Abstract
The neurotransmitter noradrenaline (NA) mediates arousal, attention and mood, and exerts anti-inflammatory and neuroprotective effects. Alterations of monoamine signalling were reported in multiple sclerosis (MS) and psychiatric illness and may account for the high prevalence of comorbid depression and fatigue in MS patients. We assessed central noradrenaline transporter (NAT) availability using positron emission tomography (PET) and the NAT selective radiotracer S,S-[11C]O-methylreboxetine in immunotherapy-naïve patients with relapsing–remitting MS (RRMS; n = 11) compared to healthy controls (HC; n = 12), and its association to lesion load, time since manifestation, the expanded disability status scale (EDSS), the fatigue scale Würzburger Erschöpfungsinventar bei MS (WEIMuS) and Beck Depression Inventory (BDI). We found NAT availability to be increased in the thalamus, amygdala, putamen and pons/midbrain of MS patients. No relation to clinical or psychometric variables was found. These first data indicate higher NAT availability in subcortical brain regions of immunotherapy-naïve RRMS patients. If these changes of noradrenergic neurotransmission predispose to psychiatric symptoms or associate with disease activity needs to be investigated in longitudinal studies or a larger sample which allows subgroup analyses.
Similar content being viewed by others
Introduction
Multiple sclerosis (MS) is a chronic inflammatory immune-mediated disease of the central nervous system that is characterized by demyelination and axonal injury, predominantly affecting young adults. Besides physical disability, neuropsychological symptoms such as fatigue and depression frequently co-occur in MS patients1,2,3, leading to a substantially decreased quality of life4.
Autonomic and cognitive functions such as arousal, attention, drive and mood are substantially modulated by the central noradrenaline (NA) system5. Apart from its role as a classical neurotransmitter, NA functions as an endogenous immunomodulator that exerts anti-inflammatory and neuroprotective effects6. Disruptions of the central NA system have been linked to neurodegeneration in Alzheimer disease and Parkinson disease7,8, to the pathophysiology and symptoms of neuropsychiatric conditions such as depression9, anxiety10 and attention deficit/hyperactivity disorder11 and are thought to co-mediate neuro-inflammation in multiple sclerosis12. In vitro, NA reduces the expression of pro-inflammatory cytokines in glia cells and neurons13,14 and exerts neuroprotective effects by increasing the concentration of neurotrophins15. In experimental autoimmune encephalitis (EAE), NA levels were reported to be decreased16, and lesioning experiments of the locus coeruleus (as the main source of NA) led to the deterioration of clinical symptoms, while increasing central NA by NA reuptake inhibition reduced EAE severity6. This finding aligns with decreased NA concentrations found in the post-mortem brains of MS patients16, and the inverse relation of NA cerebrospinal fluid concentrations with disease duration and activity in multiple sclerosis17. The function of NA both in the context of immunomodulation and psychiatric symptoms may support its role as a candidate linking states of chronic neuro-inflammation and degeneration on the one hand to the comorbid psychiatric conditions frequently seen in multiple sclerosis on the other.
NAT is a critical modulator of NA signalling by limiting NA concentrations in the synaptic cleft by its reuptake into the presynaptic neuron18. Options to assess the activity of NA signalling in vivo in humans are limited. One method is the quantification of NA transporter (NAT) availability, using specific radiotracers and positron emission tomography (PET). There are no data on NAT availability in the living human brain of MS patients.
To assess central NAT availability in vivo, PET was applied using the highly selective radiotracer (S,S)-[11C]O-methylreboxetine ([11C]MRB)19,20. We expected (i) changes of central NAT availability in immunotherapy-naïve MS patients compared to healthy controls (HC) and explored (ii) whether there is an association of NAT with physical disability, time since manifestation and psychometric measures of fatigue and depressive symptoms in MS patients.
Results
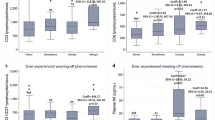
Subject characteristics are summarized in Table 1. Würzburger Erschöpfungsinventar bei MS scores (WEIMuS), but not the Beck Depression Inventory (BDI) were significantly higher in relapsing–remitting MS (RRMS) patients (see Table 1). Compared to HC, RRMS patients showed higher NAT Distribution Volume Ratios (DVR) in almost all subcortical regions specifically analyzed, reaching statistical significance in the thalamus (p = 0.001), putamen (p = 0.002), amygdala (p = 0.014) and pons/midbrain (p = 0.008) (Fig. 1 and Table 2). In contrast, NAT DVR was lower in the hypothalamus, although this difference was not significant (p = 0.12). NAT DVR of cortical regions (orbito-frontal cortex p = 0.87; dorsolateral prefrontal cortex p = 0.42; anterior cingulate cortex p = 0.64) and of the locus coeruleus (LC, p = 0.53) did not differ between the groups. Although the LC anatomically partly overlaps with the pons/midbrain region, statistical significance appears to be driven by pons/midbrain. NAT DVR were not associated with the EDSS, time since manifestation, psychometric scales or lesion volumes (see Table 3).
Group comparison of noradrenaline transporter (NAT) availability of patients with relapsing–remitting multiple sclerosis (RRMS; n = 11) and healthy controls (HC; n = 12). RRMS patients showed significantly higher NAT DVR in the thalamus, amygdala, pons/midbrain and putamen. Lines and whiskers indicate mean with SD for normally distributed data or, otherwise, median with interquartile range.
Discussion
To the best of our knowledge, this is the first study to investigate the activity of CNS noradrenergic signalling in vivo in immunotherapy-naïve MS patients by quantifying NA transporter availability, and its relation to physical disability and psychometric measures of depressive symptoms and fatigue. MS patients showed higher NAT availability than HC which reached statistical significance in subcortical brain regions involving the thalamus, parts of the limbic system (amygdala, putamen) and brainstem areas (pons/midbrain). Remarkably, the only region that displayed (numerically, but non-significantly) lower availability was the hypothalamus. Whereas BDI scores were low and did not differ between the groups, the WEIMuS questionnaire revealed higher fatigue in MS patients than in healthy controls. There was no association of NAT availability with physical disability, time since manifestation, the extent of depressive symptoms, fatigue or lesion load. MS-typical lesions did not overlap with analysed brain areas and were not considered as co-variates; even if present, the small sample size would have limited the statistical power of such analysis.
Previous PET studies in multiple sclerosis focused on neuroinflammation by measuring translocator protein or adenosine receptors to detect activated microglia21,22, on neurodegeneration by assessing glucose and choline metabolism, on myelin content targeting myelin sheaths23,24,25, or lately, on synaptic density26. Our hypothesis of changes in noradrenergic signalling in MS patients was based on a wealth of data from preclinical models6,27, some clinical studies28,29 and our earlier finding of altered serotonin transporter availability in limbic and paralimbic regions of MS patients, which relates to psychometric measures of depression and fatigue30.
We found NAT availability to be higher in MS patients in almost all subcortical regions of interest. Dysregulation of the noradrenergic system in MS was peripherally shown by decreased NA production in peripheral blood mononuclear cells of MS patients in remission31 or on long-term treatment with interferon beta32. Centrally, NA degradation products in the cerebrospinal fluid of MS patients correlate negatively with the number of relapses and disease duration17, and decreased noradrenaline concentrations and locus coeruleus neuronal density were found in post-mortem brains of MS patients16. Taken together, these observations suggest overall decreased tissue NA concentrations in EAE and MS, which were attributed to an attenuated synthesis, increased metabolism or enhanced reuptake of NA16,33. Higher NAT availability observed in most brain areas of our study would reconcile the notion of generally lower NA tissue concentrations described in previous biochemical analyses16. The increased availability of the NA transporter in patients could hence reflect reduced occupancy by endogenous NA, or alternatively explain lower NA concentrations in MS by enhanced NA reuptake. In this context, the lower NAT availability in the hypothalamus merits further discussion, which stands out even though the difference did not reach statistical significance. MS is associated with hyperactivity of the hypothalamic–pituitary–adrenal (HPA) axis34,35, and one important driver of HPA axis activity is noradrenergic stimulation by brainstem afferents, terminating in the hypothalamus. A negative association between hypothalamic NAT availability and HPA axis responsiveness could recently be shown in other entities with pronounced stress axis activity36. Although we cannot support this interpretation by functional data from the same cohort, the lower hypothalamic NAT availability may reflect more pronounced NA transporter occupancy by higher synaptic concentrations of NA or lower NA reuptake in this specific target area.
NAT is located on the presynaptic membrane and parts of the axon close to the dendrites, reflecting axonal integrity37. Since neurodegeneration and axonal damage is associated with the subsequent loss of noradrenergic projections, lowered NAT availability as seen in neurodegenerative diseases such as Parkinson’s disease38 and Alzheimer’s disease7, or in the aging human brain39, is explained by this mechanism. Hence, decreased NAT availability, or a negative correlation between NAT availability and physical impairment or time since manifestation seemed likely. A possible explanation for the absence of these associations is the selection of MS patients at an early stage of the disease, with a low median EDSS of 1.5 and only 11 months since MS manifestation, in whom marked axonal injury and neurodegeneration are less likely40. Also, the EDSS measures physical abilities which are not primarily regulated by NA41.
We found group differences in the thalamus, putamen, amygdala and parts of the brainstem. Interestingly, an altered activation or connectivity of these brain regions was lately associated with central fatigue42,43 or depressive symptoms in multiple sclerosis44. NA as a neurotransmitter modulates drive, alertness and arousal, emotion and behaviour5, and the clinical efficacy of NAT inhibition in psychiatric diseases such as depression45, attention deficit/hyperactivity disorder11 as well as in MS-related depression and fatigue46 further supports an involvement of the noradrenergic system in the pathophysiology of these entities. Based on one study which showed higher NAT availability in the thalamus of patients with major depression9, a positive correlation between NAT availability and BDI scores in MS was presumable. Even though our results indicate higher thalamic NAT availability in MS, they did not reveal a relation to the BDI or fatigue scores. Similarly, Moriguchi et al. did find an association with attention, but failed to show a relation of NAT availability to the Hamilton depression score. With clinically relevant depression as an exclusion criterion, and indeed negligibly low BDI scores, our group probably lacks the symptom heterogeneity that is necessary to show such a relation. Despite higher fatigue scores in MS patients, they did not depict a relation to the NAT availability either.
Another question is if the NAT group differences we found are region-specific. Although it is tempting to attribute NAT changes in MS specifically to the limbic system (amygdala, striatum) or brain regions essential for the regulation of vigilance and arousal (thalamus, brainstem), it must be noted that NAT tended to be higher in almost all brain regions of the patient cohort. However, some of the analysed regions such as the hippocampus or insula have low NAT availabilities, a poorer signal-to-noise ratio and high variability19; hence, statistical power possibly was not sufficient to detect intergroup differences in all regions specificied in this small sample. Yet, exploratory analyses of these brain regions are of interest since they belong to noradrenergic circuits which are involved in emotion regulation and memory processing5,47,48.
Conclusion
Our study indicates changes of noradrenergic signalling in immunotherapy-naïve RRMS patients. These first data require replication with larger sample sizes which allow subgroup analyses, e.g., also including patients with severe fatigue, a higher variability of depressive symptoms or cognitive deficits. Such studies might also include combined PET-MRI methods, e.g. for the assessment of neuromelanin as a marker of catecholaminergic neurons in relation to NAT availability49. Since the noradrenergic system is involved both in chronic inflammatory states and neuropsychiatric disorders50, NAT may constitute a promising candidate linking neuroinflammation with neuropsychiatric symptoms.
Methods
Participants
Twenty-three individuals were enrolled: eleven patients with definite relapsing–remitting MS (RRMS) according to both the criteria of Poser51 and the revised McDonald criteria52; and twelve age- and sex-matched healthy controls (HC; for subject characteristics see Table 1). RRMS patients were recruited from the outpatient clinic of the Department of Neurology, University of Leipzig. Exclusion criteria were psychiatric or neurologic disorders other than MS, a past or current history of psychopharmacotherapy, other autoimmune, chronic inflammatory or endocrine disease and hormonally active therapies (except for oral contraceptives), as well as pregnant and breastfeeding women. To screen for sub-threshold depressive symptoms, the Beck Depression Inventory (BDI) was performed at the first visit53. Fatigue was evaluated using the MS fatigue scale Würzburger Erschöpfungsinventar bei MS (WEIMuS)54. RRMS patients were naïve to antidepressants and disease-modifying therapy, and their most recent relapse or last treatment with corticosteroids had to be at least eight weeks before enrollment. Disease severity was rated using the Expanded Disability Status Scale (EDSS)55. Disease duration, or time since manifestation, is considered as the interval between the first symptoms were reported, in other words the first manifestation of the disease verified by residual neurological signs or correlating findings on evoked potentials or MRI, and PET data acquisition. Time since diagnosis designates the interval between the diagnosis of MS was made and PET imaging. All patients who agreed to disease modifying therapy (DMT) were treated as soon as possible after study completion. However, since we practice a system of shared decision making in the clinic, some patients decline DMT and prefer a strategy of watchful waiting. This resulted in 2 of 11 patients without treatment, who were included in the study at a routine follow-up visit. The study was conducted in accordance with the International Council for Harmonisation Guideline for Good Clinical Practice (ICH-GCP) and the declaration of Helsinki. It was approved by the local ethics committee of the Medical Faculty of the University of Leipzig (034–2007), and the German Bundesamt für Strahlenschutz/Federal Office for Radiation Protection (Z5-22,461/2–2013-010). All participants gave their written informed consent.
PET imaging
PET imaging using [11C]MRB was performed as described previously19. Both the MRB standard and precursor were prepared following previously described protocols56. In brief, [11C]MRB was synthesized from [11C]methyliodide ([11C]CH3I) using the TRACERLab FXC automated synthesis module (GE Healthcare, USA). The final formulated product was 98% radiochemically pure, the average injected mass was 0.021 ± 0.01 μg/kg. Dynamic PET was started after intravenous bolus injection (90 s) of 485.6 [477.2 – 489.9] MBq [11C] MRB (MS patients) and 380.6 [357.2 – 485.1] MBq (HC; see Table 1) using the ECAT EXACT HR + scanner in 3D acquisition mode (Siemens, Erlangen, Germany; intrinsic resolution at the centre 4.3 mm [full-width at half maximum, FWHM], axial resolution: 5–6 mm, field of view: 15.5 cm). Emission scan duration was 90 min, acquiring 23 frames (4 × 0.25, 4 × 1, 5 × 2, 5 × 5, 5 × 10 min). We used a 10-min transmission scan (from three 68Ga sources), which was performed prior to the emission scan, for attenuation correction. An iterative reconstruction (10 iterations, 16 subsets) was applied to a transverse image series (63 slices, 128 × 128 matrix, voxel size 2.6 × 2.6 × 2.4 mm3) with a Hann filter (cut-off 4.9 mm) for post-processing19.
Imaging data processing and analysis
PET and MRI data analyses were performed as described previously19. Briefly, individual MRI data sets of the subjects were spatially reoriented (according to the anterior commissure-posterior commissure line) onto a standard brain data set similar to the Talairach space using the image processing software PMOD version 3.5 (PMOD Technologies, Zurich, Switzerland). Hereafter, volumes of interest (VOIs) were drawn manually on consecutive transverse slices of the reoriented individual MRI data sets (Fig. 2). PET data were corrected for head motion artifacts using SPM2 software (Statistical Parametric Mapping; Wellcome Trust Centre for Neuroimaging, London, UK) and then co-registered with the individual MRI data, respective of the related VOI set, to obtain via PMOD the corresponding tissue time-activity curves (TACs) from the dynamic PET data. Kinetic modelling of these regional brain TACs was performed using the multilinear reference tissue model MRTM2 (two parameters)57 with the occipital cortex as reference regions in PMOD (version 3.5, PMOD Technologies LLC, Zurich, Switzerland). The calculated binding potential (BPND) depends linearly on the regional NAT availability and is connected to the DV ratio (DVR) of the target and the occipital cortex as the reference region by BPND = DVR-158,59. The kinetic modelling yields estimates of the BPND independent of injected tracer dose and body weight. In the final computation of the DVR, the population mean value of k2′ in the thalamus from 17 study participants (described in detail in Hesse et al.19 ) was 0.0238 ± 0.008 1/min and used as fixed washout constant k2′_fix. MRTM2 becomes linear after a certain time, called t*57. For MRB, t* equals 0. Due to low tracer activity in the first 3 PET frames, we started the multilinear regression at frame 4 (0.75 min). Head movements of MS patients and HC increased at the end of the scan (after 90 or 120 min, respectively), therefore we only used time activity curves up to 90 min (frames 4 to 23) for multi-linear regression analysis. By the use of the Lesion Segmentation Tool (LST) included in Applied Statistics for SPM, we assessed lesion load as measured by the number and total volume of lesions by means of the lesion prediction algorithm (LPA)60. In MS patients who were investigated after HC, the injected tracer activity was increased. DVR was computed based on the reference region with linear tracer kinetics which is independent of the injected tracer activity57.
Statistical analysis
Data analyses were performed using Microsoft Excel 2010 and SPSS 23. Graphs were created using GraphPad Prism 5. All data are given as median with interquartile range (IQR) or mean ± standard deviation (SD). After the exclusion of asymmetries between corresponding brain regions using the paired t-test or Wilcoxon test, DVRs were averaged side-by-side to reduce the number of variables and multiple comparison. Data distribution was analyzed using histograms and the Shapiro–Wilk test. For data which were normally distributed, the non-paired t-test and Pearson correlation were applied for inference tests or correlative analyses. For data which were not normally distributed, the Mann–Whitney-U test or Spearman-rank correlation were applied. Two-tailed significance was applied. Results were considered significant at p < 0.05.
Data availability
The datasets used and analyzed are available from the corresponding author on reasonable request.
Abbreviations
- [11C]MRB:
-
S,S-[11C]O-methylreboxetine
- BDI:
-
Beck depression inventory
- BMI:
-
Body mass index
- BPND :
-
Binding potential
- DMT:
-
Disease modifying therapy
- DRKS:
-
German clinical trials register
- DVR:
-
Distribution volume ratio
- EAE:
-
Experimental autoimmune encephalitis
- EDSS:
-
Expanded disability status scale
- FWHM:
-
Full-width at half maximum
- HC:
-
Healthy controls
- HPA:
-
Hypothalamic–pituitary–adrenal
- ICH-GCP:
-
International council for harmonisation guideline for good clinical practice
- IQR:
-
Interquartile range
- LPA:
-
Lesion prediction algorithm
- LST:
-
Lesion segmentation tool
- MRI:
-
Magnetic resonance imaging
- MRTM2:
-
Multilinear reference tissue model (two parameters)
- MS:
-
Multiple sclerosis
- NA:
-
Noradrenaline
- NAT:
-
Noradrenaline transporter
- PET:
-
Positron emission tomography
- RRMS:
-
Relapsing–remitting MS
- SD:
-
Standard deviation
- TAC:
-
Time-activity curve
- VOI:
-
Volume of interest
- WEIMuS:
-
Würzburger Erschöpfungsinventar bei MS
References
Krupp, L. B., Alvarez, L. A., LaRocca, N. G. & Scheinberg, L. C. Fatigue in multiple sclerosis. Arch. Neurol. 45, 435–437. https://doi.org/10.1001/archneur.1988.00520280085020 (1988).
Patten, S. B., Beck, C. A., Williams, J. V. A., Barbui, C. & Metz, L. M. Major depression in multiple sclerosis. A population-based perspective. Neurology 61, 1524–1527. https://doi.org/10.1212/01.wnl.0000095964.34294.b4 (2003).
von Bismarck, O. et al. Treatment choices and neuropsychological symptoms of a large cohort of early MS. Neurology Neuroimmunol. Neuroinflammat. 5, 446. https://doi.org/10.1212/NXI.0000000000000446 (2018).
Chiaravalloti, N. D. & DeLuca, J. Cognitive impairment in multiple sclerosis. The Lancet. Neurology 7, 1139–1151. https://doi.org/10.1016/S1474-4422(08)70259-X (2008).
Berridge, C. W. & Waterhouse, B. D. The locus coeruleus-noradrenergic system. Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 42, 33–84 (2003).
Simonini, M. V. et al. Increasing CNS noradrenaline reduces EAE severity. J. Neuroimmune Pharmacol. 5, 252–259. https://doi.org/10.1007/s11481-009-9182-2 (2010).
Gulyás, B. et al. The norepinephrine transporter (NET) radioligand (S, S)-18FFMeNER-D2 shows significant decreases in NET density in the human brain in Alzheimer’s disease: a post-mortem autoradiographic study. Neurochem. Int. 56, 789–798. https://doi.org/10.1016/j.neuint.2010.03.001 (2010).
Remy, P., Doder, M., Lees, A., Turjanski, N. & Brooks, D. Depression in Parkinson’s disease. Loss of dopamine and noradrenaline innervation in the limbic system. Brain128, 1314–1322. https://doi.org/10.1093/brain/awh445 (2005).
Moriguchi, S. et al. Norepinephrine transporter in major depressive disorder. A PET study. Am. J. Psychiat. 174, 36–41. https://doi.org/10.1176/appi.ajp.2016.15101334 (2017).
Goddard, A. W. et al. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress. Anxiety 27, 339–350. https://doi.org/10.1002/da.20642 (2010).
Vanicek, T. et al. The norepinephrine transporter in attention-deficit/hyperactivity disorder investigated with positron emission tomography. JAMA Psychiatry 71, 1340–1349. https://doi.org/10.1001/jamapsychiatry.2014.1226 (2014).
Feinstein, D. L., Kalinin, S. & Braun, D. Causes, consequences, and cures for neuroinflammation mediated via the locus coeruleus. Noradrenergic signaling system. J. Neurochem. 139, 154–178. https://doi.org/10.1111/jnc.13447 (2016).
Frohman, E. M., Vayuvegula, B., van den Noort, S. & Gupta, S. Norepinephrine inhibits gamma-interferon-induced MHC class II (Ia) antigen expression on cultured brain astrocytes. J. Neuroimmunol. 17, 89–101. https://doi.org/10.1016/0165-5728(88)90017-3 (1988).
Heneka, M. T. et al. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc. Natl. Acad. Sci. USA 107, 6058–6063. https://doi.org/10.1073/pnas.0909586107 (2010).
Patel, N. J., Chen, M. J. & Russo-Neustadt, A. A. Norepinephrine and nitric oxide promote cell survival signaling in hippocampal neurons. Eur. J. Pharmacol. 633, 1–9. https://doi.org/10.1016/j.ejphar.2010.01.012 (2010).
Polak, P. E., Kalinin, S. & Feinstein, D. L. Locus coeruleus damage and noradrenaline reductions in multiple sclerosis and experimental autoimmune encephalomyelitis. Brain 134, 665–677. https://doi.org/10.1093/brain/awq362 (2011).
Markianos, M. et al. Relationship of CSF neurotransmitter metabolite levels to disease severity and disability in multiple sclerosis. J. Neurochem. 108, 158–164. https://doi.org/10.1111/j.1471-4159.2008.05750.x (2009).
Benarroch, E. E. Monoamine transporters. Structure, regulation, and clinical implications. Neurology 81, 761–768. https://doi.org/10.1212/WNL.0b013e3182a1ab4a (2013).
Hesse, S. et al. Central noradrenaline transporter availability in highly obese, non-depressed individuals. Eur. J. Nucl. Med. Mol. Imag. 44, 1056–1064. https://doi.org/10.1007/s00259-016-3590-3 (2017).
Gallezot, J.-D. et al. Evaluation of (11)CMRB for assessment of occupancy of norepinephrine transporters. Studies with atomoxetine in non-human primates. NeuroImage 56, 268–279. https://doi.org/10.1016/j.neuroimage.2010.09.040 (2011).
Park, E. et al. (11)C-PBR28 imaging in multiple sclerosis patients and healthy controls: test-retest reproducibility and focal visualization of active white matter areas. Eur. J. Nucl. Med. Mol. Imag. 42, 1081–1092. https://doi.org/10.1007/s00259-015-3043-4 (2015).
Airas, L., Rissanen, E. & Rinne, J. Imaging of microglial activation in MS using PET: Research use and potential future clinical application. Mult. Scleros. 23, 496–504. https://doi.org/10.1177/1352458516674568 (2017).
Stankoff, B. et al. Imaging central nervous system myelin by positron emission tomography in multiple sclerosis using methyl-11C-2-(4’-methylaminophenyl)- 6-hydroxybenzothiazole. Ann. Neurol. 69, 673–680. https://doi.org/10.1002/ana.22320 (2011).
Pietroboni, A. M. et al. Amyloid PET as a marker of normal-appearing white matter early damage in multiple sclerosis: correlation with CSF β-amyloid levels and brain volumes. Eur. J. Nucl. Med. Mol. Imag. 46, 280–287. https://doi.org/10.1007/s00259-018-4182-1 (2019).
Faria, D. P., Copray, S., Buchpiguel, C., Dierckx, R. & deVries, E. PET imaging in multiple sclerosis. J. Neuroimmune Pharmacol. 9, 468–482. https://doi.org/10.1007/s11481-014-9544-2 (2014).
Högel, H., Rissanen, E., Vuorimaa, A. & Airas, L. Positron emission tomography imaging in evaluation of MS pathology in vivo. Mult. Scleros. 24, 1399–1412. https://doi.org/10.1177/1352458518791680 (2018).
Polak, P. E., Lin, S. X., Pelligrino, D. & Feinstein, D. L. The blood-brain barrier-permeable catechol-O-methyltransferase inhibitor dinitrocatechol suppresses experimental autoimmune encephalomyelitis. J. Neuroimmunol. 276, 135–141. https://doi.org/10.1016/j.jneuroim.2014.09.004 (2014).
Zoukos, Y., Leonard, J. P., Thomaides, T., Thompson, A. J. & Cuzner, M. L. beta-Adrenergic receptor density and function of peripheral blood mononuclear cells are increased in multiple sclerosis: a regulatory role for cortisol and interleukin-1. Ann. Neurol. 31, 657–662. https://doi.org/10.1002/ana.410310614 (1992).
Boyko, A., Melnikov, M., Zhetishev, R. & Pashenkov, M. The role of biogenic amines in the regulation of interaction between the immune and nervous systems in multiple sclerosis. Neuroimmunomodulation 23, 217–223. https://doi.org/10.1159/000449167 (2016).
Hesse, S. et al. Altered serotonin transporter availability in patients with multiple sclerosis. Eur. J. Nucl. Med. Mol. Imag. 41, 827–835. https://doi.org/10.1007/s00259-013-2636-z (2014).
Cosentino, M. et al. Catecholamine production and tyrosine hydroxylase expression in peripheral blood mononuclear cells from multiple sclerosis patients: effect of cell stimulation and possible relevance for activation-induced apoptosis. J. Neuroimmunol. 133, 233–240 (2002).
Rajda, C. et al. The norepinephrine level is decreased in the lymphocytes of long-term interferon-beta-treated multiple sclerosis patients. Multi. Scleros. 12, 265–270. https://doi.org/10.1191/135248506ms1269oa (2006).
Kaneko, Y. S. et al. Peripheral injection of lipopolysaccharide enhances expression of inflammatory cytokines in murine locus coeruleus. Possible role of increased norepinephrine turnover. J. Neurochem. 94, 393–404. https://doi.org/10.1111/j.1471-4159.2005.03209.x (2005).
Then Bergh, F., Kümpfel, T., Trenkwalder, C., Rupprecht, R. & Holsboer, F. Dysregulation of the hypothalamo-pituitary-adrenal axis is related to the clinical course of MS. Neurology 53, 772. https://doi.org/10.1212/WNL.53.4.772 (1999).
Heesen, C., Gold, S. M., Raji, A., Wiedemann, K. & Schulz, K.-H. Cognitive impairment correlates with hypothalamo-pituitary-adrenal axis dysregulation in multiple sclerosis. Psychoneuroendocrinology 27, 505–517 (2002).
Schinke, C. et al. Central noradrenaline transporter availability is linked with HPA axis responsiveness and copeptin in human obesity and non-obese controls. Stress 22, 93–102. https://doi.org/10.1080/10253890.2018.1511698 (2019).
Zhou, J. Norepinephrine transporter inhibitors and their therapeutic potential. Drugs Fut. 29, 1235–1244 (2004).
Sommerauer, M. et al. Decreased noradrenaline transporter density in the motor cortex of Parkinson’s disease patients. Move. Disord. 33, 1006–1010. https://doi.org/10.1002/mds.27411 (2018).
Ding, Y.-S. et al. PET imaging of the effects of age and cocaine on the norepinephrine transporter in the human brain using (S, S)-(11)CO-methylreboxetine and HRRT. Synapse 64, 30–38. https://doi.org/10.1002/syn.20696 (2010).
de Stefano, N. et al. Evidence of axonal damage in the early stages of multiple sclerosis and its relevance to disability. Arch. Neurol. 58, 65–70. https://doi.org/10.1001/archneur.58.1.65 (2001).
Hobart, J., Freeman, J. & Thompson, A. Kurtzke scales revisited: the application of psychometric methods to clinical intuition. Brain 123(Pt 5), 1027–1040. https://doi.org/10.1093/brain/123.5.1027 (2000).
Engström, M., Flensner, G., Landtblom, A.-M., Ek, A.-C. & Karlsson, T. Thalamo-striato-cortical determinants to fatigue in multiple sclerosis. Brain Behav. 3, 715–728. https://doi.org/10.1002/brb3.181 (2013).
Veauthier, C., Hasselmann, H., Gold, S. M. & Paul, F. the berlin treatment algorithm recommendations for tailored innovative therapeutic strategies for multiple sclerosis-related fatigue. EPMA J. 7, 25. https://doi.org/10.1186/s13167-016-0073-3 (2016).
Feinstein, A., Magalhaes, S., Richard, J.-F., Audet, B. & Moore, C. The link between multiple sclerosis and depression. Nat. Rev. Neurol. 10, 507–517. https://doi.org/10.1038/nrneurol.2014.139 (2014).
Eyding, D. et al. Reboxetine for acute treatment of major depression. Systematic review and meta-analysis of published and unpublished placebo and selective serotonin reuptake inhibitor controlled trials. BMJ (Clinical research ed.) 341, c4737. 10.1136/bmj.c4737 (2010).
Solaro, C. et al. Duloxetine is effective in treating depression in multiple sclerosis patients. An open-label multicenter study. Clin. Neuropharmacol. 36, 114–116. https://doi.org/10.1097/WNF.0b013e3182996400 (2013).
Hesse, S. et al. The association between in vivo central noradrenaline transporter availability and trait impulsivity. Psychiat. Res. Neuroimaging 267, 9–14. https://doi.org/10.1016/j.pscychresns.2017.06.013 (2017).
Tully, K. & Bolshakov, V. Y. Emotional enhancement of memory: how norepinephrine enables synaptic plasticity. Mol. Brain 3, 15. https://doi.org/10.1186/1756-6606-3-15 (2010).
Wengler, K., He, X., Abi-Dargham, A. & Horga, G. Reproducibility assessment of neuromelanin-sensitive magnetic resonance imaging protocols for region-of-interest and voxelwise analyses. NeuroImage 208, 116457. https://doi.org/10.1016/j.neuroimage.2019.116457 (2020).
Dantzer, R., O’Connor, J. C., Freund, G. G., Johnson, R. W. & Kelley, K. W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56. https://doi.org/10.1038/nrn2297 (2008).
Poser, C. M. et al. New diagnostic criteria for multiple sclerosis. Guidelines for research protocols. Ann. Neurol. 13, 227–231. https://doi.org/10.1002/ana.410130302 (1983).
Polman, C. H. et al. Diagnostic criteria for multiple sclerosis. 2010 revisions to the McDonald criteria. Ann. Neurol. 69, 292–302. https://doi.org/10.1002/ana.22366 (2011).
Beck, A. T. An inventory for measuring depression. Arch. Gen. Psychiat. 4, 561. https://doi.org/10.1001/archpsyc.1961.01710120031004 (1961).
Flachenecker, P. et al. "Fatigue" bei Multipler Sklerose. Entwicklung und Validierung des "Würzburger Erschöpfungsinventars bei MS". Nervenarzt 77, 165–174. https://doi.org/10.1007/s00115-005-1990-x (2006).
Kurtzke, J. F. Rating neurologic impairment in multiple sclerosis. An expanded disability status scale (EDSS). Neurology 33, 1444. https://doi.org/10.1212/WNL.33.11.1444 (1983).
Lin, K.-S. & Ding, Y.-S. Synthesis, enantiomeric resolution, and selective C-11 methylation of a highly selective radioligand for imaging the norepinephrine transporter with positron emission tomography. Chirality 16, 475–481. https://doi.org/10.1002/chir.20055 (2004).
Ichise, M. et al. Linearized reference tissue parametric imaging methods. Application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J. Cereb. Blood Flow Metabol. 23, 1096–1112. https://doi.org/10.1097/01.WCB.0000085441.37552.CA (2003).
Innis, R. B. et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J. Cereb. Blood Flow Metabol. 27, 1533–1539. https://doi.org/10.1038/sj.jcbfm.9600493 (2007).
Becker, G. A. et al. PET quantification of 18F-florbetaben binding to β-amyloid deposits in human brains. J. Nucl. Med. 54, 723–731. https://doi.org/10.2967/jnumed.112.107185 (2013).
Schmidt, P. Dissertation: Bayesian inference for structured additive regression models for large-scale problems (Chapter 6.1). Universitätsbibliothek der Ludwig-Maximilians-Universität, München, Germany. https://edoc.ub.uni-muenchen.de/ (2017).
Acknowledgements
The authors thank Dr. Franziska Zientek for her help in proband recruitment and support in the conduct of the study, and Elfi Boxhammer for her technical and organizational assistance. Part of the work was supported by the Federal Ministry of Education and Research (BMBF), Germany, FKZ: [01E01001] (https://www.bmbf.de). F.T.B. and M.S. were funded, in part, through TRM, by the German Federal Ministry of Education and Research [BMBF 1315883]. C.S. is participant in the BIH-Charité Junior Clinician Scientist Program funded by the Charité –Universitätsmedizin Berlin and the Berlin Institute of Health.
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
E.S. and C.S. conducted the study, were responsible for patient recruitment and care, collection and analyses of clinical data and samples, statistical analyses, drafting and manuscript editing. Sw.H. and F.T.B. designed and conducted the study and edited the manuscript. M.R., J.L., G.A.B. and O.S. were substantially involved in the collection and analyses of imaging data, design and conduction of the study and edited the manuscript. M.S. and S.H. were involved in regulatory preparations, patient recruitment and manuscript editing. D.L. and K.T.H. provided and analyzed MR imaging data and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schmidt, E., Schinke, C., Rullmann, M. et al. Changes of central noradrenaline transporter availability in immunotherapy-naïve multiple sclerosis patients. Sci Rep 10, 14651 (2020). https://doi.org/10.1038/s41598-020-70732-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-70732-5
- Springer Nature Limited
This article is cited by
-
Resting state functional connectivity modifications in monoaminergic circuits underpin fatigue development in patients with multiple sclerosis
Molecular Psychiatry (2024)
-
Monoaminergic network dysfunction and development of depression in multiple sclerosis: a longitudinal investigation
Journal of Neurology (2024)
-
Functional connectivity modifications in monoaminergic circuits occur in fatigued MS patients treated with fampridine and amantadine
Journal of Neurology (2023)