Abstract
Background Paradoxical low-flow (LF) severe aortic stenosis (AS) with preserved left ventricular ejection fraction (LVEF) may have poorer prognosis than normal-flow (NF) AS, though its pathophysiology remained unclear. In particular, LV stiffness has not been compared between LF vs NF. We used a novel echocardiography-derived index of LV stiffness to compare between these groups. Consecutive patients with medically-managed isolated severe AS (aortic valve area < 1 cm2) and preserved LVEF (>50%) were studied. Echocardiographic LV stiffness index was measured by a method previously validated against cardiac catheterization. We compared LF (stroke volume index, SVI < 35 ml/m2) and NF severe AS. Of the 352 patients, 121 (34%) were LF. Both LF and NF groups had similar demographics, valve areas and indices. Compared to NF, LF severe AS had higher LV stiffness indices (>0.11 ml−1 OR 3.067, 95% CI 1.825–5.128, p < 0.001). Increased LV stiffness was associated with concentric remodelling and more severe diastolic dysfunction, especially in LF AS. An LV stiffness index of > 0.11 ml−1 was independently associated with increased mortality, after adjusting for age, clinical and echocardiographic parameters (HR 2.283 95% CI 1.318–3.968, p = 0.003). Non-invasive echocardiographic-derived index of LV stiffness may be important in LF AS. Increased LV stiffness was related to LV concentric remodelling and diastolic dysfunction, and associated with poorer clinical outcomes in medically-managed AS.
Similar content being viewed by others
Introduction
Significant differences in echocardiographic profiles exist between paradoxical low-flow (LF) and normal-flow (NF) severe aortic stenosis (AS). This may be due to differences in pathophysiological processes in the natural history of LF compared to NF AS. Of note, left ventricular (LV) stiffness has not been evaluated in AS. Conventional measurement of LV stiffness involves using invasive cardiac catheterization and determination of the end-diastolic pressure-volume relationship by pressure-volume loop analysis1. This is impractical for routine serial evaluation in AS as it requires invasive cardiac catheterization2,3.
AS remains an important disease because of its high prevalence. It affects approximately 5% of patients over 75 years of age, and is associated with reduced survival4,5. LF AS (LV stroke volume index (SVI) < 35 mL/m2) despite preserved left ventricular ejection fraction (LVEF), termed “paradoxical low-flow”, has been increasingly recognised as a subgroup of severe AS that portends a worse prognosis compared to normal-flow6. However, the differential effects of the aortic valve pathology on the LV in LF compared to NF AS, particularly LV stiffness, remains to be elucidated7,8,9.
In addition, several studies have identified predictors of poor prognosis and outcomes in patients with severe AS10,11,12. These studies examined clinical, biochemical, and echocardiographic parameters13,14. For example, global longitudinal strain, valvuloarterial impedance, stroke work loss, aortic valve resistance, and systemic arterial compliance have been shown to be important prognostic markers of clinical outcomes in severe NF AS that predicted mortality15,16,17,18. However, these have not been demonstrated to be useful in paradoxical LF AS6.
LV stiffness remained to be evaluated to LF compared with NF AS, and prognostic markers in this subgroup of patients were poorly understood. We thus aimed to evaluate the role of an echocardiography-derived measure of LV stiffness in medically-managed LF versus NF severe AS.
Methods
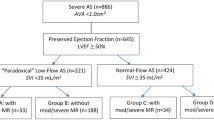
We examined the index echocardiographic studies of consecutive patients with severe AS (aortic valve area <1 cm2) from 2000–2011 with preserved LVEF (>50%) that were on medical therapy. Patients who had concomitant valvular pathology involving other valves of at least moderate severity, and patients who underwent valve replacement (surgical or transcatheter) were excluded from our study. These patients may be asymptomatic or have either declined valve replacement or were deemed to be medically unfit for the procedures. The patients were then stratified into low-flow (SVI < 35 mL/m2) and normal-flow groups. This classification of AS severity was in accordance with the American Society of Echocardiography/European Association of Cardiovascular Imaging guidelines19,20. The LV outflow track (OT) diameter was measured at the parasternal long-axis view from inner edge to inner edge. The LVOT velocity was measured with pulsed Doppler from the apical view. The LVOT diameter and LVOT velocity measurements were performed at the same annular level19. All echocardiographic measurements were made by independent and certified cardiologists.
Demographic, clinical and echocardiographic parameters were collected. Clinical outcomes in the form of all-cause mortality were collected upon chart review of subsequent follow-up visits.
All echocardiographic parameters were measured in accordance with the guidelines and standards of the American Society of Echocardiography/European Association of Cardiovascular Imaging21. Besides conventional echocardiography, tissue Doppler assessment of mitral annular systolic (S’), early (E’) and late (A’) diastolic velocities was performed. Severity of diastolic function was graded according to the American Society of Echocardiography/European Association of Cardiovascular Imaging guidelines22. Important prognostic markers of severe AS that were previously identified were also assessed.
LV stiffness was measured by means of a novel echocardiographic index, derived from the following equation2,3:
Patients were grouped based on four categories of LV geometry; determined by left ventricular mass index (LVMI) and relative wall thickness (RWT). The cut-off for LVMI was 116 g/m2 for males and 104 g/m2 for females. The cut-off for RWT was 0.43 for both sexes. If RWT and LVMI were both below the cut-off points, then the patient had normal LV geometry. Patients with high LVMI but RWT < 0.43 were defined as eccentric hypertrophy. Patients with high RWT but LVMI below the cut-off were defined as having concentric remodelling. If both LVMI and RWT were above the cut-offs, then the patient was identified as concentric hypertrophy24.
Differences in continuous variables between groups were compared using Student’s t-tests. The association between LV stiffness index and diastolic function grade was assessed using Spearman’s rank correlation. Multivariable logistic regression was used to compare differences between LF versus NF AS. We entered baseline clinical and echocardiographic parameters that were statistically significant on univariate analyses (p < 0.05) into the multivariable model. Parameters that were collinear with SVI and the LV stiffness index were excluded.
The optimized cut-off value for LV stiffness index for predicting all-cause mortality in the entire study population was determined by Youden index using receiver operating characteristic curve analysis. Differences in all-cause mortality between patients with low versus high LV stiffness were assessed using Kaplan-Meier curves and calculating the log-rank test statistic. A multivariate Cox regression model was then constructed to evaluate the effect of increased stiffness in predicting mortality and to adjust for other confounding parameters associated with mortality. We included all baseline clinical and echocardiographic parameters that were statistically significant on univariate analysis (p < 0.05), but excluded parameters that were collinear with the LV stiffness index. All statistical analyses were performed with SPSS for Windows, Version 20.0, SPSS Inc, Chicago, IL. A p-value of less than 0.05 was considered to be statistically significant.
Ethics approval for this study was obtained from National Healthcare Group Domain Specific Review Board (DSRB) prior to the conduct of this study. The study was in compliance with all DSRB requirements based on the Declaration of Helsinki, ethical principles in the Belmont report and the guidelines stipulated by the Bioethics Advisory Committee. No patient identifiers were collected and a waiver for the need for informed consent was obtained from the DSRB.
Results
Of the 352 patients with severe AS, 121 (34%) were low-flow, while the remaining 231 (66%) were normal-flow. The patients in the LF group were older (73.5 ± 13.8 vs 69.9 ± 13.5 years), but otherwise similar in terms of demographic background (Table 1). There was no significant difference in terms of LVEF and end-systolic wall stress, but end-diastolic volume (70.8 ± 17.7 vs 113.0 ± 26.0 mL) and left ventricular mass index (100.3 ± 31.4 vs 129.8 ± 36.2 g/m2) were significantly lower in the LF group (Table 1).
Patients with LF AS did not differ significantly from those with NF in aortic valve area, transaortic mean pressure gradient or peak velocity. The tissue Doppler indices of systolic and diastolic function were also not significantly different between groups. Conventional prognostic markers of AS severity such as stroke work loss and aortic valve resistance were not significantly different between the LF and NF groups. Systemic arterial compliance was lower and valvuloarterial impedance higher in LF AS compared with NF.
There was a strong negative logarithmic correlation between LV stiffness index and SVI (R2 = 0.813, Fig. 1). Receiver operating characteristic curve analysis demonstrated an area under the curve of 0.69 (95% CI 0.63–0.75, p < 0.001) and the derived optimised cut-off for LV stiffness index was >0.111 mL−1, based on Youden index. After adjusting for the confounding effect of other parameters associated with LF AS, we showed that patients with LF AS had a significantly increased odds of displaying an elevated LV stiffness index (>0.111 mL−1) compared to patients with normal-flow AS (OR 3.03, p < 0.001) (Table 2).
When comparing the various patterns of LV geometry, we found higher LV stiffness in patients with concentric remodelling and hypertrophy. This was especially noted in the patients with LF AS compared to their NF counterparts (Fig. 2). Furthermore, with increasing LV stiffness, we also demonstrated increasing severity of diastolic dysfunction (Fig. 3). This trend was statistically significant by Spearman’s rank correlation, and was more prominent in LF compared with NF AS. In fact, increased stiffness (>0.111 mL−1) was associated with older age, higher body mass index, lower left ventricular mass index and lower end-systolic wall stress. Prevalence of cardiovascular risk factors and comorbidities as well as degree of AS severity were similar (Table 3).
When comparing clinical outcomes of patients (average length of follow-up 6.2 ± 5.0 years) with high LV stiffness (>0.111 mL−1) compared with low stiffness, patients with increased LV stiffness had significantly higher mortality (42.9% vs 31.4%, Kaplan-Meier Log-rank test statistic 16.677, p < 0.001, Fig. 4). On multivariate Cox regression analysis, increased LV stiffness (>0.111 mL−1) was demonstrated to be independently associated with increased mortality (Hazard Ratio 2.283, 95% CI 1.318–3.968, p = 0.003) after adjusting for age, body mass index, LV mass index and cardiovascular risk factors such as diabetes mellitus, hypertension and hyperlipidaemia (Table 4).
Discussion
To our knowledge, this was the first study to assess differences in LV stiffness between patients with normal-flow and low-flow severe AS. The main findings of this study were that (1) patients with LF AS had higher LV stiffness than those with normal-flow AS, (2) increasing LV stiffness is related to concentric remodeling and hypertrophy, as well as more advanced diastolic dysfunction; and (3) AS subjects with increased LV stiffness had higher mortality than those with lower LV stiffness.
Paradoxical LF AS has been shown to form a significant proportion (up to 30%) of Asian cohorts13. Patients with LF AS tended to be older and have increased cardiovascular risk factors such as diabetes mellitus and hyperlipidemia. Understanding this subgroup of patients would therefore be important in the management of AS.
This novel echocardiographic index to estimate LV stiffness has been evaluated and validated against the gold standard of cardiac catheterization with pressure-volume loop analysis3. This parameter indexed the tissue Doppler assessment of diastolic function against the measured end-diastolic volume as a surrogate measure of LV stiffness. It has previously been employed to study patients with heart failure with preserved ejection fraction (HFpEF)2. Though LV stiffness has not been validated in the setting of LV pressure overload, this study proposes that the findings and application may be extrapolated to the AS population. A published study has shown that E/e’ ratio correlates with filling pressures in AS. The study demonstrated that E/e’ ratio was found to be the best single Doppler predictor of elevated filling pressures in patients with severe AS. The E/e’ ratio correlated significantly with LV pre-A pressures (r = 0.75, p < 0.001), and the LV end-diastolic pressure (r = 0.78, p < 0.001)23.
Indeed, for patients with AS, the high chronic afterload may lead to LV remodelling and fibrosis, resulting in increased stiffness and diastolic dysfunction. Increased LV stiffness from increased afterload had been previously shown in patients with essential hypertension as well as in HFpEF24,25. In HFpEF, increased LV stiffness has been shown to be associated with symptoms of dyspnea and reduced exercise tolerance, and correlated with disease progression26,27,28.
LV diastolic dysfunction has also been shown to be of increasing importance in severe AS. Prior studies had established the relationship between diastolic dysfunction and patients with severe AS with preserved LVEF29. LV diastolic dysfunction, and subsequently indices of LV myocardial and chamber stiffness had been associated with symptomatic AS, especially dyspnea30. These findings had suggested that heart failure symptoms in severe AS were driven by high LV filling pressures consequent to LV hypertrophy and diastolic dysfunction31. The actual relationship between LV remodeling and diastolic filling however is highly complex, where both early relaxation and passive LV filling later in diastole have demonstrated important roles in the development of heart failure symptoms32.
We therefore postulated that a similar phenomenon described in HFpEF may also be observed in our population of patients with LF compared to NF severe AS33,34,35. True enough, we found increased LV stiffness in patients with LF compared to NF severe AS. As LV stiffness appeared to be a prominent feature in patients with LF AS, it may also be related to the development of symptoms such as dyspnea and reduced effort tolerance. In the context of severe AS, the development of such symptoms would be a Class I indication for aortic valve replacement36. Classically, patients with severe AS who develop dyspnea would have a median survival of 2 years, although whether this holds true for patients with LF severe AS remained unclear1,3. By comparison, patients with NF tended to have lower LV stiffness, and the correlation between increasing LV stiffness and increasing degree of diastolic dysfunction was not demonstrated in the NF group.
In our study, we observed that increasing LV stiffness is predominantly related to concentric remodeling and hypertrophy. This trend was most prominent in patients with LF AS. Furthermore, increasing LV stiffness was also associated with increasing diastolic dysfunction in LF AS. We postulate that LV stiffness in LF AS may be secondary to chronic sympathetic stimulation of the beta-adrenoreceptor, associated with higher plasma concentrations of brain natriuretic peptide (BNP), as evidenced by significant LV remodelling and diastolic dysfunction37,38. This suggested that targeted therapy against LV remodeling in the form of beta-blockade (as used in heart failure) or renin-angiotensin blockade may be beneficial in LF AS to reduce harmful LV remodeling39.
The LV stiffness index also appeared to have prognostic value. Even after adjusting for age, body mass index, LV mass index and cardiovascular risk factors such as hypertension, hyperlipidaemia and diabetes, increased LV stiffness index remained independently associated with increased mortality. Regardless of flow-category, patients with higher stiffness showed significant higher mortality on subsequent follow-up. Other parameters conventionally used to predict outcomes in severe AS such as valvuloarterial impedance and systemic arterial impedance had been criticized to be highly flow-dependent14,15 and thus had a limited role in prognosis for patients with LF severe AS with preserved LVEF15,16. The LV stiffness index appeared to be more useful for prognosis in the LF subgroup.
We therefore found that an echocardiographic parameter of LV stiffness has the potential to guide prognostication and management of patients with LF severe AS. This index may be more useful compared to other more flow-dependent indices and predictors which may be less reliable in LF AS. The pathophysiological process in LF AS with preserved LVEF may parallel that of HFpEF and other disease processes where increasing LV stiffness and diastolic dysfunction results in morbidity and mortality.
Limitations
This study was retrospective and examined a moderately-sized cohort of index echocardiographic studies for patients diagnosed with isolated severe AS. It was however prospective in terms of clinical outcomes. As this was a cross-sectional study, there may have been lead-time bias as subjects were studied at different time points of the natural progression of AS. There was also no follow-up echocardiographic data, which may be useful in evaluating the progression of AS severity over time. Specific biomarkers such as brain natriuretic peptide could have been compared to the LV stiffness estimation, however this was not investigated in the present study. In addition, we did not evaluate the prognostic significance of LV stiffness in surgical management of severe AS. Although we did not examine the role of medical therapy on LV stiffness, renin-angiotensin blockade had previously been described to be associated with a lower prevalence of pathological LV remodelling in severe AS39. Nevertheless, our findings remained hypothesis-generating, and the role of medical therapy in improving LV stiffness may be an important subject for future prospective studies with serial echocardiographic analyses.
Conclusions
LV stiffness was demonstrated to be significantly higher in patients with LF AS, and this was related to the development of LV concentric remodelling, concentric hypertrophy and diastolic dysfunction. An increased LV stiffness index was associated with poorer mortality outcomes in medically-managed severe AS. This novel non-invasive echocardiographic estimation of LV stiffness may be an important prognostic tool in medically-managed AS.
References
Mirsky, I., Tajimi, T. & Peterson, K. L. The development of the entire end-systolic pressure–volume and ejection fraction-after load relations: a new concept of systolic myocardial stiffness. Circulation 76, 343–356 (1987).
Kasner, M., Sinning, D., Burkhoff, D. & Tschöpe, C. Diastolic pressure-volume quotient (DPVQ) as a novel echocardiographic index for estimation of LV stiffness in HFpEF. Clin Res Cardiol. 104(11), 955–963 (2015).
Chowdhury, S. M. et al. Echocardiographic Detection Of Increased Ventricular Diastolic Stiffness In Pediatric Heart Transplant Recipients: A Pilot Study. J Am Soc Echocardiogr 31(3), 342–348 (2017).
Otto, C. & Prendergast., B. Aortic-Valve Stenosis – From Patients at Risk to Severe Valve Obstruction. N Eng. J Med 371, 744–756 (2014).
Freeman, R. V. & Otto, C. M. Spectrum of calcific aortic valve disease. pathogenesis, disease progression, and treatment strategies. Circulation 111, 3316–3326 (2015).
Hachicha, Z., Dumesnil, J. G., Bogaty, P. & Pibarot, P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 115, 2856–2864 (2007).
Eleid, M. F. et al. Flow-Gradient Patterns In Severe Aortic Stenosis With Preserved Ejection Fraction. Circulation 128, 1781–1789 (2013).
Tribouilloy, C. et al. Low-gradient, low-flow severe aortic stenosis with preserved left ventricular ejection fraction: characteristics, outcome, and implications of surgery. J Am Coll Cardiology 65(1), 55–66 (2015).
Connolly, H. M. et al. Aortic valve replacement for aortic stenosis with severe left ventricular dysfunction: prognostic indicators. Circulation 95, 2395–2400 (1997).
Rosenhek, R., Klaar, U. & Schemper, M. Mild and moderate aortic stenosis. Natural history and risk stratification by echocardiography. Eur Heart J. 25, 199–205 (2004).
Pellikka, P. A., Sarano, M. E. & Nishimura, R. A. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 111, 3290–3295 (2005).
Rosenhek, R., Binder, T. & Porenta, G. Predictors of outcome in severe asymptomatic aortic valve stenosis. N Engl J Med 343, 611–617 (2000).
Ngiam, J. N. et al. Comparing characteristics and clinical and echocardiographic outcomes in low-flow vs normal-flow severe aortic stenosis with preserved ejection fraction in an Asian population. Echocardiography 34(5), 638–648 (2017).
Poh, K. K. et al. Left ventricular fluid dynamics in heart failure: echocardiographic measurement and utilities of vortex formation time. Eur Heart J Cardiovasc Imaging 13(5), 385–393 (2012).
Kearney, L. G. et al. Global longitudinal strain is a strong independent predictor of all-cause mortality with aortic stenosis. Eur Heart J Cardiovasc Imaging 13, 827–833 (2012).
Delgado, V. et al. Strain analysis in patients with severe aortic stenosis and preserved left ventricular ejection fraction undergoing surgical valve replacement. Eur Heart J 30, 3037–3047 (2009).
Cramariuc, D., Cioffi, G. & Rieck, A. E. Low-flow aortic stenosis in asymptomatic patients: valvular arterial impedance and systolic function from the SEAS substudy. J Am Coll Cardiol Img. 2, 390–399 (2009).
Levy, F. et al. Does valvuloarterial impedance improve risk stratification in low-ejection fraction, low-gradient aortic stenosis? Results from a multicenter study. Eur J Echocardiogr 12, 358–363 (2011).
Baumgartner, H. et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: A focused update from the European Association of Cardiovascular imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging (2017).
Baumgartner, H. et al. ESC Scientific Document Group; 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 38(36), 2739–2791 (2017).
Lang, R. M. et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16(3), 233–271 (2015).
Nagueh, S. F. et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 10(2), 165–193 (2009).
Bruch, C. et al. Tissue Doppler imaging in patients with moderate to severe aortic valve stenosis: Clinical usefulness and diagnostic accuracy. Am Heart J 148(4), 696–702 (2004).
Hussein, M., Al-Mashhadani, A. & Essa, S. The Use of Left Ventricular Myocardial Stiffness Index as a Predictor of Myocardial Performance in Patients with Systemic Hypertension. International Journal of Medical Physics, Clinical Engineering and Radiation Oncology 3, 167–175 (2014).
Westermann, D., Kasner, M. & Steendijk, P. Role of Left Ventricular Stiffness in Heart Failure with Normal Ejection Fraction. Circulation 117, 2051–2060 (2008).
Kitzman, D. W. et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA 288(17), 2144–2150 (2002).
Phan, T. T. et al. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol. 54(5), 402–409 (2009).
Yamamoto, K., Sakata, Y., Ohtani, T., Takeda, Y. & Mano, T. Heart failure with preserved ejection fraction. Circ J 73(3), 404–410 (2009).
Dahl, J. S. et al. Left ventricular diastolic function is associated with symptom status in severe aortic valve stenosis. Circ Cardiovasc Imaging 7, 142–148 (2014).
Park, J. S. et al. Hemodynamic patterns for symptomatic presentations of severe aortic stenosis. JACC Cardiovasc Imaging 6(2), 137–146 (2013).
Kamimura, D. et al. Increased Left Ventricular Diastolic Stiffness is Associated with Heart Failure Symptoms in Aortic Stenosis Patients with Preserved Ejection Fraction. J Card Fail. 23(8), 581–588 (2017).
Carebello, B. A. The symptoms of aortic stenosis: a step closer to understanding their cause. JACC Cardiovasc Imaging 6, 147–149 (2013).
van Heerebeek, L. et al. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation 113(16), 1966–1973 (2006).
Yu, C. M. et al. Progression of systolic abnormalities in patients with “isolated” diastolic heart failure and diastolic dysfunction. Circulation 105(10), 1995–1201 (2002).
Arruda, A. L., Pellikka, P. A., Olson, T. P. & Johnson, B. D. Exercise capacity, breathing pattern, and gas exchange during exercise for patients with isolated diastolic dysfunction. J Am Soc Echocardiogr. 20(7), 838–846 (2007).
Nishimura, R. A. et al. AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129(23), 2440–2492 (2014).
Watanabe, S. et al. Myocardial stiffness is an important determinant of the plasma brain natriuretic peptide concentration in patients with both diastolic and systolic heart failure. Eur Heart J 27, 832–838 (2006).
Gibbs, M. et al. Chronic beta-adrenoreceptor activation increases cardiac cavity size through chamber remodeling and not via modifications in myocardial material properties. Am J Physiol Heart Circ Physiol 287(6), H2762–H2767 (2004).
Goh, S. S. et al. Effect of Renin-Angiotensin Blockers on Left Ventricular Remodeling in Severe Aortic Stenosis. Am J Cardiol 119(11), 1839–1845 (2017).
Author information
Authors and Affiliations
Contributions
Jinghao Nicholas Ngiam and Nicholas Chew were involved in data collection, analysis and writing of the manuscript. Benjamin Yong-Qiang Tan, Hui Wen Sim and William KF Kong were involved in data collection and writing of the manuscript. Tiong-Cheng Yeo, Shahryar Chowdhury and Kian-Keong Poh were involved in the conception of the idea, along with data analysis and writing of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ngiam, J.N., Chew, N., Tan, B.YQ. et al. Novel Echocardiography-Derived Left Ventricular Stiffness Index in Low-Flow Versus Normal-Flow Severe Aortic Stenosis with Preserved Left Ventricular Ejection Fraction. Sci Rep 10, 9086 (2020). https://doi.org/10.1038/s41598-020-65758-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-65758-8
- Springer Nature Limited
This article is cited by
-
The constricting effect of reduced coronary artery compliance on the left ventricle is an important cause of reduced diastolic function in patients with coronary heart disease
BMC Cardiovascular Disorders (2022)
-
Can we explore AF–pacemakers’ relationship using clinical and echocardiographic parameters in patients with permanent pacemaker? (Echocardiography and subclinical AF in permanent pacemaker)
The International Journal of Cardiovascular Imaging (2022)