Abstract
To examine the association between various retinal vascular measurements and microalbuminuria in patients with type 2 diabetes in a northwestern China study. Data from 911 patients with type 2 diabetes were analyzed. Novel retinal vascular measurements from the whole vascular tree were extracted using a validated fully automatic computer program. Retinal vascular measurements were analyzed continuously and categorically for associations with microalbuminuria using multiple logistic regressions, adjusted for related variables. In logistic regression adjusting for multiple variables, microalbuminuria was associated with smaller peripheral arteriolar caliber, larger peripheral venular caliber, larger arteriolar tortuosity, and smaller arteriolar fractal dimension (p = 0.028, p < 0.001, p = 0.038, p = 0.035, respectively). In further categorical analyses, microalbuminuria was related to smaller peripheral arteriolar caliber [T1 vs. T3: odds ratio (OR) 2.029; 95% confidence interval (CI) 1.186–3.473], larger peripheral venular caliber (T1 vs. T3: OR 0.609; 95% CI 0.362–1.024), and smaller arteriolar fractal dimension (T1 vs. T3: OR 1.659; 95% CI 1.028–2.675). Microalbuminuria in type 2 diabetes is associated with both retinal vascular caliber and geometry. These noninvasive vascular measurements serve as potential preclinical markers to identify populations at high risk of early kidney disease in the course of diabetes.
Similar content being viewed by others
Introduction
There are 463 million adults with diabetes mellitus worldwide in 2019, with high rates of diabetic kidney disease (DKD) reported1. It is shown that even early stages of DKD confer a substantial increase in the risk of cardiovascular disease, making the early identification of DKD of great importance2. Current biomarkers, such as albuminuria and estimated glomerular filtration rate (eGFR), are mostly functional measures and have limited predictive precision at an earlier preclinical stage3. Kidney biopsy samples can show early disease, but the biopsy procedure is too invasive for routine use3. As a result, there remains an unmet clinical need for easily accessible, non-invasive surrogate biomarkers that allow early identification of those individuals at high risk4.
The retina gives a unique chance to visualize and monitor human microcirculation optically and non-invasively. Moreover, retinal and renal microvasculature is reported to share similar physiological changes during early diabetes because of abnormal glucose metabolism and other processes5,6. In this respect, a large number of studies have been conducted in the past decades and evidences have suggested that retinal vascular changes are related to the risk of renal dysfunction in diabetes7,8,9,10.
In spite of the general associations that have been established, the results have been inconclusive11. For instance, retinal arteriolar caliber was positively associated12,13,14 or not associated15,16 with reduced renal function, while venular caliber was negatively associated17,18 or not associated14,15,16 with reduced renal function. Previous studies on retinal vasculature were usually restricted to retinal vessel from selected regions in the image. For example, caliber measurements were restricted to retinal vessels proximal to the optic disc while geometrical measurements were restricted to manually selected vascular branches19. However, a few recent studies have suggested that more peripheral retinal vessels may signify an even earlier change than central retinal vessels in microvascular complications of diabetes20. In this respect, although inspiring results were reported, to the best of our knowledge, no study has extended the measurements to include all visible vessels in the retinal image.
In this study, we examined the associations between various retinal vascular measurements and urinary albumin to creatinine ratio (ACR) in patients with type 2 diabetes. Retinal vascular measurements were extended to the most distal branches using a validated fully-automatic computer program. We hypothesized that extending the measurements to distal branches will allow a better representation of the entire vasculature and thus provide a more comprehensive understanding of the relationship between retinal vasculature and early renal function in diabetes.
Materials and methods
Study population and study design
The Northwest China Diabetes Study is a cross-sectional study of diabetes patient, who attended the First Affiliated Hospital (Xijing Hospital) of Fourth Military Medical University of China between January 2014 and August 2016. For this study, we included participants with type 2 diabetes, aged from 18 to 70 years old and further excluded those with prevalent cardiovascular diseases (defined as self-reported myocardial infarction, angina or stroke), diabetic ketoacidosis, diabetic hyperglycemic hyperosmolar state, septicemia and end-stage renal disease (estimated glomerular filtration rate ≤ 30 mL/(min 173 m2)) (n = 934). Of the 934 participants, 911 (97.5%) had gradable retinal photographs and formed the base population of this study. All data were collected with approval by the Institutional Review Board of the Air Force Medical University of China in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from all participants.
Measurement of early renal dysfunction
Participants provided a spot urine specimen generally immediately after arriving in the morning and urine albumin and creatinine were measured at the Clinical Chemistry Laboratory of Xijing Hospital. ACR was calculated from assayed albumin and creatinine levels. Microalbuminuria was defined as ACR > 2.5 mg/mmol in men and ACR > 3.5 mg/mmol in women21.
Measurement of other variables
Digital fundus photography was performed using a 45° retinal camera (Canon CR-DGI with a 10D SLR digital camera back, Canon, Tokyo, Japan) after pupil dilation. To better visualize the distal branches, macular-centered retinal images were captured. The right eye of each participant was used and when unavailable, was replaced by the left eye.
All participants underwent a face-to-face interview at clinic regarding their past medical history and current medications. Systolic and diastolic blood pressure were measured using a digital automatic BP monitor (Orion Instruments, Inc). Body mass index (BMI) was calculated from weight (kg) divided by squared height (m2). Biochemical analysis of fasting venous blood samples was performed with DX-800 Automated analyzer (Beckman Kurt Inc) for HbA1c, serum creatinine, total cholesterol, triglycerides, high-density lipoprotein (HDL), and low-density lipoprotein (LDL).
Retinal vascular measurements
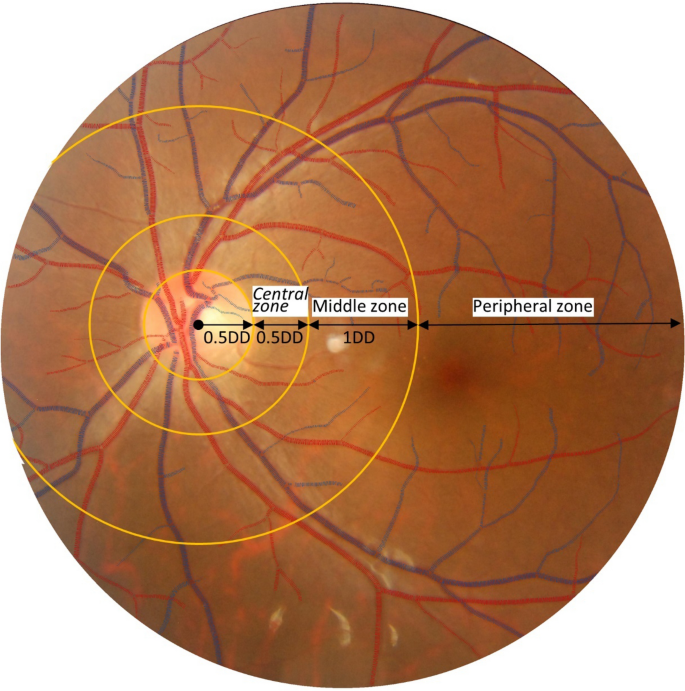
Retinal vascular measurements were performed using a fully-automated computer software (supplemental Fig. 1). The software automatically finds optic disc center, segments the whole vascular tree, distinguishes arterioles and venules, measures vessel width, and calculates tortuosity and fractal dimension. Specifically, two sets of measurements were calculated. The first set is vascular caliber measurements. Centered at the optic disc, blood vessels were categorized into three concentric zones: central zone (0.5–1.0 disc diameter, DD), middle zone (1.0–2.0 DD), and peripheral zone (> 2.0 DD) (Fig. 1). The averaged arteriolar and venular calibers were calculated in each zone and denoted as aCtr, aMdl, aPeri, vCtr, vMdl, and vPeri, respectively. The second set is vascular geometry measurements, including arteriolar and venular fractal dimension (i.e., aDf and vDf) and tortuosity (i.e., aTor and vTor). The accuracy and reproducibility of this system have been validated extensively on public datasets22.
Sample image showing the vascular caliber measurements. Centered at the optic disc, blood vessels were categorized into three concentric zones: central zone (0.5–1.0 DD), middle zone (1.0–2.0 DD), and peripheral zone (> 2.0 DD). The averaged arteriolar and venular calibers were calculated in each zone and denoted as aCtr, aMdl, aPeri, vCtr, vMdl, and vPeri, respectively.
Statistical methods
At baseline, continuous data are presented as mean ± SD and categorical data are presented as percentages. Mann–Whitney U test was used for continuous data and the χ2 test was used for categorical data to assess the differences between two groups. In logistic regression, vascular measurements were first analyzed as continuous variables and, if significance was found, further analyzed as categorical variables in tertiles. Two models were used to analyze the association between vascular measurements and microalbuminuria in logistic regression, with the first model adjusted for basic participant information (i.e., age and gender) and the second model further adjusted for covariates known to be associated with renal dysfunction (i.e., duration, smoking, BMI (kg/m2), systolic and diastolic blood pressure (mmHg), glycosylated hemoglobin (%), total cholesterol (mmol/L), HDL (mmol/L), LDL (mmol/L), and triglyceride (mmol/L)). The counterpart vessel caliber was further adjusted in the vessel caliber analysis. Findings with a p value < 0.05 were considered statistically significant. The 95% CI was given for estimates of ORs and considered statistically significant if they did not cross 1.0. All statistical analyses were performed using Statistical Package for the Social Sciences version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristic
Baseline characteristics are given in Table 1. Subjects without microalbuminuria were more likely to be younger, with shorter duration, lower BMI, SBP, DBP, and triglyceride. There were no significant differences in smoking, HbA1c, total cholesterol, HDL, and LDL between the two groups. Subjects with microalbuminuria showed a smaller arteriolar fractal dimension (aDf,) as well as larger peripheral venular caliber (vPeri).
Logistic regression
In logistic regression analyses, retinal vascular measurements were first analyzed as continuous variable (Table 2). For vascular geometry measurements, arteriolar fractal dimension was associated with microalbuminuria in Model 1 (OR 0.062; 95% CI 0.015–0.249) and Model 2 (OR 0.187; 95% CI 0.039–0.886). Arteriolar tortuosity was associated with microalbuminuria after adjustment in Model 2 (OR 32.733; 95% CI 1.217–880.629). Venular tortuosity was also associated with microalbuminuria after adjustment in Model 1 (OR 18.860, 95% CI 1.249–284.667), but the association was attenuated and no longer significant after adjusting for more risk factors in Model 2.
For vascular caliber measurements, peripheral arteriolar caliber was associated with microalbuminuria in both Model 1 (OR 0.907; 95% CI 0.852–0.965) and Model 2 (OR 0.924; 95% CI 0.861–0.992). Peripheral venular caliber was also associated with microalbuminuria in both Model 1 (OR 1.183; 95% CI 1.096–1.278) and Model 2 (OR 1.180; 95% CI 1.080–1.290). Arteriolar calibers in the central zone (OR 0.935; 95% CI 0.885–0.988) and middle zone (OR 0.945; 95% CI 0.895–0.999), as well as venular caliber in the middle zone (OR 1.113; 95% CI 1.034–1.199), were also associated with microalbuminuria in Model 1, but the association was attenuated and no longer significant after adjusting for more risk factors in Model 2.
Retinal vascular measurements showed significant association with microalbuminuria in Model 2 were further analyzed as categorical variables in tertile (Table 3). Participants at the lowest aDf tertile showed 96.1% and 65.9% increased odds of microalbuminuria compared with those at the highest aDf tertile, after adjustment in Model 1 and Model 2 (OR 1.961; 95% CI 1.297–2.964 and OR 1.659; 95% CI 1.028–2.675, respectively). No associations were found between microalbuminuria and aTor.
Participants at the lowest aPeri tertile was associated with 120.0% and 102.9% increased odds of microalbuminuria compared with those at the highest aPeri tertile, after adjustment in Model 1 and Model 2 (OR 2.200; 95% CI 1.373–3.525 and OR 2.029; 95% CI 1.186–3.473, respectively). Participants at the middle vPeri tertile showed 58.0% and 57.4% decreased odds compared with those at the highest vPeri tertile, after adjustment in Model 1 and Model 2 (OR 0.420; 95% CI 0.275–0.641 and OR 0.426; 95% CI 0.264–0.687, respectively).
Discussion
In this study, we extended the vascular measurements to the entire vascular tree and explored the associations between microalbuminuria and retinal vascular measurements in patients with type 2 diabetes. Our findings suggested that microalbuminuria is associated with retinal vasculatures, both in terms of caliber and geometry. Our study adds significant new knowledge in this study field. First, we demonstrated that peripheral vascular calibers, rather than central vascular calibers, were strongly associated with microalbuminuria. Second, we demonstrated that arteriolar geometries, rather than venular geometries, were strongly associated with microalbuminuria.
Previous studies have been focusing on central retinal vessels proximal to optic disc19. As discussed in a recent study, reducing the measurement area would result in an enormous data reduction that may miss vessel changes occurring elsewhere in the retina20. By applying a fully automatic image processing program, we extended the measurements to the entire vascular trees, especially to the peripheral zone. Our findings showed that microalbuminuria was associated with peripheral arteriolar and venular calibers, rather than vascular calibers in the central or middle zones. It is known that smaller peripheral retinal vessels host pericytes and endothelial cells, both playing a major role in the pathogenesis of microvascular complications23. Apoptosis of pericytes is noted as one of the earliest vascular changes in hyperglycaemia and eventually leads to vascular nonperfusion24. We hypothesize that these early changes in pericytes lead to observable vascular caliber changes prior to the onset of clinical complications. In further analyses as categorical variables, the smallest aPeri tertile was associated with increased odds of microalbuminuria (T1 vs. T3: OR 2.029; 95% CI 1.186–3.473). These changes in peripheral vascular caliber may serve as useful biomarkers in identifying early renal changes.
Fractal dimension, a geometrical measurement indicating the complexity of branching patterns, has been associated with the incidence of diabetic nephropathy in cross-sectional and longitudinal studies25,26. However, to the best of our knowledge, no previous studies have separately analyzed the arteriolar and venular trees. With the automatic program, we analyzed the arteriolar and venular trees separately for the first time. We demonstrated that decreased arteriolar fractal dimension was significantly associated with reduced renal function, while the venous fractal dimension was not associated with renal function. Decreased vascular fractal dimension indicates vessel rarefaction27, which was speculated to be the result of vasoconstriction induced by endothelial dysfunction28. Although the underlying differences between arterioles and venules were not clear, it is reported that the retinal blood flow is decreased in the arterioles, but increased or unchanged in the venules in early diabetes29,30,31, which might lead to different physiological changes and results in a faster damage in arterioles.
Increased retinal arteriolar tortuosity has been associated with early kidney dysfunction in type 1 diabetes subjects32. But the results were based on selected arteriolar branches and could not represent the overall retinal vascular tortuosity. We extended the measurement to the entire arteriolar and venular trees for the first time. Our results supported that microalbuminuria was associated with increased arteriolar tortuosity in continuous analyses, though no association was found in further categorical analyses. It is reported that increased tortuosity is linked to impaired vessel autoregulation33, which is the result of disturbed blood flow and endothelial dysfunction in the course of diabetes34.
There are several limitations of our study. First, given the cross-sectional nature of this study, these associations do not allow a risk stratification. To gain better insights into the pathophysiological mechanism of the earlier stages of the disease and to evaluate the prognostic value of the image biomarkers, a longitudinal follow-up is desired in the future. Second, the current vascular caliber measurement simply averaged all vascular calibers within each zone and did not take the different branching levels into consideration. In this respect, caliber measurement that can represent different vascular branching levels will probably provide more meaningful information and is more desired.
In conclusion, this study evaluated the associations between various retinal vascular measurements and renal function in a northwestern China study with type 2 diabetes mellitus. The present study extended the conventional analysis of proximal branches of central retinal vessels to the entire vascular tree. We found that peripheral vascular calibers as well as arteriolar geometries were strongly associated with microalbuminuria. These measurements may assist in identifying individuals at high risk of complications early in the course of diabetes.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Saeedi, P. et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract.157, 107843–107843. https://doi.org/10.1016/j.diabres.2019.107843 (2019).
Ravi, R., Cull, C. A., Thorne, K. I., Adler, A. I. & Holman, R. R. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes55, 1832–1839 (2006).
Ikram, M. K. et al. Retinal vascular caliber as a biomarker for diabetes microvascular complications. Diabetes Care36, 750–759. https://doi.org/10.2337/dc12-1554 (2013).
Klein, R. et al. The relationship of retinal vessel diameter to changes in diabetic nephropathy structural variables in patients with type 1 diabetes. Diabetologia53, 1638–1646. https://doi.org/10.1007/s00125-010-1763-3 (2010).
Gariano, R. F. & Gardner, T. W. Retinal angiogenesis in development and disease. Nature438, 960–966 (2005).
Nagaoka, T. & Yoshida, A. Relationship between retinal blood flow and renal function in patients with type 2 diabetes and chronic kidney disease. Diabetes Care36, 957–961 (2013).
Romero-Aroca, P. et al. Relationship between diabetic retinopathy, microalbuminuria and overt nephropathy, and twenty-year incidence follow-up of a sample of type 1 diabetic patients. J. Diabetes Complicat.26, 506–512. https://doi.org/10.1016/j.jdiacomp.2012.06.010 (2012).
Cheung, Y. L. et al. Retinal vascular geometry and 6 year incidence and progression of diabetic retinopathy. Diabetologia60, 1770–1781 (2017).
Yip, W. F. et al. Comparison of common retinal vessel caliber measurement software and a conversion algorithm. Transl. Vis. Sci. Technol.5, 11 (2016).
Moriya, T., Matsubara, M., Kishihara, E., Yoshida, Y. & Ouchi, M. Type 2 diabetic patients with diabetic retinopathy and concomitant microalbuminuria showed typical diabetic glomerulosclerosis and progressive renal dysfunction. J. Diabetes Complicat.30, 1111–1116. https://doi.org/10.1016/j.jdiacomp.2016.04.007 (2016).
Cheung, C. Y., Ikram, M. K., Klein, R. & Wong, T. Y. The clinical implications of recent studies on the structure and function of the retinal microvasculature in diabetes. Diabetologia58, 871–885. https://doi.org/10.1007/s00125-015-3511-1 (2015).
Grauslund, J. et al. Retinal vessel calibre and micro- and macrovascular complications in type 1 diabetes. Diabetologia52, 2213–2217. https://doi.org/10.1007/s00125-009-1459-8 (2009).
Broe, R. et al. Retinal vessel calibers predict long-term microvascular complications in type 1 diabetes: the danish cohort of pediatric diabetes 1987 (DCPD1987). Diabetes63, 2215–2221 (2014).
Sabanayagam, C. et al. Retinal microvascular caliber and chronic kidney disease in an Asian population. Am. J. Epidemiol.169, 625–632 (2009).
Keel, S. et al. Retinal vascular caliber and kidney function in children and adolescents with type 1 diabetes. Ophthalmic Epidemiol.24, 204–208 (2017).
Mckay, G. J. et al. Retinal microvascular parameters are not associated with reduced renal function in a study of individuals with type 2 diabetes. Sci. Rep.8, 3931 (2018).
Wong, T. Y., Shankar, A., Klein, R. & Klein, B. E. K. Retinal vessel diameters and the incidence of gross proteinuria and renal insufficiency in people with type 1 diabetes. Diabetes53, 179–184. https://doi.org/10.2337/diabetes.53.1.179 (2004).
Klein, R., Klein, B. E. K., Moss, S. E. & Wong, T. Y. Retinal vessel caliber and microvascular and macrovascular disease in type 2 diabetes: XXI: The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology114, 1884–1892 (2007).
Benitez-Aguirre, P. Z. et al. The adolescent cardio-renal intervention trial (AdDIT): retinal vascular geometry and renal function in adolescents with type 1 diabetes. Diabetologia7, 1–9 (2018).
Owen, C. G. et al. Retinal vasculometry associations with cardiometabolic risk factors in the European prospective investigation of cancer—Norfolk study. Ophthalmology126, 96–106. https://doi.org/10.1016/j.ophtha.2018.07.022 (2019).
Rakesh, A. et al. Risk of microalbuminuria and progression to macroalbuminuria in a cohort with childhood onset type 1 diabetes: prospective observational study. BMJ336, 697–701 (2008).
Xu, X. et al. Simultaneous arteriole and venule segmentation with domain-specific loss function on a new public database. Biomed. Opt. Express9, 3153–3166. https://doi.org/10.1364/BOE.9.003153 (2018).
Velayutham, V. et al. Innovative technology shows impact of glycaemic control on peripheral retinal vessels in adolescents with type 1 diabetes. Diabetologia60, 2103–2110. https://doi.org/10.1007/s00125-017-4375-3 (2017).
Wilkinson-Berka, J. L. Diabetes and retinal vascular disorders: role of the renin-angiotensin system. Expert Rev. Mol. Med.6, 1–18 (2004).
Broe, R. et al. Retinal vascular fractals predict long-term microvascular complications in type 1 diabetes mellitus: the Danish Cohort of Pediatric Diabetes 1987 (DCPD1987). Diabetologia57, 2215–2221 (2014).
Sng, C. C. et al. Fractal analysis of the retinal vasculature and chronic kidney disease. Nephrol. Dial Transplant25, 2252–2258 (2010).
Cheung, C. Y. et al. Retinal vascular fractal dimension and its relationship with cardiovascular and ocular risk factors. Am. J. Ophthalmol.154, 663-674 e661. https://doi.org/10.1016/j.ajo.2012.04.016 (2012).
Vervoort, G. et al. Elevated skeletal muscle blood flow in noncomplicated type 1 diabetes mellitus: role of nitric oxide and sympathetic tone. Hypertension34, 1080 (1999).
Nagaoka, T. et al. Impaired retinal circulation in patients with type 2 diabetes mellitus: retinal laser Doppler velocimetry study. Investig. Ophthalmol. Vis. Sci.51, 6729–6734. https://doi.org/10.1167/iovs.10-5364 (2010).
Grunwald, J. E., Riva, C. E., Sinclair, S. H., Brucker, A. J. & Petrig, B. L. Laser Doppler velocimetry study of retinal circulation in diabetes mellitus. Arch. Ophthalmol.104, 991–996. https://doi.org/10.1001/archopht.1986.01050190049038 (1986).
Patel, V., Rassam, S., Newsom, R., Wiek, J. & Kohner, E. Retinal blood flow in diabetic retinopathy. BMJ (Clin. Res. Ed.)305, 678–683. https://doi.org/10.1136/bmj.305.6855.678 (1992).
Sasongko, M. B. et al. Retinal arteriolar tortuosity is associated with retinopathy and early kidney dysfunction in type 1 diabetes. Am. J. Ophthalmol.153, 176–183171. https://doi.org/10.1016/j.ajo.2011.06.005 (2012).
Taarnhoj, N. et al. Straight versus tortuous retinal arteries in relation to blood pressure and genetics. Br. J. Ophthalmol.92, 1055–1060 (2008).
Nicholas, W. et al. Abnormalities of retinal microvascular structure and risk of mortality from ischemic heart disease and stroke. Hypertension47, 975–981 (2006).
Acknowledgements
This work was supported by National Natural Science Foundation of China (81401480), China Postdoctoral Science Foundation (2016T90929), and Basic Natural Science Research Program of Shaanxi Province (2018JM7130).
Author information
Authors and Affiliations
Contributions
X.X. and B.G. designed the study and interpreted the results. X.X., B.G., and F.S. wrote the manuscript. X.X. and W.D. analyzed the images and performed statistical analysis. B.G., M.Z., Q.W., and A.Y. collected the clinical data. B.G. supervised the data quality control and statistical analysis. X.X. and B.G. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, X., Sun, F., Wang, Q. et al. Comprehensive retinal vascular measurements: a novel association with renal function in type 2 diabetic patients in China. Sci Rep 10, 13737 (2020). https://doi.org/10.1038/s41598-020-70408-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-70408-0
- Springer Nature Limited
This article is cited by
-
Remnant cholesterol is correlated with retinal vascular morphology and diabetic retinopathy in type 2 diabetes mellitus: a cross-sectional study
Lipids in Health and Disease (2024)
-
The automatic detection of diabetic kidney disease from retinal vascular parameters combined with clinical variables using artificial intelligence in type-2 diabetes patients
BMC Medical Informatics and Decision Making (2023)
-
Comprehensive retinal vascular measurements: time in range is associated with peripheral retinal venular calibers in type 2 diabetes in China
Acta Diabetologica (2023)
-
Application of multi-label classification models for the diagnosis of diabetic complications
BMC Medical Informatics and Decision Making (2021)
-
Retinal image measurements and their association with chronic kidney disease in Chinese patients with type 2 diabetes: the NCD study
Acta Diabetologica (2021)