Abstract
Cardiac and aortic characteristics are crucial for cardiovascular disease detection. However, noninvasive estimation of aortic hemodynamics and cardiac contractility is still challenging. This paper investigated the potential of estimating aortic systolic pressure (aSBP), cardiac output (CO), and end-systolic elastance (Ees) from cuff-pressure and pulse wave velocity (PWV) using regression analysis. The importance of incorporating ejection fraction (EF) as additional input for estimating Ees was also assessed. The models, including Random Forest, Support Vector Regressor, Ridge, Gradient Boosting, were trained/validated using synthetic data (n = 4,018) from an in-silico model. When cuff-pressure and PWV were used as inputs, the normalized-RMSEs/correlations for aSBP, CO, and Ees (best-performing models) were 3.36 ± 0.74%/0.99, 7.60 ± 0.68%/0.96, and 16.96 ± 0.64%/0.37, respectively. Using EF as additional input for estimating Ees significantly improved the predictions (7.00 ± 0.78%/0.92). Results showed that the use of noninvasive pressure measurements allows estimating aSBP and CO with acceptable accuracy. In contrast, Ees cannot be predicted from pressure signals alone. Addition of the EF information greatly improves the estimated Ees. Accuracy of the model-derived aSBP compared to in-vivo aSBP (n = 783) was very satisfactory (5.26 ± 2.30%/0.97). Future in-vivo evaluation of CO and Ees estimations remains to be conducted. This novel methodology has potential to improve the noninvasive monitoring of aortic hemodynamics and cardiac contractility.
Similar content being viewed by others
Introduction
Clinical parameters directly measured in the heart or at the root of the aorta are crucial for detection, diagnosis, prognosis, treatment, and management of cardiovascular diseases1,2,3,4. Aortic hemodynamics, such as aortic systolic blood pressure (aSBP) and cardiac output (CO), are direct and more informative parameters for assessing cardiovascular health than corresponding measurements obtained at the peripheral arteries1,5,6. However, the gold standard techniques for measuring aSBP and CO are catheter-based and expensive7,8. Furthermore, there is a need for noninvasive estimation of cardiac contractility. End-systolic elastance (Ees), i.e., the slope of the end-systolic pressure–volume relation (ESPVR), is a pivotal determinant of left ventricular (LV) systolic performance and a powerful index of the arterio-ventricular interaction4,9,10. Despite its clinical importance, the clinical use of this measure is limited by the need for invasive acquisition of multiple LV pressure–volume loops under varying loading conditions11.
Peripheral blood pressure (BP) measurements acquired by cuff sphygmomanometry have a fundamental role in the everyday clinical setting12. Recognizing the important differences between peripheral and central aortic pressures, significant efforts were oriented towards the noninvasive estimation of aortic hemodynamics, in particular aSBP, based on peripheral pressure measurements13. Among commonly used approaches for obtaining aSBP are generalized transfer functions (GTFs)14,15,16, moving average models17,18 and pulse wave analysis-based methods8,19,20. Nevertheless, the totality of them relies on the acquisition of the entire peripheral pressure waveform which can be tedious and susceptible to errors21.
Prediction of CO constitutes a more challenging task due to its dependency on the patient-specific arterial dimensions22. Noninvasive CO monitoring has been addressed using single-beat pulse contour analysis23,24,25 which, however, allows for the derivation of only an uncalibrated estimation instead of the absolute CO value. Finally, notable studies have been developed and validated against invasive techniques for estimating Ees for a single cardiac cycle26,27. The first fully noninvasive method was introduced by Chen et al.26. They proposed a simple equation to derive Ees from pressure arm-cuffs, echo-Doppler cardiography and electrocardiograms.
Despite the good precision of previous techniques, there has been no holistic and complete study to investigate the possibility of estimating aortic hemodynamics and cardiac contractility using readily available noninvasive measurements on the same population. This is mainly attributed to two inherent limitations, i.e., the lack of invasive data in a large scale and the ethical limitation to perform invasive measurements on a healthy population, if no diagnostic reason has been provided.
Cardiovascular models hold a valuable position for addressing the challenge of limited access on in-vivo data28,29. They constitute a faithful representation of the real cardiovasculature and allow the study of pathophysiological mechanisms and diseases30,31. Furthermore, they can provide a complete set of parameters to describe the system, whereas the simulated signals are noise-free.
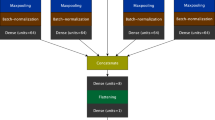
The present study aimed to evaluate whether aortic hemodynamics (i.e., aSBP and CO) and cardiac contractility (i.e., Ees) can be accurately predicted by the use of brachial systolic blood pressure (brSBP) and diastolic blood pressure (brDBP), heart rate (HR), carotid-to-femoral pulse wave velocity (cfPWV), and, if necessary, ejection fraction (EF). These quantities were chosen as they are readily available in clinical practice and have been shown to provide information on the cardiovascular state2,3,4,32. To overcome the aforementioned limitations, we performed our experiments using synthetic data (n = 4,018), which were generated using a previously validated one-dimensional (1-D) mathematical model of the cardiovascular system28. Regression analysis was performed to establish the relationship between the noninvasive measurements (brSBP, brDBP, HR, cfPWV, (and EF)) and the invasive quantities of interest (aSBP, CO, and Ees). The regression pipeline of the present study is presented in Fig. 1. A ten-fold cross validation (CV) scheme was employed for the training/testing of the proposed approach. We evaluated four models including Random Forest33, Support Vector Regressor (SVR)34, Ridge35, and Gradient Boosting36. In addition, averaging of the multiple predictions was performed. Two approaches were investigated: (i) prediction of aSBP, CO, and Ees using brSBP, brDBP, HR, and cfPWV as inputs, and (ii) prediction of Ees using brSBP, brDBP, HR, cfPWV, and EF. The accuracy of our prediction was evaluated by comparing the model-derived values with the reference simulated data. The accuracy of the aSBP model was subsequently validated using a large clinical dataset including in-vivo hemodynamic measurements (n = 783). Lack of CO and Ees in-vivo data impeded the clinical evaluation of the corresponding models.
Schematic illustration of the regression pipeline. brSBP Brachial systolic blood pressure, brDBP brachial diastolic blood pressure, HR heart rate, cfPWV carotid-to-femoral pulse wave velocity, and EF ejection fraction were used as features for predicting aortic systolic blood pressure (aSBP), cardiac output (CO), and end-systolic elastance (Ees). Regression models were trained to map the input data to the respective target data of interest. The methodology presented here was followed for each regression process (in terms of set of inputs, model, and output).
Results
Table 1 aggregates the cardiovascular parameters of the in-silico study population. The comparisons between the model-derived predictions and the reference data are presented below for each of the targeted outputs.
Prediction of aSBP, CO, and Ees from brSBP, brDBP, HR, and cfPWV
For the four models, the comparison between the predicted aSBP and the actual aSBP is presented in Table 2. The average difference (in absolute value) between the model-aSBP and the reference aSBP was less than 5 mmHg in 87% of the total cases for Random Forest, 89% for SVR, 75% for Ridge, and 88% for Gradient Boosting, respectively. Accuracy, correlation and agreement of model-CO estimates in comparison to the reference data are summarized in Table 3. The difference between model-CO and reference CO was less than 0.3/0.5 L min−1 in 62/84% of the population for Random Forest, 65/86% for SVR, 50/74% for Ridge, and 63/85% for Gradient Boosting. Finally, the Ees predictions are compared to the reference data in Table 4. High errors were reported for all of the regression models, whereas correlation between the estimated and the reference data was significantly poor.
Prediction of Ees from brSBP, brDBP, HR, cfPWV, and EF
The statistics of the second regression analysis for Ees, i.e., after additional knowledge of EF, are presented in Table 5. Differences between the predicted Ees and the actual Ees were found to be less than 0.05/0.20 mmHg mL−1 in the 47/78%, 51/81%, 39/70%, and 47/78% of the entire population, for Random Forest, SVR, Ridge, and Gradient Boosting, respectively.
The scatterplots and Bland–Altman graphs for the best performing models are provided in Figs. 2 and 3. The plotted data are corrupted with random noise (see “Blending the dataset with random noise” in “Methods”). Table 6 presents the frequency of selection for each hyperparameter value over the tenfold CV for the best performing model. For the aSBP and Ees estimators, we observed an apparent consistency for the values of the C and gamma hyperparameters. Concretely, C and gamma were set at 100 and 0.001 for aSBP, and 10 and 0.001 for Ees, respectively, in the totality of the 10 folds. Such a consistency is not evident for the CO estimator where C was set at 100 for the 60% of the times. Nevertheless, gamma was again consistently selected to be 0.001.
Comparison between predicted and reference data. Scatterplots and Bland–Altman plots between: (A, B) the predicted aSBP and the reference aSBP, and (C, D) the predicted CO and the reference CO. The solid line of the scatterplots represents equality. In Bland–Altman plots, limits of agreement (LoA), within which 95% of errors are expected to lie, are defined by the two horizontal dashed lines.
Comparison between predicted and reference data. Scatterplots and Bland–Altman plots between: (A, B) the predicted Ees and the reference Ees without ejection fraction as regression input, and (C, D) the predicted Ees and the reference Ees with ejection fraction as regression input. The solid line of the scatterplots represents equality. In Bland–Altman plots, limits of agreement (LoA), within which 95% of errors are expected to lie, are defined by the two horizontal dashed lines.
Sensitivity analysis for the training size
The training size, that is, the number of data instances used for training, plays a major role on the accuracy of the predictions. To investigate the sensitivity to the number of training data, the training size was modified from 95 to 15% of the total number of cases (Fig. 4). For all models except for Ridge, the RMSEs were increased gradually with decreasing training size. For the Random Forest, SVR, and Gradient Boosting, the RMSEs of the aSBP predictions were less than 4.20 mmHg. Using Ridge, the RMSE varied at a lesser extent, while it was consistently higher compared to the rest of the models. For the CO predictions, all RMSE values were less than 0.50 L min−1. In particular, RMSE for SVR did not exceed 0.38 L min−1, even when only the 15% of the entire population was used for the training. Finally, all RMSEs of Ees estimations were equal or below 0.20 mmHg mL−1.
Feature importance evaluation
Figure 5 presents the correlation matrix reporting the inter-feature correlations, and the correlations between the inputs and the target outputs. Table 7 presents the average importances of the input features, sorted in a descending order for predicting aSBP, CO, and Ees, respectively. For estimating aSBP, brSBP was found to be a critical contributor; the importance level (0.98) indicated that brSBP should be sufficient for estimating aSBP. The features of brSBP and cfPWV were the dominant contributors in the estimation of CO. Finally, EF was found to play the most significant role in the Ees prediction, followed by brDBP and HR. To further verify the sensitivity of the model’s performance to the input features, we present the RMSE variation for different subsets of input features (only for the best performing models) (Table 8).
For aSBP, it was shown again that the brSBP is the most pivotal predictor of aSBP; when brSBP was removed from the input features, the RMSE increased significantly. On the contrary, a precise prediction of CO requires the use of at least one of the brachial BP values; exclusion of the latter resulted to a deterioration of the model’s performance. Finally, Ees appears to be mainly sensitive to EF which significantly contributes to the accuracy of the Ees estimation. Results of the hypothesis testing for the ordinary least squares (OLS) regression coefficients are summarized in Table 9. All of the specified coefficients were statistically significantly different from zero.
In-vivo evaluation of the aSBP estimations
After the in-silico validation, the performance of the aSBP estimator was evaluated anew using clinical data. The population included both women (n = 136) and men (n = 647). The descriptive and clinical characteristics of the clinical population are presented in Table 10.
The comparisons between the predicted aSBP and the reference aSBP are presented below. First, we assessed the capacity of an SVR model, which was trained using only in-silico data, to make an accurate prediction for the human population (Fig. 6A,B). Then, we compared the latter’s performance with an SVR model which was trained using in-vivo data (Fig. 6C,D). The regression statistics between the model predictions and the reference data are summarized in Table 11. For the in-vivo data, the hypothesis testing’s results for the OLS regression coefficients are presented in Table 12. Figure 7 provides the correlation matrix for the in-vivo dataset.
Comparison between predicted and reference clinical data. Scatterplots and Bland–Altman plots between: the predicted aSBP and the reference aSBP for SVR trained using in-silico data (A, B), and for SVR trained using in-vivo data (C, D). The solid line of the scatterplots represents equality. In Bland–Altman plots, the limits of agreement (LoA), within which 95% of errors are expected to lie, are defined by the two horizontal dashed lines.
Discussion
The present study demonstrated that accurate estimations of central hemodynamics (namely, aSBP and CO) and left ventricular Ees from readily available noninvasive clinical measurements can be obtained by using machine learning models. Our basic hypothesis was whether brSBP, brDBP (cuff BP), HR, and cfPWV provide sufficient information to predict aSBP, CO, and Ees. However, for the determination of Ees, data from peripheral pressure waves fall short to provide a precise estimate. Our results indicated that additional information, such as the EF, which is directly measured in the heart (rather than the periphery) may improve the noninvasive Ees predictions. To our best knowledge, this is the first work to evaluate the use of machine learning models in predicting cardiac contractility.
The best performing prediction model for all three target outputs was SVR which outperformed the other models accomplishing the highest accuracy. The Ees estimation was effectively achieved only with the inclusion of EF in the set of input features. In order to evaluate the robustness of our regression models, sensitivity to the training size was investigated. The RMSE was gradually increased with decreasing the number or training samples for Random Forest, SVR, and Gradient boosting. Variations were less distinct for Ridge. Despite the increase in RMSE with changes in the training size, the errors lied within acceptable limits37,38,39,40,41 for Random Forest, SVR, and Gradient Boosting.
Moreover, we tested the performance of an ensemble predictor which used averaging of the single models’ predictions. The ensemble prediction model did not outperform the best performing single prediction model (SVR). However, such an approach may benefit the estimations’ accuracy by reducing the variance of the predictor and thus may improve the model’s generalization ability42. To avoid overwhelm the reader with an exhaustive report of several other approaches, we did not explore other ensemble learning techniques. Such an extensive exploration of different ensemble techniques would be out of the scope of this study.
Following the in-silico validation, in-vivo validation was performed only for the aSBP. The aSBP predictions were found to be precise in the both investigated scenarios, i.e., SVR trained with in-silico data, and SVR trained with in-vivo data. The accuracy was slightly higher in the second scenario despite the smaller size of the training dataset. This is expected if we consider that the in-vivo data may contain more physiologically relevant content and thus be more informative compared to the in-silico data in the training of the model. Interestingly, the hyperparameter tuning led to the same selection for the hyperparameters C = 100 (selected 100% of the times) and gamma = 0.001 (selected 100% of the times) when the SVR model was validated using the in-vivo population. These findings may verify that the in-silico predictive models can be rather informative for the design of clinical studies.
The principal reason that brSBP, brDBP, HR, and cfPWV were selected as the model inputs was the simplicity of their measurement in a clinical setting. Brachial cuff pressure constitutes a readily available and cost-efficient measurement in traditional medicine. At the same time, the use of pressure-based cfPWV is steadily increasing, as a result of numerous studies demonstrating its importance as an independent predictor of cardiovascular disease43,44,45. The convenience and the cost-efficiency of the aforementioned measurements render them suitable for easy, noninvasive, regular medical check-ups.
Based on the feature importances’ assessment, the aSBP prediction was found to be determined mainly from the brSBP. The strong dependency between aSBP and brSBP errors is to be expected, given that the two values are strongly related to mean BP, which is practically the same in both the aorta and the brachial artery. Moreover, brSBP seemed to be a significant predictor of CO. Resistance, and thus mean BP, is a strong determinant of CO. Given that brSBP is related to mean BP, this means that brSBP is indirectly related to CO. In addition, cfPWV is a measure of arterial compliance, which is also determinant of stroke volume and thus CO. Finally, EF and Ees have been reported to be positively correlated46 and this further explains the high importance level of EF for predicting Ees. The results using different subsets of the input features further verified each feature’s contribution to the predictions of the target output variables.
Prior work proposed by Xiao et al.47 used an artificial neural network (ANN) to predict aSBP from invasive radial SBP and DBP, and HR. The differences between the predicted aSBP and the measured aSBP were found to be low and equal to − 0.30 ± 5.90 mmHg. Despite providing accurate results, invasive radial BP is not commonly measured on a regular basis, and thus its modelling imposes a substantial limitation on the clinical application of their proposed model. When an ANN with the same configuration, as the one reported in the study of Xiao et al., was employed to estimate aSBP in our study, the results indicated a similarly good prediction performance. Concretely, the employment of the ANN using only the in-silico data (n = 4,018) achieved an RMSE = 3.79 ± 1.88 mmHg and r = 0.99 (p < 0.001). Training/testing the ANN with only the in-vivo data (n = 783) achieved an RMSE = 3.38 ± 1.09 and r = 0.97 (p < 0.001). In the case of the in-vivo data, we observed that the accuracy is slightly improved by the use of ANN compared to the best performing configuration (SVR achieved an RMSE = 3.53 ± 1.27 mmHg, r = 0.97, p < 0.001).
In general, the majority of previous aSBP estimators relies on features extraction from the pressure waveforms47,48. In our approach, apart from peripheral SBP and DBP, and HR, we incorporated the cfPWV measurement. The idea was that cfPWV being an index of aortic stiffening would improve the performance of the model and strengthen the clinical relevance of our results. However, feature importances indicated that brSBP may be sufficient for estimating aSBP. Using only brSBP, brDBP, and HR as inputs would not alter significantly the accuracy of the estimation of aSBP (using the in-silico data); namely, the RMSE would slightly increase from 3.13 to 3.31 mmHg for the best performing model. In the case that only brSBP and brDBP were used as input features, the accuracy would deteriorate with a RMSE of 3.46 mmHg which could still be acceptable. The use of only brSBP as an input, however, would essentially increase the error at 5.33 mmHg. For the clinical dataset, the same errors were equal to 3.52 mmHg (brSBP, brDBP, HR as inputs) and 4.11 mmHg (brSBP, brDBP as inputs). Finally, using only the brSBP predictor would lead to an RMSE = 4.20 mmHg.
In addition to prediction models for aSBP, estimation of CO from arterial BP characteristics has been a fertile area of research. Dabanloo et al.25 has evaluated the performance of neural networks in predicting CO from invasive arterial pressure waves. Upon comparison between the predicted CO and thermodilution-derived CO, their best performing model provided a mean absolute error equal to 0.54 L min−1 and a correlation coefficient of 0.89. Nevertheless, their model made use of the entire pressure waveform, from which input features were extracted, whereas it provided only an uncalibrated estimation of CO rather than its absolute value.
The results presented in this study are also compliant with the findings of Bikia et al.49, who suggested that brachial BP and cfPWV can be used to predict central SBP and CO (RMSE equal to 2.46 mmHg and 0.36 L min−1, respectively). Following an inverse problem-solving approach, a generalized model of the cardiovascular system was adjusted to quasi- patient-specific standards using measurements of brSBP, brDBP, HR, and cfPWV. Additional geometrical information on the aortic diameter size of each subject was also integrated. The aortic diameter was approximated using previously published age- and BSA-related data50. A similar approximation of the aortic geometry could be embedded in the present study and improve the accuracy of the results. Therefore, employment of machine learning on clinical data could be further reinforced with the inclusion of additional input features such as age, height, and weight. However, given that the errors are already rather low, it is not anticipated that such an improvement would be of particular clinical significance.
Additionally, this study aimed to effectively predict Ees while utilizing a small number of required inputs. Chen et al.26 proposed a method to estimate Ees from cuff pressure, stroke volume, and EF. Their method provided accurate predictions of Ees with differences equal to 0.43 ± 0.50 mmHg mL−1. In contrast to Chen’s approach, we excluded stroke volume from our input vector and, on the other hand, we introduced cfPWV which constitutes an index of aortic stiffness and thus a powerful index of arterio-ventricular coupling51. In an attempt to remove EF from the set of inputs, Ees was found to be poorly predicted. This underachieving performance may be rather expected given that a specific combination of brachial SBP and DBP, and cfPWV might not be unique for only a particular Ees value. Importantly, our study emphasized on the significance of EF in accurately predicting Ees.
The use of EF is further encouraged from the fact that EF constitutes a noninvasive parameter which can be derived via several cardiac imaging modalities. The Simpson’s method52 has been the most commonly used technique; however, it might underestimate EF when compared to the magnetic resonance imaging (MRI), which has been shown to be the gold standard noninvasive technique for assessing LV function and thus EF53. Of course, the latter are not considered as convenient and cost-efficient as a cuff- or tonometry-based pressure measurement. It is likely that the EF-related information may be derived from another measured parameter which is directly or indirectly related to the cardiac contractility, e.g. electrical signals of cardiac events54. Further investigation towards this direction will be conducted in future work.
It should be noted that the aim of the current study is not to propose necessarily a tool that could provide simultaneous predictions of aSBP, CO, and Ees. The models developed in this study could be considered as independent predictors for each of the target parameters in different clinical occasions. In particular, aSBP and CO are major hemodynamical indices that are often useful to the clinician and their noninvasive estimation is highly desirable in a routine clinical examination. On the other hand, Ees is less often required. Currently, Ees is measured invasively with the acquisition of the left ventricular pressure–volume loops. The invasive nature of this technique severely limits the use of Ees in clinical practice.
The booming of data has led to efforts of transferring one type of information to another using machine learning models. Specifically, in relation to patho-physiology, the advancement on measuring and imaging techniques has encouraged the employment of machine learning for estimating clinical pathophysiological indices and validating their results. This promising area of research could not exclude applications on cardiovascular health25,47,55,56. High correlation between peripheral pressure and central aortic pressure indicates the potential to estimate the latter from the former. However, the correlations for a complete set of cardiovascular variables have not been thoroughly investigated. In this work, we performed a first study to elucidate which input parameters (noninvasive measurements) are considered necessary when machine learning is employed for predicting aortic hemodynamics and contractility index (invasive measurements). A major advantage of the present study pertains to the well-balanced dataset that was used for the training/testing scheme. The use of synthetic data allowed for covering a wide range of hemodynamical characteristics, whereas it provided us with access to cardiovascular quantities which are difficult to obtain noninvasively in the real clinical setting, i.e., aortic BP or Ees.
Cardiovascular models have attracted great interest due to the increasing impact of cardiovascular disease. They have provided a valuable alternative for the assessment of pressure and flow in the entire arterial network providing additional pathophysiological insights, which are difficult to acquire in-vivo. Numerous previous studies have used in-silico data for the estimation of aortic BP, cardiac output, aortic PWV and many more56,57,58,59,60. Importantly, in-silico studies allow for the preliminary evaluation of predictive models across a wide range of cardiovascular parameters61 in a quick and cost-efficient way, while their results can be rather informative of the design of clinical studies62,63.
Several limitations need to be acknowledged. The data used for the training/testing scheme were derived from a simulator instead of a real human population. While synthetic data can mimic numerous properties of the real clinical data, they do not copy the original content in an identical way. Nevertheless, the goal here was to define the minimum necessary input information that is required to estimate aortic hemodynamics and Ees. Thus, despite that the use of synthetic data might not lead to exactly the same results with the results coming from clinical data, it should not undermine the reliability of the study’s findings. The latter has been verified by the in-vivo validation of our aSBP estimations. Clinical validation was not possible for the CO and Ees estimators, due to the lack of the respective data. At the initial stage of our research, we found it reasonable to start with an in-silico validation of our predictive models, instead of collecting measurements of CO and Ees in a large cohort. In addition, the cost and the complexity of the respective measurements would make it difficult to incorporate them in the current study. Future work should include the use of real-world data for all parameters that will finally verify the application of the proposed method in the clinical setting. Finally, the proposed models have been designed and tested on data coming from a generalized model of the cardiovascular system which was developed according to published data28. Hence, the precision of the predictions might be compromised in the case of pathological conditions, such as atherosclerosis, aneurysm or aortic valve disease. It is of great importance that in-vivo validation of the models should be conducted using pathological clinical data as well.
In summary, this study showed that the use of noninvasive arm-cuff pressure and PWV alone potentially allows for the estimation of aSBP and CO with acceptable accuracy. This might not be the case for Ees prediction. Nevertheless, the estimated Ees can be greatly improved when EF is used as an additional input in the prediction model. Following validation on in-vivo invasive data, this approach may provide a promising potential in the prediction of aortic hemodynamics and left ventricular contractility using unintrusive, readily available standard clinical measurements.
Methods
A regression pipeline was applied for estimating aortic hemodynamics and LV contractility index. The schematic representation of the methodology is presented in Fig. 1. The input data comprised brSBP, brDBP, HR, cfPWV, and EF for every subject. These data were fed to the regression models to estimate aSBP, CO, and Ees. First, brSBP, brDBP, HR, and cfPWV were used as input predictors for all three outputs, i.e., aSBP, CO, and Ees. A second regression analysis was performed using EF as an additional input feature only for the estimation of Ees. The outputs of each testing set were blinded and kept as the ground truth against which our predictions were later compared.
Brief description of the in-silico model of cardiovascular dynamics
In the present study, we used a 1-D in-silico model of the cardiovascular system, that has been previously described and validated against in-vivo data28,29. The arterial tree includes the main arteries of the systemic circulation, as well as the cerebral circulation and the coronary circulation. In summary, the governing equations of the model are derived by integrating the longitudinal momentum and continuity equations over the arterial cross section. Pressure and flow are acquired across the arterial tree by solving the governing equations employing an implicit finite-difference scheme. Local arterial compliance is calculated, provided that pulse wave velocity (PWV) is approximated as an inverse power function of the arterial lumen diameter28. Three-element Windkessel models64 are coupled to the distal vessels to account for the peripheral resistance. The contractility of the LV is modeled using a time-varying elastance model4,9. This elastance model considers a linear ESPVR characterized by its slope, the end-systolic elastance (Ees), and its intercept, the dead volume, Vd, as well as a linear end-diastolic pressure–volume relation characterized by its slope, the end-diastolic elastance (Eed).
Synthetic population generation
A database of 4,018 synthetic hemodynamic cases was created. The 1-D cardiovascular model ran using different combinations of arbitrary input parameters. The distributions of the input parameters were based on physiologically relevant data from the literature. The cardiovascular parameters were chosen to represent healthy individuals. Due to the limited amount of probabilistic information, the sampling was selected to be random Gaussian. The values of Ees and Eed ranged within [1.03, 3.50] mmHg mL−1 and [0.05, 0.20] mmHg mL−1, respectively65,66,67. HR varied between 60 and 100 bpm. The LV filling pressure lied between 7.00 and 23.00 mmHg according to68. The dead volume (Vd) and the time of maximal elastance (tmax) were kept unchanged. Their selected values were equal to the mean values of Vd = 15.00 mL and tmax = 340.00 ms as reported by previously published works28,69. Arterial geometry was modified to simulate different body types by adapting the length and the diameter of the arterial vessels. The heights covered a range of [150.00, 200.00] cm while the limits for aortic diameter were set to [1.90, 4.00] cm50,70. Total peripheral resistance varied within 0.50–2.00 mmHg s mL−171. Total arterial compliance was chosen within the range of [0.10, 3.80] mL mmHg−1 in order to account for a wide range of different values of arterial tree stiffness72,73. It should be noted that evidence of nonuniform aortic stiffening was integrated for the elderly and hypertensive virtual subjects, following the methodology described by Bikia et al.49.
Virtual Database
The parameters of interest were estimated from the 1-D model-derived pressure and flow waves (simulation’s outputs). Concretely, synthetic brSBP, brDBP as well as HR data were obtained from the pressure wave at the left brachial artery. Similarly, aSBP was derived from the pressure waveform at the aortic root. CfPWV was derived using the tangential method74. The method computed the intersection (foot) of two tangents, i.e., the line passing tangentially through the systolic upstroke and the horizontal line passing through the point of minimum pressure. Subsequently, the pulse transit time was estimated between the foot of the wave at the two sites, namely, between the carotid artery and the femoral artery. The length between the two arterial sites was calculated by summing the lengths of the arterial segments within the transmission path. Finally, the cfPWV was estimated by dividing the arterial length of the path by the pulse transit time. Given that the ESPVR was known, the EF was derived by dividing the blood volume that is ejected within each heartbeat, i.e., the stroke volume (SV), by the end-diastolic volume (EDV). The value of the Ees was defined as the slope of the ESPVR. Then, all simulated information was discarded, except for the “measured” brSBP, brDBP, HR, cfPWV, and EF (inputs) and the aSBP, CO, and Ees data (outputs). The total dataset (organized in pairs of inputs and outputs) was kept for the training/testing process.
Blending the dataset with random noise
The synthetic data were corrupted with random noise in order to represent a more realistic data collection. The introduced noise was equivalent to a random relative error within the range of [− 6.00, 6.00] % with respect to the actual value. This magnitude of error was selected based on published data from previous studies75.
Clinical database
For the clinical validation of the aSBP estimations, we used clinical data from 783 subjects who underwent noninvasive cardiovascular assessment for research purposes, at the First University Department of Cardiology (Hippokration General Hospital, Athens, Greece). Anonymized data were analyzed in compliance with the Declaration of Helsinki of the World Medical Association and the National Regulations for clinical research.
The carotid-to-femoral pulse wave velocity (cfPWV) was measured in every subject as previously described76,77,78. In brief, cfPWV measurement was performed using the SphygmoCor apparatus (AtCor Medical Pty Ltd, West Ryde, Australia). First, short-term continuous arterial pressure waveforms were recorded by use of a hand-held tonometer (Millar, Houston, USA), simultaneously with ECG acquisition (for the synchronization of the continuous pressure waves recorded at the carotid and the femoral artery). Then, the recorded pressure waveforms were processed by proprietary software that automatically computes pulse transit time from the carotid to the femoral artery using the tangential method 74. Finally, cfPWV was calculated by the ratio of the distance between the two recording sites (calculated as the length from the suprasternal notch to femoral artery minus the length from the carotid artery to the suprasternal notch) to the pulse transit time. CfPWV measurements were performed with the subject at the supine position after 5 min resting period.
Noninvasive estimation of the aortic pressure waveforms was performed by the SphygmoCor System (AtCor Medical Pty Ltd), as previously described79,80. Radial pressure waves were first recorded by applanation tonometry and central pressure waves were derived by use of validated transfer functions81. Multiple recordings were performed in every subject to accomplish optimal quality control criteria (quality index: > 85%). Calibration of the recorded pulse waves was performed using the brachial systolic and diastolic BPs, which were measured by cuff sphygmomanometry. The accuracy of this apparatus has been previously evaluated by comparing the estimated aortic BPs with intra-aortic catheter-based BP measurements79. Furthermore, the reproducibility of this technique has been also found to be acceptable under several different conditions and populations82.
Regression analysis
Four regression models were trained/tested to estimate the corresponding target outputs. The models that were employed were Random Forest33, SVR34, Ridge35, and Gradient Boosting36. By definition, a regression model comprises the following components: (i) the unknown hyperparameters, β, (ii) the independent variables, Xi, and (iii) the dependent variable, Yi. In this analysis, the objective was to investigate whether the regression model can estimate aSBP, CO, and Ees from single-beat input predictors (brSBP, brDBP, HR, cfPWV, (EF)). The training/testing scheme was based on a tenfold CV scheme83 (Fig. 8). Concretely, all cases were divided into ten equal sets in a random manner. In each fold, one set was left out being the testing group, and the rest of sets were used as the training group to tune the parameters of the models. Hyperparameter tuning was performed internally in each fold using GridSearch with a tenfold CV in order to optimize the β parameters of each fold’s model (Fig. 8). The hyperparameters that were chosen to be optimized are reported in the Table 13. The hyperparameters’ values that are not reported in Table 13 were set to their default value.
We investigated two approaches: (i) one to predict aSBP, CO, and Ees using brSBP, brDBP, HR, and cfPWV, and (ii) a second one to predict solely Ees using brSBP, brDBP, HR, cfPWV, and EF. Consequently, we evaluated the accuracy of each regression model for every target variable on a subject level. Additionally, averaging of the multiple predictions was tested as an ensemble learning approach. The training/testing pipeline was implemented using the Scikit-learn library84 in a Python programming environment. The pandas and numpy packages were also used85,86.
In-silico validation of the model-derived predictions
We first assessed the performance of each regression model for every target variable on a subject level for the virtual population. Ten-fold CV as described above was used to evaluate the accuracy of the trained models. Moreover, we calculated the percentages of the cases whose aSBP errors met the international standards (< 5 ± 8 mmHg) of the European Society of Hypertension International Protocol37. The error threshold for CO was set to 0.3 and 0.5 L min−1 based on the objective criteria suggested by Critchley and Critchley87. Finally, given that the only clinically acceptable technique for measuring Ees is the invasive end-systolic pressure–volume relationship, there are not meta-analyses using Ees data. In this respect, for the Ees values within the range of [1, 4.5] mmHg mL−1, thresholds of 0.05 and 0.20 mmHg mL−1 should be adequate to provide an accurate estimation of Ees.
Sensitivity analysis for the training size
In order to assess the effect of the number of training samples on our models’ accuracy, sensitivity analysis was performed. Concretely, the regression analysis was repeated after decreasing the training size from 95 to 15% of the total number of cases. For each training size, the predictions were evaluated in terms of RMSE between the estimated and reference data. Hyperparameter tuning was implemented for each different training set under consideration.
In-vivo validation of the model-derived aSBP predictions
Moreover, in-vivo validation was performed only for the best performing aSBP estimator, i.e., SVR. The validation was realized in two steps. First, we trained/tested an SVR model using only in-vivo data following the experimental design described in Fig. 8. Consequently, an SVR model was trained with the totality of the in-silico data (n = 4,018) and, then, was tested on the in-vivo data (n = 783), as depicted in Fig. 9. During training, hyperparameter tuning was performed using GridSearch with tenfold CV.
Feature importance evaluation
We assessed the importance of each input feature using the scores returned by the Random forest model. The average importance of each feature was then calculated by averaging the scores from every fold k (k = 1, 2, … 10).
Statistical analysis
The algorithms and the statistical analysis were implemented in Python (Python Software Foundation, Python Language Reference, version 3.6.8, Available at https://www.python.org). We performed OLS estimation of the regression coefficients using each of the target parameters, i.e., aSBP, CO, and Ees, as dependent variable and brSBP, brDBP, cfPWV, HR, and EF (only for Ees) as independent variables (using statsmodels library88). Hypothesis testing for each regression coefficient was realized using the t-stastistic. The agreement, bias and precision between the method-derived predictions and the real values were evaluated by using the Pearson’s correlation coefficient (r), the coefficient of determination (R2), the root mean square error (RMSE), and the normalized root mean square error (nRMSE). The computed nRMSE was based on the difference between the minimum and maximum values of the dependent variable. Bias and limits of agreement as described by89 were reported. The level of statistical significance was set at p < 0.05.
Data availability
The virtual database generated and analyzed in the present study is available from the corresponding author on a reasonable request.
References
Waddell, T. K., Dart, A. M., Medley, T. L., Cameron, J. D. & Kingwell, B. A. Carotid pressure is a better predictor of coronary artery disease severity than brachial pressure. Hypertension 38, 927–931 (2001).
Safar, M. E. et al. Central pulse pressure and mortality in end-stage renal disease. Hypertension 39, 735–738 (2002).
Berkenstadt, H. et al. Stroke volume variation as a predictor of fluid responsiveness in patients undergoing brain surgery. Anesth. Analg. 92, 984–989 (2001).
Sagawa, K., Suga, H., Shoukas, A. A. & Bakalar, K. M. End-systolic pressure/volume ratio: a new index of ventricular contractility. Am. J. Cardiol. 40, 748–753 (1977).
Song-Tao, A., Yan-Yan, Q. & Li-Xia, W. The severity of coronary artery disease evaluated by central systolic pressure and fractional diastolic pressure. N. Am. J. Med. Sci. 2, 218–220 (2010).
Lees, N., Hamilton, M. & Rhodes, A. Clinical review: goal-directed therapy in high risk surgical patients. Crit. Care 13, 231 (2009).
Nishimura, R. A. & Carabello, B. A. Hemodynamics in the cardiac catheterization laboratory of the 21st century. Circulation 125, 2138–2150 (2012).
Ganter, M. T. et al. Continuous cardiac output measurement by un-calibrated pulse wave analysis and pulmonary artery catheter in patients with septic shock. J. Clin. Monit. Comput. 30, 13–22 (2016).
Hiroyuki, S. & Kiichi, S. Instantaneous pressure-volume relationships and their ratio in the excised supported canine left ventricle. Circ. Res. 35, 117–126 (1974).
Suga, H., Sagawa, K. & Shoukas, A. A. Load independence of the instantaneous pressure-volume ratio of the canine left ventricle and effects of epinephrine and heart rate on the ratio. Circ. Res. 32, 314–322 (1973).
Sagawa, K. The end-systolic pressure-volume relation of the ventricle: definition, modifications and clinical use. Circulation 63, 1223–1227 (1981).
Williams, B. et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 39, 3021–3104 (2018).
Papaioannou, T. G., Protogerou, A. D., Stamatelopoulos, K. S., Vavuranakis, M. & Stefanadis, C. Non-invasive methods and techniques for central blood pressure estimation: procedures, validation, reproducibility and limitations. Curr. Pharm. Des. 15, 245–253 (2009).
Hope, S. A., Tay, D. B., Meredith, I. T. & Cameron, J. D. Use of arterial transfer functions for the derivation of aortic waveform characteristics. J. Hypertens. 21, 1299–1305 (2003).
Stok, W. J., Westerhof, B. E. & Karemaker, J. M. Changes in finger-aorta pressure transfer function during and after exercise. J. Appl. Physiol. 101, 1207–1214 (2006).
Fetics, B., Nevo, E., Chen, C.-H. & Kass, D. M. Parametric model derivation of transfer function for noninvasive estimation of aortic pressure by radial tonometry. IEEE Trans. Biomed. Eng. 46, 698–706 (1999).
Williams, B. et al. Development and validation of a novel method to derive central aortic systolic pressure from the radial pressure waveform using an N-point moving average method. J. Am. Coll. Cardiol. 57, 951–961 (2011).
Shih, Y.-T., Cheng, H.-M., Sung, S.-H., Hu, W.-C. & Chen, C.-H. Application of the N-point moving average method for brachial pressure waveform-derived estimation of central aortic systolic pressure. Hypertension 63, 865–870 (2014).
Udy, A. A., Altukroni, M., Jarett, P., Roberts, J. A. & Lipman, J. A comparison of pulse contour wave analysis and ultrasonic cardiac output monitoring in the critically ill. Anaesth. Intensive Care 40, 631–637 (2012).
Jansen, J. R. C. et al. A comparison of cardiac output derived from the arterial pressure wave against thermodilution in cardiac surgery patients †. Br. J. Anaesth. 87, 212–222 (2001).
Langwieser, N. et al. Radial artery applanation tonometry for continuous noninvasive arterial blood pressure monitoring in the cardiac intensive care unit. Clin. Res. Cardiol. 104, 518–524 (2015).
Christie, J. et al. Determination of stroke volume and cardiac output during exercise: comparison of two-dimensional and Doppler echocardiography, Fick oximetry, and thermodilution. Circulation 76, 539–547 (1987).
Swamy, G. & Mukkamala, R. Estimation of the aortic pressure waveform and beat-to-beat relative cardiac output changes from multiple peripheral artery pressure waveforms. IEEE Trans. Biomed. Eng. 55, 1521–1529 (2008).
Fazeli, N. & Hahn, J.-O. Estimation of cardiac output and peripheral resistance using square-wave-approximated aortic flow signal. Front. Physiol. 3, 298 (2012).
Dabanloo, N. J., Adaei, F. & Nasrabadi, A. M. The Performance of Neural Network in the Estimation of Cardiac Output Using Arterial Blood Pressure Waveforms. (2011).
Chen, C. H. et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J. Am. Coll. Cardiol. 38, 2028–2034 (2001).
Shishido, T. et al. Single-beat estimation of end-systolic elastance using bilinearly approximated time-varying elastance curve. Circulation 102(16), 1983–1989. https://doi.org/10.1161/01.CIR.102.16.1983 (2000).
Reymond, P., Merenda, F., Perren, F., Rüfenacht, D. & Stergiopulos, N. Validation of a one-dimensional model of the systemic arterial tree. Am. J. Physiol. Heart Circ. Physiol. 297, H208-222 (2009).
Reymond, P., Bohraus, Y., Perren, F., Lazeyras, F. & Stergiopulos, N. Validation of a patient-specific one-dimensional model of the systemic arterial tree. Am. J. Physiol. Heart Circ. Physiol. 301, H1173-1182 (2011).
Reymond, P., Westerhof, N. & Stergiopulos, N. Systolic hypertension mechanisms: effect of global and local proximal aorta stiffening on pulse pressure. Ann. Biomed. Eng. 40, 742–749 (2012).
Heusinkveld, M. H. G. et al. Augmentation index is not a proxy for wave reflection magnitude: mechanistic analysis using a computational model. J. Appl. Physiol. 127, 491–500 (2019).
Avolio, A. Central aortic blood pressure and cardiovascular risk: a paradigm shift?. Hypertension 51, 1470–1471 (2008).
Liaw, A. & Wiener, M. Classification and Regression by randomForest. R News 2, 18–22 (2002).
Smola, A. J. & Schölkopf, B. A tutorial on support vector regression. Stat. Comput. 14, 199–222 (2004).
Robert, T. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B 58, 267–288 (1996).
Natekin, A. & Knoll, A. Gradient boosting machines, a tutorial. Front. Neurorobot. 7, 21 (2013).
O’Brien, E. et al. European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Pressure Monit. 15, 23–38 (2010).
Critchley, L. A. H., Huang, L. & Zhang, J. Continuous cardiac output monitoring: what do validation studies tell us?. Curr. Anesthesiol. Rep. 4, 242–250 (2014).
Nishikawa, T. & Dohi, S. Errors in the measurement of cardiac output by thermodilution. Can. J. Anaesth. 40, 142–153 (1993).
Nitenberg, A., Antony, I. & Loiseau, A. Left ventricular contractile performance, ventriculoarterial coupling, and left ventricular efficiency in hypertensive patients with left ventricular hypertrophy. Am. J. Hypertens. 11, 1188–1198 (1998).
Popović, Z. et al. Partial left ventriculectomy improves left ventricular end systolic elastance in patients with idiopathic dilated cardiomyopathy. Heart 83, 316–319 (2000).
Dietterich, T. G. Ensemble Methods in Machine Learning. In Multiple Classifier Systems (ed. Dietterich, T. G.) 1–15 (Springer, Berlin Heidelberg, 2000).
Joo, H. J. et al. The relationship between pulse wave velocity and coronary artery stenosis and percutaneous coronary intervention: a retrospective observational study. BMC Cardiovasc. Disord. 17, 1–10 (2017).
Muiesan, M. L. et al. Pulse wave velocity and cardiovascular risk stratification in a general population: the Vobarno study. J. Hypertens. 28, 1935–1943 (2010).
Khoshdel, A. R., Carney, S. L., Nair, B. R. & Gillies, A. Better management of cardiovascular diseases by pulse wave velocity: combining clinical practice with clinical research using evidence-based medicine. Clin. Med. Res. 5, 45–52 (2007).
Monge García, M. I. et al. Determinants of left ventricular ejection fraction and a novel method to improve its assessment of myocardial contractility. Ann. Intensive Care 9, 48 (2019).
Xiao, H., Qasem, A., Butlin, M. & Avolio, A. Estimation of aortic systolic blood pressure from radial systolic and diastolic blood pressures alone using artificial neural networks. J. Hypertens. 35, 1577–1585 (2017).
Ghasemi, Z. et al. Estimation of cardiovascular risk predictors from non-invasively measured diametric pulse volume waveforms via multiple measurement information fusion. Sci. Rep. 8, 1–11 (2018).
Bikia, V. et al. Noninvasive cardiac output and central systolic pressure from cuff-pressure and pulse wave velocity: a model-based study. IEEE J. Biomed. Health Inform. https://doi.org/10.1109/JBHI.2019.2956604 (2019).
Wolak, A. et al. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area. JACC Cardiovasc. Imaging 1, 200–209 (2008).
Antonini-Canterin, F. et al. Arterial stiffness and ventricular stiffness: a couple of diseases or a coupling disease? A review from the cardiologist’s point of view. Eur. J. Echocardiogr. 10, 36–43 (2009).
Otterstad, J. E. Measuring left ventricular volume and ejection fraction with the biplane Simpson’s method. Heart 88, 559–560 (2002).
Simpson, R. et al. Comparing echocardiography and cardiac magnetic resonance measures of ejection fraction: implications for HFMRF research. In British Cardiovascular Imaging Meeting 2018 A3.1-A3 (BMJ Publishing Group Ltd and British Cardiovascular Society, 2018). https://doi.org/10.1136/heartjnl-2018-BCVI.6.
Reant, P. et al. Systolic time intervals as simple echocardiographic parameters of left ventricular systolic performance: correlation with ejection fraction and longitudinal two-dimensional strain. Eur. J. Echocardiogr. 11, 834–844 (2010).
Howard, J. P. et al. Artificial intelligence for aortic pressure waveform analysis during coronary angiography. JACC 12, 2093–2101 (2019).
Huttunen, J. M. J., Kärkkäinen, L. & Lindholm, H. Pulse transit time estimation of aortic pulse wave velocity and blood pressure using machine learning and simulated training data. PLoS Comput. Biol. 15, e1007259 (2019).
Stergiopulos, N., Westerhof, B. E. & Westerhof, N. Physical basis of pressure transfer from periphery to aorta: a model-based study. Am. J. Physiol.-Heart Circ. Physiol. 274, H1386–H1392 (1998).
Trachet, B. et al. Numerical validation of a new method to assess aortic pulse wave velocity from a single recording of a brachial artery waveform with an occluding cuff. Ann. Biomed. Eng. 38, 876–888 (2010).
Papaioannou, T. G., Vardoulis, O. & Stergiopulos, N. The, “systolic volume balance” method for the noninvasive estimation of cardiac output based on pressure wave analysis. Am. J. Physiol.-Heart Circ. Physiol. 302, H2064–H2073 (2012).
Huttunen, J. M. J., Kärkkäinen, L., Honkala, M. & Lindholm, H. Deep learning for prediction of cardiac indices from photoplethysmographic waveform: a virtual database approach. Int. J. Numer. Methods Biomed. Eng. 36, e3303 (2020).
Shi, Y., Lawford, P. & Hose, R. Review of zero-D and 1-D models of blood flow in the cardiovascular system. BioMed. Eng. OnLine 10, 33 (2011).
Willemet, M., Vennin, S. & Alastruey, J. Computational assessment of hemodynamics-based diagnostic tools using a database of virtual subjects: application to three case studies. J. Biomech. 49, 3908–3914 (2016).
Vennin, S. et al. Identifying hemodynamic determinants of pulse pressure: a combined numerical and physiological approach. Hypertension 70, 1176–1182 (2017).
Westerhof, N., Lankhaar, J.-W. & Westerhof, B. E. The arterial Windkessel. Med. Biol. Eng. Comput. 47, 131–141 (2009).
Chen, C.-H. et al. Coupled systolic-ventricular and vascular stiffening with age. J. Am. Coll. Cardiol. 32, 1221–1227 (1998).
Pak, P. H., Maughan, W. L., Baughman, K. L., Kieval, R. S. & Kass, D. A. Mechanism of acute mechanical benefit From VDD pacing in hypertrophied heart: similarity of responses in hypertrophic cardiomyopathy and hypertensive heart disease. Circulation 98, 242–248 (1998).
Feldman, M. D. et al. Acute cardiovascular effects of OPC-18790 in patients with congestive heart failure: time- and dose-dependence analysis based on pressure-volume relations. Circulation 93, 474–483 (1996).
Senzaki, H., Chen, C.-H. & Kass, D. A. Single-beat estimation of end-systolic pressure-volume relation in humans: a new method with the potential for noninvasive application. Circulation 94, 2497–2506 (1996).
Starling, M. R. et al. The relationship of various measures of end-systole to left ventricular maximum time-varying elastance in man. Circulation 76, 32–43 (1987).
Devereux, R. B. et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons ≥15 years of age. Am. J. Cardiol. 110, 1189–1194 (2012).
Lu, Z. & Mukkamala, R. Continuous cardiac output monitoring in humans by invasive and noninvasive peripheral blood pressure waveform analysis. J. Appl. Physiol. 101, 598–608 (2006).
Langewouters, G. J. Visco-elasticity of the Human Aorta in Vitro in Relation to Pressure and Age (University of Amsterdam, Amsterdam, 1982).
Segers, P. et al. Three- and four-element Windkessel models: assessment of their fitting performance in a large cohort of healthy middle-aged individuals. Proc. Inst. Mech. Eng. https://doi.org/10.1243/09544119JEIM287 (2008).
Vardoulis, O., Papaioannou, T. G. & Stergiopulos, N. Validation of a novel and existing algorithms for the estimation of pulse transit time: advancing the accuracy in pulse wave velocity measurement. Am. J. Physiol.-Heart Circ. Physiol. 304, H1558–H1567 (2013).
Liu, J. et al. Patient-specific oscillometric blood pressure measurement: validation for accuracy and repeatability. IEEE J. Transl. Eng. Health Med. 5, 1900110 (2017).
Papaioannou, T. G. et al. The influence of resting heart rate on pulse wave velocity measurement is mediated by blood pressure and depends on aortic stiffness levels: insights from the Corinthia study. Physiol. Meas. 40, 055005 (2019).
Tousoulis, D. et al. Acute exposure to diesel affects inflammation and vascular function. Eur. J. Prev. Cardiol. https://doi.org/10.1177/2047487319898020 (2020).
Papaioannou, T. G. et al. Arterial stiffness and subclinical aortic damage of reclassified subjects as stage 1 hypertension according to the new 2017 ACC/AHA blood pressure guidelines. VASA 48, 236–243 (2019).
Papaioannou, T. G. et al. Accuracy of commercial devices and methods for noninvasive estimation of aortic systolic blood pressure a systematic review and meta-analysis of invasive validation studies. J. Hypertens. 34, 1237–1248 (2016).
Ioakeimidis, N. et al. Acute effect of heat-not-burn versus standard cigarette smoking on arterial stiffness and wave reflections in young smokers. Eur. J. Prev. Cardiol. https://doi.org/10.1177/2047487320918365 (2020).
Karamanoglu, M., O’Rourke, M. F., Avolio, A. P. & Kelly, R. P. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur. Heart J. 14, 160–167 (1993).
Siebenhofer, A., Kemp, C., Sutton, A. & Williams, B. The reproducibility of central aortic blood pressure measurements in healthy subjects using applanation tonometry and sphygmocardiography. J. Hum. Hypertens. 13, 625–629 (1999).
Wong, T.-T. Performance evaluation of classification algorithms by k-fold and leave-one-out cross validation. Pattern Recogn. 48, 2839–2846 (2015).
Pedregosa, F. et al. Scikit-learn: Machine Learning in Python. JMLR 12, 2825–2830 (2011).
McKinney, W. Data Structures for Statistical Computing in Python. In Proceedings of the 9th Python in Science Conference 51–56 (2010). https://doi.org/10.25080/Majora-92bf1922-00a.
Oliphant, T. E. A guide to NumPy (Trelgol Publishing, USA, 2006).
Critchley, L. A. H. & Critchley, J. A. J. H. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J. Clin. Monit. Comput. 15, 85–91 (1999).
Seabold, S. & Perktold, J. statsmodels: Econometric and statistical modeling with python. (2010).
Bland, J. M. & Altman, D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1, 307–310 (1986).
Author information
Authors and Affiliations
Contributions
V.B. and N.S. conceived and designed the experimental protocol; V.B. designed and generated the virtual database; V.B. developed the algorithms and analyzed the data; E.O., G.S., D.T. designed and wrote the clinical protocol; E.O., G.S., D.T. performed and evaluated the clinical measurements; V.B. drafted the manuscript; V.B., T.G.P, S.P., G.R., E.O., G.S., D.T., and N.S. edited and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bikia, V., Papaioannou, T.G., Pagoulatou, S. et al. Noninvasive estimation of aortic hemodynamics and cardiac contractility using machine learning. Sci Rep 10, 15015 (2020). https://doi.org/10.1038/s41598-020-72147-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72147-8
- Springer Nature Limited
This article is cited by
-
Novel theory and potential applications of central diastolic pressure decay time constant
Scientific Reports (2024)
-
Machine Learning Identification Framework of Hemodynamics of Blood Flow in Patient-Specific Coronary Arteries with Abnormality
Journal of Cardiovascular Translational Research (2023)
-
The Critical Role of Lumped Parameter Models in Patient-Specific Cardiovascular Simulations
Archives of Computational Methods in Engineering (2022)