Abstract
The aim of this study was to explore the microbial communities of endodontic infections at their apical portion by 16S rRNA Illumina sequencing and delineate the core microbiome of root canal infections and that of their associated clinical symptomatology. Samples were collected from fifteen subjects presenting one tooth with a root canal infection, and their associated symptoms were recorded. Samples were collected from the apical third of roots using a #10 K file and then amplified using multiple displacement amplification and PCR-amplified with universal primers. Amplicons were sequenced (V3–V4 hypervariable region of the 16S rRNA gene) using MiSeq (Illumina, CA). The microbial composition of the samples was determined using QIIME and HOMINGS. Data were analyzed using t tests and ANOVA. A total of 1,038,656 good quality sequences were obtained, and OTUs were assigned to 10 bacterial phyla, led by Bacteroidetes (51.2%) and Firmicutes (27.1%), and 94 genera were represented primarily by Prevotella (17.9%) and Bacteroidaceae G-1 (14.3%). Symptomatic teeth were associated with higher levels of Porphyromonas (p < 0.05) and Prevotella. P. endodontalis and P. oris were present in both cores. The present study demonstrated the complexity of the root canal microbiome and the “common denominators” of root canal infections and identified taxa whose virulence properties should be further explored. The polymicrobial etiology of endodontic infections has long been established. However, few studies have focused on expanding the breadth and depth of coverage of microbiome-infected root canals at their apical portion.
Similar content being viewed by others
Introduction
Microbiological evaluations of infected root canals have expanded our knowledge on the topic1,2,3,4, confirmed the predominance of anaerobic species, revealed previously unrecognized bacterial diversity1,2,3,5,6 and showed that the complexity of the microbial consortium influences the pathogenesis of periradicular conditions4,7. In infected root canals, microbial communities remain as surface-associated biofilms8,9. The bacterial biofilm requires treating root canals to prevent and/or heal apical periodontitis10.
There is overwhelming evidence to support that an unspecific microbial community is able to induce periapical lesion development4,7,11. However, as stated by Tatikonda et al.12, there are a few studies that focus on analyzing “the pulp canal segments”. The infected apical third of root canals maintains a distinct array of microorganisms from its coronal segment13,14. Nevertheless, few studies have focused on the analysis of the most apical portion of endodontic infections. This gap in the current literature has precluded a better picture of the root canal core microbiome and its associated clinical parameters.
The cloistered root canal space interferes in its microbial colonization (10, 12). The microbial status of infected root canals was demonstrated by studies that have progressed depending on the evolution of microbiological methods. Recently, culture-independent strategies, such as next-generation sequencing, have improved this knowledge. Additionally, molecular studies have revealed significant differences in the prevalence of certain pathogens, demonstrating that geographical and individual characteristics may influence bacterial community profiles1,2,5,15,16,17,18. Next-generation sequencing (NGS) has revolutionized genomic research19,20. The ultramodern MiSeq platform is ideal for rapid and cost-effective genetic analysis21. However, MiSeq sequencing may not always reach species-level taxonomic resolution. To improve this limitation, HOMINGS22,23,24 utilized the speed and efficiency of next-generation sequencing combined with the refinement of bacterial species-level identification based on 16S rDNA comparisons17.
Therefore, knowing that the apical portion of the root canal infection harbors a distinct microbiome from its coronal segment, this study aimed to explore the microbiome of the apical portion of the root canal through metagenetics approaches and its association with clinical symptomatology.
Materials and methods
Study population
Study participants were 15 patients referred to the dental school to receive endodontic care. The exclusion criteria for this study was antibiotic therapy up to 3 months before starting endodontic therapy, systemic diseases, and pregnancy. All participants signed the Free Agreement Formulary. The Ethics Committee of the FUMG approved this study (ETIC 122⁄08). Clinical samples were taken from 15 teeth (single and multirooted) with pulp necrosis and apical periodontitis that were diagnosed by clinical (presence of tissue swelling, percussion sensitivity, symptomatic or asymptomatic) and radiographic analyses, in addition to pulp sensibility tests.
Additionally, if the tooth was symptomatic, the following parameters were recorded: onset of pain (time and duration) and quality of pain (throbbing, stabbing, dull) and whether teeth were single or multirooted. The final diagnosis was made based on those findings. All teeth selected for sampling presented pulpal necrosis, as well as no history of trauma, periodontal involvement, or previous root canal treatment. The clinical parameter of each tooth is described in Supplemental Table 1.
Root canal samples
The selection and preparation of the teeth, as well as the sample collection, was performed, as previously described1,2, by the same experienced endodontist. Briefly, the tooth was cleaned with pumice and isolated with a rubber dam. The teeth were decontaminated and disinfected with a 30% hydrogen peroxide solution (H2O2) and then with 2.5% sodium hypochlorite solution (NaOCl). The access cavity was prepared with a high speed sterile carbide bur, and before the pulp chamber was exposed, the cleaning of the tooth and rubber dam was repeated as previously described. The samples were taken by filing the root canal walls with a sterile #10 K-type hand file (Maillefer, Ballaigues, Switzerland). The file was introduced into the canal to the level of the tooth apex. The tooth length was defined using an apex locator (Root ZXII ®; J. Morita-USA, Irvine, CA, USA). In multirooted teeth, samples were collected from the largest root canal. After removal from the canal, the final 4 mm of the file was cut using a sterile pair of surgical scissors and placed in a microcentrifuge tube containing 20 μl of alkaline lysis buffer (400 mM KOH, 100 mM dithiothreitol, 10 mM EDTA). After 10 min of incubation on ice, 20 μl of neutralization solution (400 mM HCl, 600 mM Tris–HCl, pH 0.6) was added. Samples were kept at 4 °C until analysis.
Multiple displacement amplification (MDA) of root canal samples
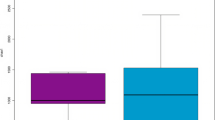
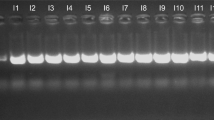
To ensure the availability of adequate DNA for analysis, Multiple displacement amplification (MDA) was performed prior to sequencing, as previously described1,2,25. The Illustra GenomiPhi V2 DNA Amplification Kit (GE Healthcare, Salt Lake City, UT, USA) was used, and DNA measurement prior to and after amplification was performed using the Picogreen™ dsDNA quantification assay (Invitrogen, Carlsbad, CA, USA). An example of the similarity of samples before and after MDA can be observed in Supplemental Figure 1.
Illumina sequencing of barcoded 16S rRNA gene amplicons
Sample DNA was analyzed by sequencing the 16S rRNA gene V3–V4 hypervariable region using MiSeq (Illumina, CA), according to the protocol described by Caporaso et al.26. In brief, 10–50 ng of DNA was PCR-amplified using the 341F/806R universal primers targeting the V3–V4 hypervariable region: 341F (forward) AATGATACGGCGACCACCGAGATCTACACTATGGTAATTGTCCTACGGGAGGCAGCAG; 806R (reverse) CAAGCAGAAGACGGCATACGAGATTCCCTTGTCTCC AGTCAGTCAGCCGGACTACHVGGGTWTCTAAT, where the ‘TCCCTTGTCTCC’ region represents the appropriate barcode sequences and the underlined bases make the PCR products Illumina sequencing compatible22,23,24. PCR samples were purified using AMPure beads, and 100 ng of each barcoded library was pooled, purified and quantified using a bioanalyzer and qPCR. Then, 12 pM of each library mixture library spiked with 20% PhiX was loaded onto the MiSeq and sequenced.
Sequencing analytical pipeline
The reads generated using MiSeq were analyzed using the QIIME pipeline27. In brief, the quality control of the reads was performed using FastQC. The paired-end reads were merged using Flash. The libraries were split in QIIME according to the barcodes used in the sequencing run, low-quality reads were filtered out, and chimeras were removed using UCHIME. Operational taxonomic units (OTUs) were picked using the Human Oral Microbiome Database (HOMD) v13.2 as a reference database28 using a 97% similarity threshold. Taxonomy was assigned using the Ribosomal Database Project (RDP) classifier trained on the HOMD v13.2 database with assignments required to meet a > 80% confidence threshold.
Because MiSeq sequencing may not always reach species-level taxonomic resolution, in the present study, we complemented it with HOMINGS22,23,24 an in silico 16S rDNA probe analysis that allows for species-level identification of sequencing datasets generated with MiSeq (https://homings.forsyth.org)17. Species-specific, 16S rRNA-based oligonucleotide “probes” were used in a Perl program based on a text string search to identify the frequency of oral bacterial targets. HOMINGS comprises 671 oligonucleotide probes of 17–40 bases that target 538 individual oral bacterial species/phylotypes or, in some cases, a few closely related taxa22,23,24.
Data analysis
Taxa detected by HOMINGS were mapped to species- and genus-level targets (v2.0, https://homings.forsyth.org/bacterialtaxa.html). Analyses were performed at the species level or by summing the relative abundance of the taxa detected to the genus or phylum level.
The HOMINGS taxa detected at ≥ 0.1% relative abundance in ≥ 50% of all samples were taken to constitute the microbial communities, which were subdivided based on the mean relative abundance of the taxa in symptomatic and asymptomatic samples29. Taxa that were present in ≥ 50% of samples in any of the clinical categories but were not part of the microbial communities considering all samples constituted microbial communities of those conditions. These taxa were subgrouped based on the mean relative abundance of the taxa in symptomatic and asymptomatic samples. Data analyses were performed using R 3.2.1 (https://cran.r-project.org/). The microbial composition of the symptomatic and asymptomatic samples was compared using t tests and ANOVA. Due to the exploratory nature of the study, no adjustments for multiple comparisons were performed.
Results
Study participants had a mean age of 39.8 years old (SD = 13.8; range: 12–69 years old), and most of them were female (n = 10). Most of the sampled teeth were multirooted (n = 12) and were symptomatic (n = 9) or presented with a closed cavity (n = 11).
A total of 1,038,656 sequences were obtained from the 15 samples after quality control (median: 29,328 reads). A total of 946 OTUs were identified and assigned to 10 phyla, 94 genera and 311 species using the QIIME pipeline. The most abundant phylum detected was Bacteroidetes (51.2%), followed by Firmicutes (27.1%) and Actinobacteria (11.5%) (Fig. 1a). The most prominent genera were Prevotella (17.9%) and Bacteroidaceae G-1 (14.3%) (Fig. 1b), while Bacteroidaceae [G-1] sp oral taxon (ot) 272 (12.6%), Parvimonas micra (6.2%), Porphyromonas endodontalis (3.4%), and Bacteroidetes [G-5] sp ot 511 (2.5%) were the most numerous species/phylotypes (Fig. 1c). Despite being a close-ended analysis, HOMINGS detected the most representative taxa in the samples, covering, on average, 84.1% of the post QC MiSeq reads (range: 57–93%).
Bar charts of the composition of each of the samples examined and their average representation at the phylum (A), genus (B) and species levels (C). Species-level results were obtained using HOMINGS. The percentage of reads that were not identified by HOMINGS (i.e., unassigned reads) was not plotted.
Principal coordinate analysis (PCoA) suggested that the status of the cavity at the time of sampling (open/closed cavity) not only can influence (or be influenced by) the microbial composition of the local biofilm, but this effect is of a lesser magnitude regarding the presence of symptoms because limited clustering was observed in those cases (Fig. 2a, b). The phyla Bacteroidetes and Firmicutes predominated in symptomatic and asymptomatic cases (Fig. 3a). In the presence of symptoms, higher levels of Prevotella (absence × presence, 11.5% × 22.2%), Bacteroidaceae [G-1] (10.9% × 16.6%), Porphyromonas (0.1% × 12.9%; p < 0.05), Parvimonas (2.6% × 9.9%) and Dialister (2.7% × 4.9%) were observed (Fig. 3b). Bacteroidaceae [G-1] sp ot 272 (9.9% × 14.4%), P. micra (2.4% × 8.8%), Prevotella oris (0.1% × 7.9%, p < 0.05), P. endodontalis (0.0% × 5.6%), Porphyromonas sp ot 395 (0.0% × 4.9%) and D. invisus (0.2% × 3.6%; p < 0.05) were the predominant species/phylotypes (Fig. 3c).
Line plots of the microbial composition of the samples in which symptoms were present (blue) or absent (pink), at the phylum (A), genus (B) and species levels (C). Graphs show the mean relative abundance for phyla that were 0.01% different, genera that were 0.1% different, and species that were 0.2% different. Taxa were sorted according to relative abundance in the positive group. ***Taxa with statistically significant differences (p ≤ 0.05).
The presence of an open cavity at the time of sampling (Supplemental Figure 2a, b, c) was associated with higher levels of Bacteroidetes (p < 0.05), whereas teeth that were closed harbored more Actinobacteria (p < 0.05). Furthermore, open teeth presented a higher abundance of Bacteroidaceae [G-1] sp (closed × open; 8.6% × 30.1%), Prevotella sp (16.0% × 23.2%), Porphyromonas sp (5.7% × 13.7%), Dialister (2.3% × 8.6%, p < 0.05) and Filifactor (0.3% × 1.5%, p < 0.05). In particular, those types of teeth had higher levels of Bacteroidaceae G1 ot 272 (7.6% × 26.3%), P. endodontalis (0.9% × 10.2%), P. oris (3.6% × 8.0%), D. invisus (1.5% × 4.2%) and D. pneumosintes (0.8% × 4.0%) (Supplemental Figure 2). Teeth with closed cavities were rich in Atopobium (13.2% × 0.4%, p < 0.05) and Parvimonas (9.3% × 0.6%), particularly P. micra (9.3% × 0.6%).
Finally, we investigated the core microbiome according to the symptomatology (Fig. 4). Overall, it was composed of Bacteriodaceae sp ot 272, Filifactor alocis, Fretibacterium fastidiosum, Peptostreptococcus stomatitis, Pseudoramibacter alactolyticus, D. invisus and D. pneumosintes. The presence of asymptomatic teeth at the time of sampling was associated with a more diverse core than that of symptomatic cases. P. endodontalis and P. oris were consistently associated with symptoms.
Core microbiome of the samples analyzed according to the clinical symptomatology studied. The HOMINGS probes that were present with ≥ 0.1% relative abundance in ≥ 50% of all samples constitute the core microbiome (green). Samples were divided into two categories based on the absence (0) or presence (1) of symptoms. The core microbiome was subdivided into 2 groups based on the mean relative abundance of the taxa in samples in each clinical category (presence or absence of symptomatology). Furthermore, taxa that were present in ≥ 50% of samples in a single category but were not part of the core microbiome considering all samples constitute the core microbiomes. Taxa in the category core microbiomes were subgrouped based on the mean relative abundance of the taxa in samples in each clinical category. Taxa in bold were present in ≥ 75% of all samples (core) or samples in the indicated category (category cores).
Discussion
NGS community profiling, such as pyrosequencing (the first-generation NGS approach) and Illumina (the second-generation NGS approach), allows studies to determine the microbial composition and relative abundance of taxa10. Pyrosequencing is a method of DNA sequencing based on the “sequencing by synthesis” principle, while Illumina improves this method in the sequencing of homopolymeric regions. Illumina technology operates by reversible terminator chemistry, presenting lower sequencing error rates and lower cost than the former method21,30.
In this survey, we employed MiSeq sequencing (Illumina)31,32,33 to explore the microbiome at the apical portion of endodontic infections. We obtained 1,038,656 sequences and 946 OTUs from the 15 samples (mean: 62,895 reads; range: 14,971–166,232), far surpassing pyrosequencing studies, which yield 6,000–10,000 average reads per sample5,34 and 18716, 33920 and 803 OTUs5. Recently, employing the MiSeq platform (Illumina), Sánchez-Sanhueza et al.35 obtained 2,248,552 reads and 86 OTUs from the 24 root canal samples.
In this study, the phyla that prevailed were similar to those in previous reports developed in Asia, Europe and Africa5,6,20,34,36. Conversely, reports from individuals residing on the American continent, such as the individuals who made up this study, have found Proteobacteria11,16,35,37. These contradictory results suggest that geographical conditions are not directly related to the microbial pattern since many other factors may influence its composition35.
Further, we combined this comprehensive microbial analysis with HOMINGS species-level resolution, which represents a significant step forward in the field because most NGS-based studies of the endodontic microbiome fail to achieve species-level identification16,20,34,38. Overall, the most predominant genera were Prevotella and Bacteroidaceae G-1, which is in agreement with previous studies20,36,38. Species-level analysis indicated that Bacteroidaceae [G-1] sp ot 272, P. micra, P. oris, P. endodontalis, and Bacteroidetes [G-5] sp ot 511 were the most numerous species/phylotypes. Several of those taxa have been detected in association with pulpal pathology with secondary infections or have been demonstrated to be recalcitrant to treatment2,16. Collectively, these findings support a pathogenic role for those organisms.
The rationale for the study of symptoms of endodontic infections stems from the fact that the local microbial insult can have relevant clinical implications. We observed that the presence of symptoms has an impact on the root canal microbiome. Similar patterns have been shown previously when acute and chronic root canal infections were compared6. Alternatively, they might be the result of the local environmental pressures that select distinct microorganisms in each condition. Studies have endeavored to characterize the microbiota profile of different clinical endodontic conditions, such as asymptomatic or symptomatic teeth6,15 and the status of the coronal cavity (open/closed cavity)39. However, the findings were highly variable and at times, contradictory10. These contradictions could be attributed to differences in HVR(s) sequenced, collection methods, and OTU picking strategies.
Similar to other authors6,34, we found considerable variability across samples, as they presented complex combinations of taxa. Thus, the study of the microbial communities of the clinical symptomatology analyzed should help clarify taxa that are consistently associated with root canal infections. Overall, infections mainly comprise of Bacteriodaceae sp oral taxon 272, F. alocis, F. fastidiosum, P. stomatitis, P. alactolyticus, P. micra, D. invisus and D. pneumosintes. The symptom cores were characterized by P. oris and P. endodontalis, which is in line with previous reports6,7,40,41,42,43. Studies have reported that Prevotella intermedia, Prevotella nigrescens, Porphymonas gingivalis, and Porphymonas endodontalis are frequently detected by the use of molecular biology techniques in teeth with necrotic pulps 1,15,43. Collectively, these results suggest a prominent pathogenic role for those organisms. For instance, F. alocis, F. fastidiosum, P. micra and Dialister species have been associated with periapical infections15. In addition, recent studies have proposed them as candidate pathogens in periodontal diseases44,45. Although the study of their pathogenic mechanisms is in its infancy, it has been shown that F. alocis modulates microbiome and host proteome changes46, inhibits complement activation48 and induces neutrophil degranulation and chemotaxis48, while D. pneumosintes has been isolated from local49 and systemic infections50. In our core analysis, we were also able to identify new candidate pathogens, such as Bacteriodetes sp ot 272, 365 and Prevotella sp ot 526. The potential role of those phylotypes in periapical disease supports their further characterization in cultivation studies to determine their metabolism and virulence properties.
Most studies of the endodontic microbiota have employed paper points for sample collection5,15. Although convenient, this approach has limitations. First, they yield samples that conceivably are not representative of the apical portion of the infection, as they are likely to absorb material from the entire extension of the root canal. Second, their DNA yield appears to favor the collection of host DNA, which is detrimental to bacterial DNA44. Third, as demonstrated previously51, they can contain contaminating organisms, and their use “as a sampling tool for microbial profiling of clinical samples by open-ended techniques such as sequencing or DGGE should be avoided”. The fact that paper points might not reach the tooth apex to collect biofilm is problematic for researcher outcomes. This is a privileged location where bacteria are poised to initiate and sustain host responses that ultimately lead to signs and symptoms; hence, a prime site for studying the pathogenesis of these infections. In addition, the apical and middle coronal microbiomes present distinct profiles14. However, most studies that focused on this location employed extracted teeth, which preclude the routine evaluation of the microbiome of endodontic diseases. Thus, we inserted a K file into the canal to a level of the tooth apex and used the final 4 mm to analyze the microbiome, as described previously1,2.
In this study, one potential limitation was MDA used to increase the sample biomass prior to sequencing, as some have demonstrated its potential to introduce bias52. However, such potential was never demonstrated in 16S RNA sequencing studies but in metagenomics analyses. Furthermore, several recent studies have relied on MDA to study clinical isolates of P. gingivalis53 and uncultured phylotypes54, generating important insights into their metabolism and pathogenicity. Our group55 and others56 have used this technique to study endodontic infections, confirming previous findings obtained without MDA and expanding the knowledge about the different aspects of these conditions. Finally, the results presented in Supplemental Figure 1 demonstrate the similarity of the microbial profiles of amplified and non-amplified samples.
Another limitation of this study is the sample size. Nevertheless, Shin et al.10 critically reviewed the 12 peer-reviewed articles that specifically used different NGS technologies to assess the intracanal polymicrobial communities from 2010 to 2017. The total sample size ranged from 7 to 48 samples, with a mean of 19 samples, but only five articles presented a sample size higher than that of this study.
The knowledge raised by this study is the root canal microbiome complexity, the “common denominators” of root canal infections and that symptoms impact the root canal microbiome. Moreover, the virulence properties of identified taxa remain unknown.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Data availability
Datasets related to this article can be found at https://homings.forsyth.org/Genus%20probe%20list%20for%20website_v2.0.pdf.
References
Brito, L. C. et al. Use of multiple-displacement amplification and checkerboard DNA-DNA hybridization to examine the microbiota of endodontic infections. J. Clin. Microbiol. 45, 3039–3049 (2007).
Brito, L. C. et al. Microbiologic profile of endodontic infections from HIV− and HIV+ patients using multiple-displacement amplification and checkerboard DNA-DNA hybridization. Oral Dis. 18, 558–567 (2012).
Siqueira, J. F. Jr. & Rôças, I. N. Diversity of endodontic microbiota revisited. J. Dent. Res. 88, 969–981 (2009).
Sundqvist, G. Ecology of the root canal flora. J. Endod. 18, 427–430 (1992).
Hong, B. Y. et al. Microbial analysis in primary and persistent endodontic infections by using pyrosequencing. J. Endod. 39, 1136–1140 (2013).
Santos, A. L. et al. Comparing the bacterial diversity of acute and chronic dental root canal infections. PLoS ONE 6, e28088 (2011).
Rôças, I. N. & Siqueira, J. F. Jr. Root canal microbiota of teeth with chronic apical periodontitis. J. Clin. Microbiol. 46, 3599–3606 (2008).
Donlan, R. M. & Costerton, J. W. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15, 167–193 (2002).
Costerton, J. W., Stewart, P. S. & Greenberg, E. P. Bacterial biofilms: a common cause of persistent infections. Science 284, 1318–1322 (1999).
Shin, J. M. et al. Deciphering endodontic microbial communities by next-generation sequencing. J. Endod. 44, 1080–1087 (2018).
Saber, M. H. et al. Bacterial flora of dental periradicular lesions analyzed by the 454-pyrosequencing technology. J. Endod. 38, 1484–1488 (2012).
Tatikonda, A. et al. Evaluation of bacteriological profile in the apical root segment of the patients with primary apical periodontitis. J. Contemp. Dent. Pract. 18, 44–48 (2017).
Alves, F. R. et al. Bacterial community profiling of cryogenically ground samples from the apical and coronal root segments of teeth with apical periodontitis. J. Endod. 35, 486–492 (2009).
Ozok, A. R. et al. Ecology of the microbiome of the infected root canal system: a comparison between apical and coronal root segments. Int. Endod. J. 45, 530–541 (2012).
Gomes, B. P. et al. Microbial analysis of canals of rootfilled teeth with periapical lesions using polymerase chain reaction. J. Endod. 34, 537–540 (2008).
Siqueira, J. F. Jr., Alves, F. R. & Rôças, I. N. Pyrosequencing analysis of the apical root canal microbiota. J. Endod. 37, 1499–2150 (2011).
Gomes, B. P., Berber, V. B., Kokaras, A. S., Chen, T. & Paster, B. J. Microbiomes of endodontic-periodontal lesions before and after chemomechanical preparation. J. Endod. 41, 1975–1984 (2015).
Tzanetakis, G. N. et al. Comparison of bacterial community composition of primary and persistent endodontic infections using pyrosequencing. J. Endod. 41, 1226–1233 (2015).
Huttenhower, C. et al. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Jones, S. E. & Lennon, J. T. Dormancy contributes to the maintenance of microbial diversity. Proc. Natl. Acad. Sci. USA 107, 5881–5886 (2010).
Di Bella, J. M., Bao, Y., Gloor, G. B., Burton, J. P. & Reid, G. High throughput sequencing methods and analysis for microbiome research. J. Microbiol. Methods 95, 401–414 (2013).
Belstrom, D. et al. Comparative analysis of bacterial profiles in unstimulated and stimulated saliva samples. J. Oral Microbiol. 8, 3402 (2016).
Belstrom, D. et al. Bacterial composition in whole saliva from patients with severe hyposalivation—a case-control study. Oral Dis. 22, 330–337 (2016).
Belstrom, D., Paster, B. J., Fiehn, N. E., Bardow, A. & Holmstrup, P. Salivary bacterial fingerprints of established oral disease revealed by the Human Oral Microbe Identification using Next Generation Sequencing (HOMINGS) technique. J. Oral Microbiol. 8, 30170 (2016).
Teles, F., Haffajee, A. D. & Socransky, S. S. Multiple displacement amplification as an aid in checkerboard DNA–DNA hybridization. Oral Microbiol. Immunol. 22, 118–125 (2007).
Caporaso, J. G. et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. PNAS 108, 4516–4522 (2011).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336 (2010).
Chen, T., Yu, W. H., Izard, J., Baranova, O. V., Lakshmanan, A., Dewhirst, F. E. The Human Oral Microbiome Database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database (Oxford), baq013 (2010).
Abusleme, L. et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 7, 1016–1025 (2013).
Mardis, E. R. Next-generation sequencing platforms. Annu. Rev. Anal. Chem. (Palo Alto Calif) 6, 287–303 (2013).
Caporaso, J. G. et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6, 1621–1624 (2012).
Frey, K. G. et al. Comparison of three next-generation sequencing platforms for metagenomic sequencing and identification of pathogens in blood. BMC Genomics 15, 96 (2014).
Smith, D. P. & Peay, K. G. Sequence depth, not PCR replication, improves ecological inference from next generation DNA sequencing. PLoS ONE 9, 1–12 (2014).
Hsiao, W. W. et al. Microbial transformation from normal oral microbiota to acute endodontic infections. BMC Genomics 13, 345 (2012).
Sánchez-Sanhueza, G. H. et al. Metagenomic study of bacterial microbiota in persistent endodontic infections using Next-generation sequencing. Int. Endod. J. 51, 1336–1348 (2018).
Vengerfeldt, V. et al. Highly diverse microbiota in dental root canals in cases of apical periodontitis (data of Illumina sequencing). J. Endod. 40, 1778–1783 (2014).
Siqueira, J. F. Jr., Antunes, H. S., Rôças, I. N., Rachid, C. T. & Alves, F. R. Microbiome in the apical root canal system of teeth with post-treatment apical periodontitis. PLoS ONE 11, e0162887 (2016).
Li, L. et al. Analyzing endodontic infections by deep coverage pyrosequencing. J. Dent. Res. 89, 980–984 (2010).
Sassone, L. M. et al. A microbiological profile of unexposed and exposed pulp space of primary endodontic infections by checkerboard DNA-DNA hybridization. J. Endod. 38, 889–893 (2012).
Ribeiro, A. C., Matarazzo, F., Faveri, M., Zezell, D. M. & Mayer, M. P. Exploring bacterial diversity of endodontic microbiota by cloning and sequencing 16S rRNA. J. Endod. 37, 922–926 (2011).
Sakamoto, M., Rôças, I. N., Siqueira, J. F. Jr. & Benno, Y. Molecular analysis of bacteria in asymptomatic and symptomatic endodontic infections. Oral Microbiol. Immunol. 21, 112–122 (2006).
Darveau, R. P., Hajishengallis, G. & Curtis, M. A. Porphyromonas gingivalis as a potential community activist for disease. J. Dent. Res. 91, 816–820 (2012).
Gomes, B. P. et al. Porphyromonas gingivalis, Porphyromonas endodontalis, Prevotella intermedia and Prevotella nigrescens in endodontic lesions detected by culture and by PCR. Oral Microbiol. Immunol. 20, 211–215 (2005).
Pérez-Chaparro, P. J. et al. Evaluation of human and microbial DNA content in subgingival plaque samples collected by paper points or curette. J. Microbiol. Methods 111, 19–20 (2015).
Teles, R., Teles, F., Frias-Lopez, J., Paster, B. & Haffajee, A. Lessons learned and unlearned in periodontal microbiology. Periodontol 2000(62), 95–162 (2013).
Aruni, W., Chioma, O. & Fletcher, H. M. Filifactor alocis: the newly discovered kid on the block with special talents. J. Dent. Res. 93, 725–732 (2014).
Jusko, M. et al. FACIN, a double-edged sword of the emerging periodontal pathogen Filifactor alocis: a metabolic enzyme moonlighting as a complement inhibitor. J. Immunol. 197, 3245–3259 (2016).
Armstrong, C. L. et al. Filifactor alocis promotes neutrophil degranulation and chemotactic activity. Infect. Immun. 84, 3423–3433 (2016).
Drago, L., Vassena, C., Saibene, A. M., Del Fabbro, M. & Felisati, G. A case of coinfection in a chronic maxillary sinusitis of odontogenic origin: identification of Dialister pneumosintes. J. Endod. 39, 1084–1087 (2013).
Pierre Lepargneur, J., Dubreuil, L. & Levy, J. Isolation of Dialister pneumosintes isolated from a bacteremia of vaginal origin. Anaerobe 12, 274–275 (2006).
van der Horst, J. et al. Sterile paper points as a bacterial DNA-contamination source in microbiome profiles of clinical samples. J. Dent. 41, 1297–1301 (2013).
Direito, S. O. L., Zaura, E., Little, M., Ehrenfreund, P. & Roling, W. F. M. Systematic evaluation of bias in microbial community profiles induced by whole genome amplification. Environ. Microbiol. 16, 643–657 (2014).
McLean, J. S. et al. Genome of the pathogen Porphyromonas gingivalis recovered from a biofilm in a hospital sink using a high-throughput single-cell genomics platform, rm. Genome Res. 23, 867–877 (2013).
McLean, J. S. et al. Candidate phylum TM6 genome recovered from a hospital sink biofilm provides genomic insights into this uncultivated phylum. Proc. Natl. Acad. Sci. USA 110, E2390-2399 (2013).
Henriques, L. C. et al. Microbial ecosystem analysis in root canal infections refractory to endodontic treatment. J. Endod. 42, 1239–1245 (2016).
Rôças, I. N., Alves, F. R., Santos, A. L., Rosado, A. S. & Siqueira, J. F. Jr. Apical root canal microbiota as determined by reverse-capture checkerboard analysis of cryogenically ground root samples from teeth with apical periodontitis. J. Endod. 36, 1617–1621 (2010).
Acknowledgements
This work was supported in part by the Krakow Harvard/Forsyth Endowed Endodontic Research Fund (to LCNB and FRFT) and the National Institutes of Health/National Institute of Dental and Craniofacial Research (R01-DE024767 to FRFT). NIH/NIDCR R01 DE024767 - Targeted Cultivation of New Periodontal Pathogens. This study was also supported by research grants from the Brazilian Support Agencies for Superior Education (CAPES and CNPq) PRPQ/UFMG, and the post gradution program- School of Dentistry, UFMG. APRS is a CNPq fellow. We want to thank the members of the Forsyth Institute Sequencing Core (Bruce Paster, Jon Mcafferty, Keerthana Krishnan, Sean Cotton, Alexis Kokaras and George Chen) for their technical assistance.
Author information
Authors and Affiliations
Contributions
W.L.F.T., F.R.S.T. and A.P.R.B.—wrote the main manuscript text. L.C.N.B., J.D.H. and K.M.—prepared figures. L.C.N.B. and C.T.L.—performed experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Brito, L.C.N., Doolittle-Hall, J., Lee, CT. et al. The apical root canal system microbial communities determined by next-generation sequencing. Sci Rep 10, 10932 (2020). https://doi.org/10.1038/s41598-020-67828-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-67828-3
- Springer Nature Limited
This article is cited by
-
Identification of potential microbial risk factors associated with fecal indicator exceedances at recreational beaches
Environmental Microbiome (2024)
-
Bacterial diversity in primary infected root canals of a Chinese cohort: analysis of 16 S rDNA sequencing
BMC Oral Health (2023)