery without axillary clearance yielded similar OS (75% vs 73%; P = 0.77) at a median follow-up of 6.6 years11. In Martelli’s study, the 15-year crude cumulative incidence of breast cancer death was 14.0% in the no axillary dissection group and 13.6% in the axillary dissection group (P = 0.657)21. However, in our study, 302 patients who underwent breast-conserving surgery did not receive radiotherapy. The IBTR rate in patients who underwent BCSNR was significantly higher than in patients who underwent other operations. This result is similar to those of the PRIME II study25. In that study, 658 women who underwent breast-conserving surgery were randomly assigned to receive whole-breast irradiation and 668 did not receive whole-breast irradiation. After 5 years of follow-up, the IBTR rate was 1.3% in women who underwent radiotherapy and 4.1% in those assigned no radiotherapy (P = 0.0002). The authors of that study suggested that the IBTR rate was low enough for the omission of radiotherapy to be considered for some patients. The CALGB 9343 trial reached similar conclusions14. In that clinical trial, 636 patients (age ≥ 70 years) who underwent lumpectomies were randomly assigned to receive tamoxifen plus radiation therapy or tamoxifen alone. After 10 years of follow-up, 98% of the patients receiving tamoxifen plus radiation were free from local and regional recurrences compared to 90% of those receiving tamoxifen alone (95% CI 85–93%).
Furthermore, we found that HR status, molecular subtype, age, and T stage were related to the prognosis of patients. HR status was significantly associated with DFS by univariate analysis, and the prognoses of patients with HR-positive disease were better than those with HR-negative disease. Molecular subtype was significantly correlated with DFS by multivariable analysis, and the prognoses of the luminal type subgroups were significantly better than those of the poor molecular type subgroups. Similar to the findings of Syed et al.9, the higher the HR-positive rate in elderly patients, the better the prognosis was. T stage was significantly correlated with OS by multivariate analysis, and the prognoses of patients with small tumor size were significantly better than those of patients with large tumor size. This result is similar to those reported by Hongchao et al.26, in which the risk of breast cancer-related death was dependent on T status, and patients with early-T stage disease had better prognoses than those with late-T stage disease. We found that the risk of death in patients aged 75–79 years was significantly lower than that in patients aged 70–74 years, in line with previous reports10; as patient age increased, breast cancer-related mortality decreased. Univariate Cox regression showed that age had no significant correlation with DFS, but it was related to OS, indicating that the prognosis of elderly breast cancer patients may be closely related to their own basic diseases and physical conditions, but not to breast cancer disease itself and the operation method we choose. There was no significant difference in OS between patients over 80 years old and those 70–74 years old, which may be due to the confounding factor of non-breast cancer-related death.
In our study, we evaluated the recurrence, metastasis, and survival results of elderly patients with breast cancer who underwent BCSNR. We further compared these patients with those who underwent surgery involving other methods, including breast-conserving surgery plus axillary lymph nodes dissection or sentinel lymph node biopsy and mastectomy plus axillary lymph nodes dissection or sentinel lymph node biopsy. We have demonstrated that BCSNR is safe and reliable for elderly patients with breast cancer. Future studies should include more patients and better match the clinical characteristics of patients who receive BCSNR with those who have other operations. Additionally, prolonged follow-up periods could further support the results of future studies.
In conclusion, we found that elderly patients with breast cancer treated with BCSNR had similar DFS and OS to patients who underwent larger scope operations. Therefore, BCSNR is a feasible treatment modality for patients with breast cancer ≥ 70 years old with clinically negative axillary lymph nodes.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. https://doi.org/10.3322/caac.21492 (2018).
Cleries, R. et al. Assessing predicted age-specific breast cancer mortality rates in 27 European countries by 2020. Clin. Transl. Oncol. 20, 313–321. https://doi.org/10.1007/s12094-017-1718-y (2018).
Hery, C., Ferlay, J., Boniol, M. & Autier, P. Changes in breast cancer incidence and mortality in middle-aged and elderly women in 28 countries with Caucasian majority populations. Ann. Oncol. 19, 1009–1018. https://doi.org/10.1093/annonc/mdm593 (2008).
Fan, L. et al. Breast cancer in China. Lancet Oncol. 15, e279-289. https://doi.org/10.1016/S1470-2045(13)70567-9 (2014).
DeSantis, C., Ma, J., Bryan, L. & Jemal, A. Breast cancer statistics, 2013. CA Cancer J. Clin. 64, 52–62. https://doi.org/10.3322/caac.21203 (2014).
Kiderlen, M. et al. Impact of comorbidity on outcome of older breast cancer patients: A FOCUS cohort study. Breast Cancer Res. Treat. 145, 185–192. https://doi.org/10.1007/s10549-014-2917-7 (2014).
de Glas, N. A. et al. Postoperative complications and survival of elderly breast cancer patients: A FOCUS study analysis. Breast Cancer Res. Treat. 138, 561–569. https://doi.org/10.1007/s10549-013-2462-9 (2013).
Jenkins, E. O. et al. Age-specific changes in intrinsic breast cancer subtypes: A focus on older women. Oncologist 19, 1076–1083. https://doi.org/10.1634/theoncologist.2014-0184 (2014).
Syed, B. M. et al. Long-term clinical outcome of oestrogen receptor-positive operable primary breast cancer in older women: A large series from a single centre. Br. J. Cancer 104, 1393–1400. https://doi.org/10.1038/bjc.2011.105 (2011).
Ali, A. M., Greenberg, D., Wishart, G. C. & Pharoah, P. Patient and tumour characteristics, management, and age-specific survival in women with breast cancer in the East of England. Br. J. Cancer 104, 564–570. https://doi.org/10.1038/bjc.2011.14 (2011).
International Breast Cancer Study, G et al. Randomized trial comparing axillary clearance versus no axillary clearance in older patients with breast cancer: First results of International Breast Cancer Study Group Trial 10–93. J. Clin. Oncol. 24, 337–344. https://doi.org/10.1200/JCO.2005.01.5784 (2006).
Early Breast Cancer Trialists’ Collaborative, G et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378, 1707–1716. https://doi.org/10.1016/S0140-6736(11)61629-2 (2011).
Killander, F. et al. No breast cancer subgroup can be spared postoperative radiotherapy after breast-conserving surgery. Fifteen-year results from the Swedish Breast Cancer Group randomised trial, SweBCG 91 RT. Eur. J. Cancer 67, 57–65. https://doi.org/10.1016/j.ejca.2016.08.001 (2016).
Hughes, K. S. et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: Long-term follow-up of CALGB 9343. J. Clin. Oncol. 31, 2382–2387. https://doi.org/10.1200/JCO.2012.45.2615 (2013).
Zhong, Y. et al. Breast-conserving surgery without axillary lymph node surgery or radiotherapy is safe for HER2-positive and triple negative breast cancer patients over 70 years of age. Breast Cancer Res. Treat. 182, 117–126. https://doi.org/10.1007/s10549-020-05686-3 (2020).
Wolff, A. C. et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 31, 3997–4013. https://doi.org/10.1200/JCO.2013.50.9984 (2013).
Swerdlow, S. H. WHO Classification of Tumours of the Breast, 4th edn (2012).
Hery, C., Ferlay, J., Boniol, M. & Autier, P. Quantification of changes in breast cancer incidence and mortality since 1990 in 35 countries with Caucasian-majority populations. Ann. Oncol. 19, 1187–1194. https://doi.org/10.1093/annonc/mdn025 (2008).
Schonberg, M. A. et al. Breast cancer among the oldest old: Tumor characteristics, treatment choices, and survival. J. Clin. Oncol. 28, 2038–2045. https://doi.org/10.1200/JCO.2009.25.9796 (2010).
Johnston, S. J. et al. A randomised trial of primary tamoxifen versus mastectomy plus adjuvant tamoxifen in fit elderly women with invasive breast carcinoma of high oestrogen receptor content: Long-term results at 20 years of follow-up. Ann. Oncol. 23, 2296–2300. https://doi.org/10.1093/annonc/mdr630 (2012).
Martelli, G. et al. Axillary dissection versus no axillary dissection in elderly patients with breast cancer and no palpable axillary nodes: Results after 15 years of follow-up. Ann. Surg. Oncol. 18, 125–133. https://doi.org/10.1245/s10434-010-1217-7 (2011).
Welsh, J. L. et al. Predicting nodal positivity in women 70 years of age and older with hormone receptor-positive breast cancer to aid incorporation of a society of surgical oncology choosing wisely guideline into clinical practice. Ann. Surg. Oncol. 24, 2881–2888. https://doi.org/10.1245/s10434-017-5932-1 (2017).
Hughes, K. S. et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N. Engl. J. Med. 351, 971–977. https://doi.org/10.1056/NEJMoa040587 (2004).
Fyles, A. W. et al. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N. Engl. J. Med. 351, 963–970. https://doi.org/10.1056/NEJMoa040595 (2004).
Kunkler, I. H. et al. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): A randomised controlled trial. Lancet Oncol. 16, 266–273. https://doi.org/10.1016/S1470-2045(14)71221-5 (2015).
Pan, H. et al. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med. 377, 1836–1846. https://doi.org/10.1056/NEJMoa1701830 (2017).
Funding
This study was supported by the Natural Science Foundation of Beijing Municipality (Grant number: 7172168) and Fundamental Research Funds for the Central Universities (3332018020). The authors declare no competing interests.
Author information
Authors and Affiliations
Contributions
Y.Z. and Y.X. wrote the main manuscript text; Y.Z., F.M., Y.L. and J.G. collected and arranged patients’ data; S.S., B.P., C.W. conducted statistical analysis; L.P., X.H., and X.W. prepared tables and figures; Q.S. supported quality management and directed team.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhong, Y., Xu, Y., Zhou, Y. et al. Omitting radiotherapy is safe in breast cancer patients ≥ 70 years old after breast-conserving surgery without axillary lymph node operation. Sci Rep 10, 19481 (2020). https://doi.org/10.1038/s41598-020-76663-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-76663-5
- Springer Nature Limited