Abstract
Upward shifts of alpine treelines and shrub expansion are occurring under climate change, and Abies faxoniana (AF) and Rhododendron lapponicum (RL) may become distributed at higher altitudes. How do abiotic factors and litter quality modulate the effects of soil fauna on carbon release in this context? A field decomposition experiment involving the foliar litter of AF and RL was conducted along an elevation gradient encompassing coniferous forest, alpine shrubland and alpine meadow by using litterbags with different mesh sizes (3 and 0.04 mm). The objective was to determine the influences of soil fauna, litter quality and abiotic factors on species-specific carbon release and their contributions during litter decomposition. Our findings demonstrated that higher soil fauna abundance and diversity facilitated litter carbon release. The contribution rates of soil fauna to carbon release (Cfau) decreased with elevation increasing and decomposition time. Cfau are influenced by soil faunal diversity, dominant fauna groups (Collembola, Oribatida, Mesostigmata), and abiotic factors (temperature). Soil fauna significantly and directly regulated carbon release, abiotic factors indirectly regulated carbon release via altering soil fauna community composition and litter quality. This study improve our understanding in the mechanisms of decomposer contributions to carbon cycling in the context of global climate change.
Similar content being viewed by others
Introduction
Plant litter decomposition is a fundamental ecological process, linking nutrient cycling and energy flows in food webs and the structure and dynamics of ecosystems1,2. At the global scale, climate, litter quality and decomposers are considered the most important factors controlling litter decomposition3. Recent empirical studies have highlighted the need to explicitly acknowledge the role of decomposers, and it is believed that soil fauna enhance litter decomposition at both global and biome scales (average increment 27%)3,4. The soil fauna is an important component of ecosystems due to their functional roles in biogeochemical cycles in accelerating the rates of litter decomposition and nutrient transformation5,6.
Soil fauna play important roles in litter decomposition, mainly through the digestion of substrates, expansion of substrate surface area via fragmentation, and promotion of the rate of microbial inoculation into materials1,7. Direct increases in litter inputs to the soil or stimulation of microbial litter decomposition by soil fauna could have important implications for the formation of persistent soil organic matter and its C:N balance8,9. For example, the presence of isopods results in greater mobilization of various nutrients and litter-derived dissolved organic carbon, whereas the activity of Collembola has been found to increase the availability of litter-derived C to the soil microbial community10. Soil fauna generally exert a positive effect on the loss of litter mass11,12. However, accurate and quantitative predictions of the effects of soil fauna composition and diversity on C release remain lacking. In particular, the functional roles of soil fauna in alpine ecosystems are poorly understood.
Alpine ecosystems are considered thermally limited. However, due to human activities, average global temperature has increased, and treeline advance has occurred worldwide over the last century13,14. As part of global climate change, in alpine ecosystems, temperatures are increasing and precipitation regimes and patterns of snow cover are changing15. The plant community composition and diversity may shift accordingly, which is likely to influence litter mass loss and litter quality in the longer term16. Altered microclimates and associated changes in plant composition may decouple associations between plants and soil decomposer communities17. Therefore, the interactions between litter and decomposer communities in response to climate change appear to be uncertain. Although it is well understood how climate and substrate quality influence litter decomposition, the functional roles of decomposers in biogeochemical cycles remain unclear.
The alpine forest-tundra ecotone is the zone that extends from closed subalpine forest (timberline) to the upper boundary of the tree distribution. This ecotone typically appears as a patchy transition that consists of several intermediate vegetation zones and components, such as dark coniferous forest (CF), alpine shrubland (AS) and alpine meadow (AM)18. Litter decomposition in alpine forest-tundra ecotones is considered reduced relative to that at other lower altitudes due to thermal limitation. Previously, we studied the litter decomposition rates of representative plants (0.16–1.70) g/yr19 and the release of carbon and nutrients during litter decomposition20,21, mainly focusing on abiotic factors, such as freeze-thaw cycles, during the long snow-cover season. However, litter decomposition is also regulated by biotic factors17, and we previously found that soil fauna were more abundant in AS and CFs than in AMs across an alpine forest-tundra ecotone22,23,24. Additionally, we evaluated the contributions of soil fauna to lignin and cellulose degradation25. However, how soil fauna affect litter C release remains unclear.
There is a need to understand the roles of soil fauna in biogeochemical cycles in the context of global change. Thus, it is necessary to take soil fauna into account to adequately model the impacts of climate change on regional and global carbon dynamics5. Some research suggests that altitudinal gradients often produce climatic effects and influence the effects of soil fauna on decomposition3,26. Plant community composition, soil type and the micro-environment vary greatly across an alpine forest-tundra ecotone. We aimed to investigate how the contributions of soil fauna to decomposition are modulated by litter quality and abiotic factors. Treeline advance and shrub expansion into alpine ecosystems under climate change have been demonstrated13,14, which may led to upward shifts in the distributions of plant species, litter quality alterations, and variations in abiotic factors. Therefore, in this study, a field decomposition experiment involving foliar litter from Abies faxoniana and Rhododendron lapponicum was conducted along an elevational gradient encompassing CF, AS and AM using litterbags with different mesh sizes (3 and 0.04 mm). The objectives of our study were (1) to determine the abundance and species diversity of the soil fauna community and, more importantly, its contribution to C release during the decomposition of A. faxoniana and R. lapponicum foliar litter and (2) to explore how litter quality and abiotic factors modulate the effects of soil fauna on C release.
Results
Litter mass loss and carbon release
The remaining litter mass exhibited a pattern of exponential decay over decomposition time. The mass loss from A. faxoniana foliar litter under the different treatments (3-mm and 0.04-mm meshes) was 44.46% and 40.44% in CF, 45.08% and 41.67% in AS, and 38.9% and 33.29% in AM (Fig. 1a), and the mass loss from R. lapponicum litter was 41.8% and 33.48% in CF, 36.22% and 35.63% in AS, and 31.51% and 30.43% in AM (Fig. 1b). The presence of soil fauna accelerated litter decomposition and carbon release in both types of litter (P < 0.05) (Table 1). The C release rates were significantly higher when soil fauna were involved in the decomposition of A. faxoniana (R2 = 0.882, P < 0.001) and R. lapponicum (R2 = 0.778, P < 0.001) litter than when soil fauna were excluded (Fig. 1c,d).
Regression plots of remaining mass and carbon release rate of A. faxoniana and R. lapponicum foliar litter with decomposition time across the forest-tundra ecotone. Different colours and different line styles represent curves fit within different litter decomposition treatments (n = 2). Unbroken and broken lines indicate litter with and without soil fauna, respectively, and the green, blue and pink symbols represent the remaining mass or carbon release rate in coniferous forest (CF), alpine shrubland (AS) and alpine meadow (AM), respectively.
Soil fauna community composition and diversity
Across the entire ecotone, vegetation type and micro-environment significantly affected the composition of the soil fauna community (Fig. 2). A. faxoniana litter exhibited higher numbers of both soil fauna individuals and groups than did the R. lapponicum litter. A total of 1982 individuals representing 46 soil fauna groups were collected from the A. faxoniana litterbags, whereas the R. lapponicum litterbags contained 1272 individuals representing 36 groups. The highest number of soil fauna groups was found in CF, and the highest number of soil fauna individuals were observed in AS. The lowest numbers of both individuals and groups were found in AM. Collembola, Mesostigmata, Oribatid and Prostigmata were the predominant groups and accounted for 41.3%, 23.0%, 16.8% and 9.8%, respectively, of the total individuals in the A. faxoniana litter and 34.97%, 23.66%, 28.03% and 5.31%, respectively, of the total individuals in the R. lapponicum litter. Collembola was the most dominant component of the meso-fauna, and the number of individuals of this group decreased from CF to AM, whereas the numbers of Mesostigmata and Oribatid individuals increased from CF to AM. Isotomidae and Chironomidae were the only groups present in AS and CF (Tables S3, S4).
Principal component (a) and redundancy analysis (b) results of soil fauna diversity and abundance in association with selected physical and chemical properties during decomposition. Each point represents one sample of the soil fauna community from coniferous forest (green circles), alpine shrubland (blue circles), and alpine meadow (pink circles). Soil fauna community labels: Coll., Collembola; Ori., Oribatida; Meso., Mesostigmata; Pros., Prostigmata; Dipl., Diplura; Dipt., Diptera, H: Shannon-Wiener index, J: Pielou index, C: Simpson index, and D: Margalef index.
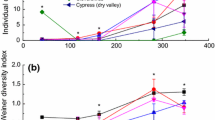
Vegetation type and decomposition time had significant effects on the diversity of the soil fauna communities (H, J, C, D) (Fig. 3). The value of the Shannon-Wiener, Pielou and Margalef indiceof the soil fauna were significantly higher in CF and AS than in AM, whereas the Simpson index values showed the opposite pattern. The PCA results revealed the relationship between soil fauna diversity and community composition during litter decomposition (Fig. 2a; Table 2). The cumulative contribution rate reached 82.8% (PC1: 68.8%, PC2: 14%), and the composition of the predominant group explained most of the variation in soil fauna diversity.
Diversity indices of soil fauna communities at three elevations in six key decomposition stages. H: Shannon-Wiener index, J: Pielou index, C: Simpson index, and D: Margalef index. Asterisks indicate statistical significance: *, **, and *** indicate significance at P < 0.05, P < 0.01, and P < 0.001, respectively; ns = not significant.
Contribution of soil fauna to C release
Over the entire field incubation period, the C release rates from A. faxoniana litter decomposition driven by soil fauna (Cfau) in CF, AS and AM were 25.86%, 20.23% and 12.3% (Fig. 4a), respectively, whereas those for R. lapponicum litter were 17.84%, 16.22% and 11.96%, respectively (Fig. 4b). The daily average C release (Pfau) contributions of the soil fauna during A. faxoniana litter decomposition in CF, AS and AM were 0.25%, 0.2% and 0.16% respectively (Fig. 4c), and those during R. lapponicum litter decomposition were 0.21%, 0.23% and 0.19%, respectively (Fig. 4d). Soil fauna (0.31, P < 0.01) and litter quality (−0.54, P < 0.001) significantly and directly affected C release during decomposition (Fig. 5a) and the C contribution of soil fauna (Fig. 5b). C release was significantly higher in A. faxoniana litter than in R. lapponicum litter, which was mainly due to differences between the two types of litter in N, P concentration. Abiotic factors indirectly regulated C release via altering the temporal dynamics of the soil fauna community and litter quality (Fig. 5a,b), which decreased from CF to AM (P < 0.05) for both species. Cfau and Pfau were highest in the early decomposition stages and decreased with increasing decomposition time. However, Cfau and Pfau showed different patterns over the course of decomposition between the two litter species, with the Cfau and Pfau of A. faxoniana litter decreasing from 136 days onward and those of R. lapponicum litter decreasing from 212 days onward.
The contribution percentage (%) of soil fauna to carbon release in different decomposition stages (mean ± SE, n = 5). (a) A. faxoniana − Cfau; (b) R. lapponicum − Cfau; (c) A. faxoniana − Pfau; (d) R. lapponicum - Pfau. Different uppercase letters indicate significant differences (P < 0.05) among decomposition stages at the same elevation within a species. Different lowercase letters indicate significant differences (P < 0.05) among elevations within the same decomposition stage and within a species.
Structural equation models describing the influences of environmental conditions, soil fauna community composition and litter quality on carbon loss (a) and the contribution of soil fauna to carbon loss (b). Note that two environmental conditions and litter quality components of the PCA were included in the SEM because both had eigenvalues > 1. Pearson correlations among soil fauna variables, environmental conditions, and litter quality variables and their corresponding PC axes. Soil fauna variables, PC1: Eigenvalues1 = 2.1; Coll = 0.69**; Pros = −0.01; Mcso = 0.63**; Orib = 0.48**; Dipl = 0.17; Dipt = 0.28; H = 0.97**; J = 0.77**; C = −0.89**; D = 0.95**. Environmental factors, PC1: Eigenvalues1 = 1.58; MT = 0.84**, FFTC = −0.54**, PAT = 0.56**, NAT = 0.71**, SP = −0.82**; PC2: Eigenvalues2 = 1.04; MT = 0.37*, FFTC = −0.72**, PAT = −0.45**, NAT = −0.44**, SP = 0.16; Litter quality model, PC1: Eigenvalues1 = 1.64; C = 0.58, N = −0.88*, P = −0.97**, Lignin = 0.45, Cellulose = −0.49; PC2: Eigenvalues2 = 1.19; C = −0.54, N = −0.41, P = −0.17, Lignin = −0.88*,Cellulose = 0.61. Solid and dotted lines denote positive and negative effects, respectively, and a bold line denotes a significantly relationship. Asterisks indicate statistical significance: *, **, and *** indicate significance at P < 0.05, P < 0.01, and P < 0.001, respectively.
Redundancy analysis (RDA) revealed the mass loss and C release rates driven by soil fauna in association with soil faunal diversity and abiotic factors during decomposition. These factors explained 33.7% of the total variance in the composition of soil fauna community (Fig. 2b; Table 2). The first canonical axis was mainly determined by Cfau, elevation and NAT, which together explained 26.4% of the total variation, whereas the second canonical axis explained 7.3% of the total variation and included mean temperature (MT: eigenvector of 0.3). Similarly, in the SEM analysis (Fig. 5), the presence of soil fauna had a significant effect on C release, and Cfau and Pfau displayed positive relationships with each of the soil fauna diversity indices (Fig. 5; H = 0.97, J = 0.77, C = −0.89, D = 0.95; all P < 0.01) and dominant groups (Collembola: 0.69 = ;, Oribatida: 0.48, Mesostigmata: 0.63; all P < 0.01).
Discussion
Soil fauna accelerate litter carbon release
Plant litter carbon release is an important biogeochemical process in terrestrial ecosystems27. The extensive manipulation experiment by Wall et al.5 indicated that soil fauna significantly increases litter mass loss in most biomes. Soil fauna have been found to enhance litter decomposition (average increment ~27%) at both global and biome scales3, but little is known about their impact on carbon release during litter decomposition and their influence on ecosystem carbon cycling. Our findings indicated that litter mass loss and C release rates were significantly accelerated when soil fauna were allowed access to the litter. We integrated the contribution of soil fauna into models of carbon release (Fig. 5), and the structural equation models indicated that soil fauna had direct significant positive effects on Cfau and Pfau (0.31 and 0.26, all P < 0.01). Inclusion of biome-specific soil fauna effects on litter decomposition is advocated as a means to reduce the unexplained variation in large-scale decomposition models3. According to the meta-analysis of Frouz et al.28, only part of the litter consumed by soil fauna is assimilated; the rest is returned to the soil as faeces. The percentage of food that is assimilated varies substantially among various groups of soil macrofauna.
Faunal activity accelerated the removal of litter from the litter layer but had no effect on overall mineralization because most of the removed material was stored in the mineral layer. Soil fauna can cause physical fragmentation, stimulation of microbial activity and transportation of fungal and bacterial propagules, thereby promoting C release due to litter decay29. By feeding on litter, soil fauna typically redistribute organic matter in the soil profile by modifying the leaching or movement of particulate organic matter. Fauna may affect the amount and quality of dissolved organic matter entering the soil from decomposing litter and modify the priming effect. In addition, soil fauna can increase the release of DOC by accelerating the turnover of microbial biomass30. DOC can be rapidly leached from litter bags and then used by soil microorganisms outside the bags, and a portion of the C that disappears from litter bags is released into the atmosphere through the respiration of soil fauna and microorganisms. Our study revealed that soil fauna can promote carbon release in the preliminary stage of decomposition, especially within the first 212 decay days. A meta-analysis revealed that microarthropods generally exert a positive effect on litter mass loss12. Many other studies have similarly indicated that soil fauna consume and break up litter in the early decomposition stages. For example, Collembola directly contributes to C transport in the litter-soil environment8, and Prostigmata is an initial coloniser during litter decomposition and is usually present in the early stages of decomposition31. In contrast, Oribatida shows a preference for later stages of decomposition. However, Collembola significantly increases the mineralization of SOC at the late stage of incubation, when litter labile C is depleted, although there is no evidence that Collembola affects the litter decomposition rate or microbial biomass32. In contrast, soil fauna were shown to support ecosystem productivity and soil carbon accrual in tallgrass prairie, where microarthropod suppression slowed litter mass loss and decreased the litter carbon input into the soil during the first 18 months of decomposition4. Therefore, soil fauna simultaneously promote not only the removal of litter from soil surface but also the accumulation of their faeces and fragmented litter in soil30.
In this study, soil fauna community abundance and diversity were the primary factors affecting carbon release. The PCA and RDA analyses indicated that Cfau and Pfau displayed positive relationships with the soil fauna diversity indices (Fig. 5; H, J, C, D) and dominant groups (P < 0.01). Similarly, mass loss was significantly correlated with the abundance of groups such as total Acari, Collembolans, and Mesostigmata mites. The faunal contribution to decomposition varies markedly among environmental gradients that differ in litter faunal diversity26. Correlational research has revealed strong impacts of soil fauna on carbon turnover27; these fauna include not only earthworms and other macrofauna but also microfauna such as Collembola. For example, introduced earthworms can double microaggregate formation and substantially stabilise new C in topsoil33,34. The presence of a single Collembola species may enhance microbial biomass by 56%35, and litter decay rates can be increased by up to 30% due to Collembola grazing36. In this study, we analyzed only microarthropod community taxa. Other soil fauna groups, such as Anelida, Enchytraeidae and earthworms, may play important roles in decomposition even if they (particularly Enchytraeidae) are not detected.
Litter quality control carbon release
Litter quality is a primary determinant of the resistance of litter to decomposition1. The initial chemical composition of litter and the chemical changes that occur during decomposition directly affect carbon release rates at large scales. Furthermore, litter quantity and quality affect the structure and functions of soil communities37, which indirectly affect carbon release. Whether litter quality is a significant determinant of litter decomposition depends on both the soil community composition and the length of field exposure because decomposition rate is dependent on mobility. Previous research found that soil organisms of all size classes responded positively to increased litter quality38. In the present study, the numbers of individuals and groups of soil fauna in A. faxoniana litter were higher than those in R. lapponicum litter. This difference mainly occurred because soil fauna favour litter with low C/N and lignin/N ratios (Hättenschwiler et al.2; Bradford et al.17; Berg et al., 1993). Because the initial chemical composition of R. lapponicum was more resistant to the decomposition process than was that of A. faxoniana litter (Table S2), the impact of soil fauna on C release was greater for A. faxoniana litter than for R. lapponicum litter. Litter species identity is an important determinant of the abundance and diversity of soil fauna2,39. We found that litter quality had significant negative effects on Cfau and Pfau (−0.54 and −0.67, P < 0.001) in the structural equation models (Fig. 5), which highlights the importance of the relationship between litter quality and decomposition. At the regional scale, litter chemistry becomes the most important determinant of litter decomposition rate. In the context of treeline advance or tundra expansion, plant species shifts along elevation lead to variations in plant litter composition that affect the interaction of litter species-specific decomposition and decomposer diversity. Therefore, the mechanisms of litter decomposition and C transformation into soil in alpine ecosystems under climate change are complicated.
The effects of abiotic factors on carbon release
We found that the decomposition rate (k) of A. faxoniana and R. lapponicum was 0.209–0.243 yr−1 and 0.173–0.189 yr−1, respectively; representing on average 43% and 35% of the total decomposition within 554 days. These results indicate that a long time is required for these litters to decay completely in alpine areas subjected to to thermal limitation. RDA and SEM analyses indicated that elevation, NAT and MT were the main abiotic factors affecting Cfau and Pfau (Fig. 2). In addition, SEM indicated that elevation had direct significant effects on the soil fauna communities (−0.46, all P < 0.001) and that abiotic factors had direct significant effects on both litter quality and soil fauna community (−0.46 and 0.23, all P < 0.01). All of the soil fauna diversity indices and dominant groups positively related to mean temperature and negatively relate to elevation.
We found that the soil macro-faunal communities of alpine shrubland and coniferous forest were more similar to each other than to the macro-faunal communities of alpine meadow. The dominant macro-faunal groups of alpine meadow (Lumbricida, Formicidae, Scarabaeidae, Bibionidae) differed from those of forest and shrubland (Staphylinidae, Lithobiida, Geophilomorph). However, meso-faunal groups (Nematoda, Enchytraeidae, Eutardigrada, Isotomidae, Harpacticoida Oribatuloidae) have been shown to be similar among these three vegetation types23. This is not only because the vegetation communities and soil type are more similar between alpine shrubland and coniferous forest than between alpine meadow but also because abiotic factors, such as MT and NAT, are more similar between alpine shrubland and coniferous forest.
Home-field advantage (HFA) is the notion that plant litter decomposes more rapidly underneath plants from its home environment than underneath plants from other areas. Litter mass loss has been shown to be, on average, 8% more rapid at home than away40,41,42. We speculate that HFA exists in the studied alpine forest-tundra ecotone, as A. faxoniana and R. lapponicum were the predominant species in CF and AS, respectively. Decomposer communities are specialized to break down litter from the plants they associate with43; this specialization may be related to differences in the abundance of some faunal functional groups or to the species-specific trophic behaviour of soil fauna41. We suggest that the magnitude of HFA effect on litter mass loss may be underestimated, since litterbags restrict the removal of litter from litterbags by soil fauna, and that HFA may increase with greater differences in litter quality between two litter types. Moreover, HFA could potentially be disrupted by timberline advance or tundra expansion42. According to the present results, if the distributions of A. faxoniana and R. lapponicum shift with elevation, soil faunas will slow down the litter C release at a higher altitudes. Hence, an altered climate and associated changes in vegetation composition may decouple the associations between plants and soil decomposer communities1,44, with consequences for ecosystem processes such as decomposition and carbon cycling. Furthermore, the impacts of climate change on decomposition are likely to depend on the local compositions of the fungal and invertebrate decomposer communities36.
Conclusion
Litter decomposition is known to generally be controlled by climate, litter quality and decomposer characteristics5,15, but the relative importance of these drivers can differ among scales and ecosystems. This study highlighted the important roles of soil fauna in litter decomposition, and the findings support the hypothesis that higher soil fauna abundance and diversity facilitate litter carbon release across an alpine forest-tundra ecotone. Furthermore, the findings provide insight into the relative importance of abiotic factors, litter quality and soil fauna in influencing litter carbon release rates and on how abiotic factors and litter quality modulate the effects of soil fauna on such rates. The evaluation of biotic factors that control litter decomposition has important implications for the long-term functioning of carbon sequestration. The relationship between decomposition and soil fauna with timberline advance or tundra expansion under climate change deserves further attention. More long-term studies on soil faunal control of carbon release during decomposition in alpine ecosystems are needed.
Materials and Methods
Site description
The study was conducted at the Long-term Research Station of Alpine Forest Ecosystems (longitude, 102°41′32″ to 102°41′54″E; latitude, 31°51′48″ to 31°51′53″N; approximately 3900–4200 m above sea level (a.s.l.), with pronounced vertical zonality), which is located in the Miyaluo Nature Reserve, Sichuan Province, southwest China. The study area is a transitional zone situated between the Tibetan Plateau and the Sichuan Basin, and it is covered by mixed coniferous broadleaved forests, dark coniferous forest, alpine shrubland and alpine meadow from the valley to the ridge, with a snow belt above 4500 m a.s.l. The weather is cool in summer and cold in winter. The annual mean air temperature ranges from approximately 6–12 °C, and the absolute maximum and minimum air temperatures are 12.6 °C in July and −8 °C in January, respectively. In the alpine zone, snow-cover season begins in November and lasts until the end of April (approximately 6 or 7 months).
Three 50-m-wide transects, which were separated by more than 1 km, were established perpendicular to the contour line in the forest-tundra ecotone. The vegetation types in these three transects, along which permanent sample plots were established in 2008, vary from CF (3900–4000 m a.s.l.) to AS (4000–4200 m a.s.l.) to AM (>4200 m a.s.l.) (Fig. S1). The dominant tree species in the CF and AS are A. faxoniana and R. lapponicum, respectively. The upward shifts of the alpine treeline and shrub expansion may be leading to shifts in A. faxoniana and R. lapponicum distributions toward higher altitude. The forest understory is cold and wet, with thick moss and humus layers, and the coverage and height of the woody shrubs decrease from the shrubland to the meadow. The shrubland is primarily characterized by vegetation consisting of woody shrub species and long-lived perennial herbaceous plants, and the alpine treeline, which is located near the upper elevational boundary of CF, is at approximately 4000 m a.s.l.20. The soils are Cryumbreps and Histosols (United States Department of Agriculture Soil Taxonomy), and soil subsamples were sent to laboratory for standard soil physicochemical analyses (Table S1).
Experimental design and sampling
Freshly fallen leaf litter from two woody species (A. faxoniana and R. lapponicum) was collected in October 2012 across the alpine forest-tundra ecotone. These two plant types represent the predominant tree and shrub species in the area. To avoid structural damage to the litter during oven drying, the freshly fallen litter was first air dried for more than two weeks at room temperature. The contribution of the soil fauna to C release via litter decomposition was determined using litterbags of two different mesh sizes45. Samples of 10 g each were placed in 20 × 20 cm nylon litterbags with a mesh size of 0.04 mm on the bottom and a mesh size of either 0.04 mm (to exclude soil fauna) or 3.00 mm (to allow soil fauna to enter) on the top. A total of 370 litterbags (two species × two mesh types × three elevations × five replicates × six sampling dates + 10 samples of variable initial quality) were placed throughout the forest-tundra ecotone. Subsamples of the foliar litter from each species were oven dried at 65 °C for 72 h to determine their initial quality (Table S2).
All litter in each litterbag was spread as flat as possible to maintain contact with the soil, and each litterbag was labelled with an aluminium tag to identify the corresponding treatment. Button temperature dataloggers (iButton DS1921, Maxim/Dallas Semiconductor, Sunnyvale, CA, USA) were wrapped in plastic to protect them from moisture and then placed inside the litterbags to automatically monitor the temperature every 3 h. The processes of freezing and thawing can be viewed as the accumulation and release of thermal energy46; therefore, the sampling dates were selected based on changes in the freezing and thawing dynamics determined from previous field observations20. Litterbags were randomly sampled from each sample plot on 4 July 2013, 7 September 2013, 23 November 2013, 12 March 2014, 4 September 2014, and 4 November 2014.
A freeze-thaw cycle was defined as a decrease in temperature to below 0 °C and maintenance of sub-zero temperatures for at least 3 h followed by an increase to above 0 °C and maintenance of above-zero temperatures for at least 3 h, and vice versa47. The daily mean temperatures (MTs) for each stage were calculated separately (Fig. S2). To better characterize the temperature changes, the positive accumulated temperature (PAT) and negative accumulated temperature (NAT) were calculated. In addition, the depth of the snow cover at each elevation was measured.
Fauna inventory
Data were analysed at the Laboratory of Ecological Forestry Engineering of Sichuan Province of the Institute of Ecology and Forests, Sichuan Agricultural University, Chengdu, China. The collected litterbags were immediately placed in modified Tullgren extractors to remove litter invertebrates48; this collection strategy requires convenient access to the sites and efficient extraction techniques. All extracted faunal samples were preserved in 75% ethanol and then sorted under a dissecting microscope into broad taxonomic groups; the Pic torial Keys for the Soil Animals of China were used to identify each sample to the family level49,50, and the taxonomic identification was wide applied in many studies26,39.
Litter chemistry analyses
After separation, the surface debris was removed from the collected litterbags, and the litter inside was oven dried at 65 °C to a constant weight. After weighing, all the samples were ground and milled for chemistry analyses. Carbon content was determined using the dichromate oxidation ferrous sulfate titration method. Nitrogen and phosphorus contents were determined using the Kjeldahl method and molybdenum-blue colorimetric method, respectively. Total phenolic content was measured using the Folin-Ciocalteu method. Cellulose and lignin concentrations were measured using the Van Soest method25.
Calculations and statistical analyses
For the foliar litter in each type of litterbag, C release rates (LC) throughout the one and a half years of the litter decomposition experiment were calculated, and the release rates due to soil fauna in each period (Cfau) were determined. The contribution of the soil fauna to C release associated with litter decomposition for each period (Pfau) was calculated as follows:
where M0 is the initial oven-dried litter mass (g); Mi is the dry mass of the litter remaining in the bag in each sampling period (g); C0 is the initial concentration (g·kg−1); Ci is the concentration of C in the remaining litter in each sampling period; (Llt − Lst) is the difference in the C release rate between the two litterbag mesh sizes for sampling date “t”; (Ll(t-1) − Ls(t−1)) is the difference in the mass loss rate between the two litterbag mesh sizes for sampling date “(t - 1)” (t = 1, 2, 3, 4, 5, 6); Llt and Ls(t-1) are the C release rates for the large-mesh-size litterbags on sampling dates “t” and “(t-1)” (t = 1, 2, 3, 4, 5, 6), respectively; and Ll and Ls are the C release loss rates for the large- and small-mesh-size litterbags, respectively, on the last sampling date.
The Shannon-Wiener diversity index (H), Pielou evenness index (J), Simpson diversity index (C) and Margalef richness index (D) were calculated for the soil faunal community as follows:
where Pi is the relative percentage of the soil fauna of type “i” in each sampling period; S is the number of groups; and N is the total number of individuals in the soil fauna.
Independent samples t-tests were used to evaluate differences in the initial substrates between the two plant species, with an alpha level of 0.05. Repeated measures analysis of variance was performed to test for variations in mass loss and C release rate with treatment and elevational gradient over the course of litter decay, which were evaluated via exponential and linear regression during different key periods. In addition, repeated measures analysis of variance was used to evaluate carbon release rate (Cfau) and the contribution ratio of carbon release (Pfau) due to soil fauna during litter decomposition. Three-way nested analysis of variance was used to evaluate the effects of litter species, elevation and time on the diversity indices of soil fauna communities.
Principal component analysis (PCA) was employed to evaluate the effects of litter quality on the composition of the soil fauna community, and11 factors were considered. These factors included the Shannon-Wiener index (H), Pielou index (J), Simpson index (C), Margalef index (D), and the dominant soil fauna groups: Collembola, Oribatida, Mesostigmata, Prostigmata, Diplura, Diptera. and Psocoptera (which together accounted for more than 95% of the total abundance). Redundancy analysis (RDA) was used to analyze the relationships between soil fauna communities and litter decomposition. The analysis included two sets of variables: abiotic factors related to local site conditions (Elevation, MT, FFTC, PAT, NAT, SP) and litter decomposition variables (mass loss, Cfau, Pfau)31. We explored the influences of local abiotic factors, soil fauna community composition and litter quality traits on the contribution of soil fauna to C release using structural equation modelling3,7. We selected C, N, P, lignin, cellulose and phenol as indicators of litter quality20,25. Before building the model, we reduced the dimensionality of the environmental variables and litter quality data set by generating multivariate axes of variability suitable for use in SEM. PCA was also used to capture most of the variance due to environmental factors and litter quality, and only PCA axes with eigenvalues >1 were retained7. It was necessary to transform the measured data using log (x + 1). The overall goodness-of-fit of each model was evaluated by the χ2 goodness-of-fit test, the root mean square error of approximation index (RMSEA) and the goodness-of-fit index (GFI). Statistical analyses were performed with SPSS version 23.0 and AMOS version 23.0 (SPSS Inc., Chicago, IL, USA).
References
Berg, B. & Mcclaugherty, C. Plant Litter. Springer Berlin (2008).
Hättenschwiler, S., Gasser, P. & Field, C. B. Soil Animals Alter Plant Litter Diversity Effects on Decomposition. Proceedings of the National Academy of Sciences of the United States of America 102, 1519–1524 (2005).
García-palacios, P., Maestre, F. T., Kattge, J. & Wall, D. H. Climate and litter quality differently modulate the effects of soil fauna on litter decomposition across biomes. Ecology Letters 16, 1045–1053 (2013).
Soong, J. L. et al. Soil microarthropods support ecosystem productivity and soil C accrual: Evidence from a litter decomposition study in the tallgrass prairie. Soil Biology &. Biochemistry 92, 230–238 (2016).
Wall, D. H. et al. Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Global Change Biology 14, 2661 (2008).
Moore, J. C. et al. Detritus, trophic dynamics and biodiversity. Ecology Letters 7, 584–600 (2010).
García‐Palacios, P., Mckie, B. G., Handa, I. T., Frainer, A. & Hättenschwiler, S. The importance of litter traits and decomposers for litter decomposition: a comparison of aquatic and terrestrial ecosystems within and across biomes. Functional Ecology 30, 819–829 (2016).
Chamberlain, P. M., Mcnamara, N. P., Chaplow, J., Stott, A. W. & Hij, B. Translocation of surface litter carbon into soil by Collembola. Soil Biology &. Biochemistry 38, 2655–2664 (2006).
Cotrufo, M. F. et al. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nature Geoscience 8 (2015).
Silvia, P. & Gerd, W. Interactions between isopods and collembolans modulate the mobilization and transport of nutrients from urban soils. Applied Soil Ecology 39, 109–126 (2008).
Heneghan, L., Coleman, D. C., Zou, X., Crossley, D. A. & Haines, B. L. Soil Microarthropod Contributions to Decomposition Dynamics: Tropical-Temperate Comparisons of a Single Substrate. Ecology 80, 1873–1882 (1999).
Kampichler, C. & Bruckner, A. The role of microarthropods in terrestrial decomposition: a meta‐analysis of 40 years of litterbag studies. Biological Reviews of the Cambridge Philosophical Society 84, 375–389 (2010).
Holtmeier, F. K. & Broll, G. Treeline advance - driving processes and adverse factors. Landscape Online 1 (2007).
Harsch, M. A., Hulme, P. E., Mcglone, M. S. & Duncan, R. P. Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecology Letters 12, 1040 (2009).
Gavazov, K. S. Dynamics of alpine plant litter decomposition in a changing climate. Plant & Soil 337, 19–32 (2010).
Baptist, F., Yoccoz, N. G. & Choler, P. Direct and indirect control by snow cover over decomposition in alpine tundra along a snowmelt gradient. Plant & Soil 328, 397–410 (2010).
Bradford, M. A., Berg, B., Maynard, D. S., Wieder, W. R. & Wood, S. A. Understanding the dominant controls on litter decomposition. Journal of Ecology 104, 229–238 (2016).
Baker, W. L. & Weisberg, P. J. Landscape Analysis of the Forest‐Tundra Ecotone in Rocky Mountain National Park, Colorado. Professional Geographer 47, 361–375 (2010).
Yang, L. et al. Relationships between decomposition rate of leaf litter and initial quality across the alpine timberline ecotone in Western Sichuan, China. Chinese Journal of Applied Ecology 26, 3602 (2015).
Liu, Y. et al. Changes in foliar litter decomposition of woody plants with elevation across an alpine forest–tundra ecotone in eastern Tibet Plateau. Plant Ecology 217, 495–504 (2016).
Deng, C. C. et al. Leaching of dissolved organic carbon from decomposing litter during the snow cover period at an alpine timberline ectone in Western Sichuan,China. Chinese. Journal of Ecology 33, 2921–2929 (2014).
He, R. L. et al. Litter decomposition and soil faunal diversity of two understory plant debris in the alpine timberline ecotone of western Sichuan in a snow cover season. Chinese Journal of Applied Ecology 26, 723–731 (2015).
Huang, X. et al. Soil faunal diversity under typical alpine vegetations in West Sichuan. Chinese Journal of Applied Ecology 21, 181 (2010).
Runlian, H. E. et al. Invertebrate diversity in foliar litter of three shrubs in the alpine timberline of western Sichuan. Acta Ecologica Sinica 36 (2016).
Wang, L. et al. Impacts of soil fauna on lignin and cellulose degradation in litter decomposition across an alpine forest-tundra ecotone. European Journal of Soil Biology 87, 53–60 (2018).
Wang, S., Honghua, R. & Wang, B. Effects of soil microarthropods on plant litter decomposition across an elevation gradient in the Wuyi Mountains. Soil Biology &. Biochemistry 41, 891–897 (2009).
Filser, J. et al. Soil fauna: key to new carbon models. soil 2, 565–582 (2016).
Frouz, J., Roubíčková, A., Heděnec, P. & Tajovský, K. Do soil fauna really hasten litter decomposition? A meta-analysis of enclosure studies. European Journal of Soil Biology 68, 18–24 (2015).
Carrillo, Y., Ball, B. A., Bradford, M. A., Jordan, C. F. & Molina, M. Soil fauna alter the effects of litter composition on nitrogen cycling in a mineral soil. Soil Biology & Biochemistry 43, 1440–1449 (2011).
Frouz, J. Effects of soil macro- and mesofauna on litter decomposition and soil organic matter stabilization. Geoderma 332, 161–172 (2018).
Fujii, S. & Takeda, H. Succession of soil microarthropod communities during the aboveground and belowground litter decomposition processes. Soil Biology &. Biochemistry 110, 95–102 (2017).
Wang, M. et al. Effect of Collembola on mineralization of litter and soil organic matter. Biology & Fertility of Soils 53, 563–571 (2017).
Six, J., Bossuyt, H., Degryze, S. & Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil & Tillage Research 79, 7–31 (2004).
Marashi, A. R. A. & Scullion, J. Earthworm casts form stable aggregates in physically degraded soils. Biology & Fertility of Soils 37, 375–380 (2003).
Filser, J. The role of Collembola in carbon and nitrogen cycling in soil. Pedobiologia 46, 234–245 (2002).
A’Bear, A. D., Boddy, L. & Jones, T. H. Impacts of elevated temperature on the growth and functioning of decomposer fungi are influenced by grazing collembola. Global Change Biology 18, 1823–1832 (2012).
Sauvadet, M., Chauvat, M., Fanin, N., Coulibaly, S. & Bertrand, I. Comparing the effects of litter quantity and quality on soil biota structure and functioning: Application to a cultivated soil in Northern France. Applied Soil Ecology 107, 261–271 (2016).
Smith, V. C. & Bradford, M. A. Litter quality impacts on grassland litter decomposition are differently dependent on soil fauna across time. Applied Soil Ecology 24, 197–203 (2003).
Jiang, Y., Yin, X. & Wang, F. The influence of litter mixing on decomposition and soil fauna assemblages in a Pinus koraiensis mixed broad-leaved forest of the Changbai Mountains, China. European Journal of Soil Biology 55, 28–39 (2013).
Ayres, E. et al. Home-field advantage accelerates leaf litter decomposition in forests. Soil Biology &. Biochemistry 41, 606–610 (2009).
Freschet, G. T., Aerts, R. & Cornelissen, J. H. C. Multiple mechanisms for trait effects on litter decomposition: moving beyond home-field advantage with a new hypothesis. Journal of Ecology 100, 619–630 (2012).
Veen, G. F., Freschet, G. T., Ordonez, A. & Wardle, D. A. Litter quality and environmental controls of home‐field advantage effects on litter decomposition. Oikos 124, 187–195 (2015).
Veen, G. F., Sundqvist, M. K. & Wardle, D. A. Environmental factors and traits that drive plant litter decomposition do not determine home‐field advantage effects. Functional Ecology 29, 981–991 (2015).
Morriën, E., Engelkes, T., Macel, M., Meisner, A. & Putten, W. H. V. D. Climate change and invasion by intracontinental range-expanding exotic plants: the role of biotic interactions. Ann Bot 105, 843–848 (2010).
Crutsinger, G. M., Sanders, N. J. & Classen, A. T. Comparing intra- and inter-specific effects on litter decomposition in an old-field ecosystem. Basic & Applied Ecology 10, 535–543 (2009).
Bhakta, K. R. Positive degree-day factors for ice ablation on four glaciers in the Nepalese Himalayas and Qinghai-Tibetan Plateau. Bulletin of Glaciological. Research 20, 7–14 (2003).
Konestabo, H. S., Michelsen, A. & Holmstrup, M. Responses of springtail and mite populations to prolonged periods of soil freeze-thaw cycles in a sub-arctic ecosystem. Applied Soil Ecology 36, 136–146 (2007).
Wallwork, J. A. The Distribution and Diversity of Soil Fauna. Quarterly Review of Biology, 319 (1976).
Yin, W. Pictorial keys to soil animals of China. (Science press, 2000).
Bellinger, P. F., Christiansen, K. A., & Janssens, F. Checklist of the Collembola of the world. Retrieved from, http://www.collembola.org.
Acknowledgements
This work was financially supported by projects from the National Natural Science Foundation of China (31570605), the key Project of Sichuan Education Department (18ZA0393), National key research and development plan (2017YFC0505003) and Key research and development project of sichuan province (18ZDYF0307).
Author information
Authors and Affiliations
Contributions
Y.L., R.L.H., Y.M.C. and J.Z.designed the study. R.L.H., Y.M.C., L.Y., J.J.X., P.Z. and L.H.C. performed the experiments. L.F.W., R.L.H, Z.F.X., B.T., L.Z. and L.G. analyzed the data. All of the authors were involved in discussing the data. L.F.W. and Y.L. contributed to drawing the figures. Y.L. and L.F.W. drafed the manuscript, and all of the authors reviewed the manuscript. Y.L. and J.Z. approved the final version.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Y., Wang, L., He, R. et al. Higher soil fauna abundance accelerates litter carbon release across an alpine forest-tundra ecotone. Sci Rep 9, 10561 (2019). https://doi.org/10.1038/s41598-019-47072-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-47072-0
- Springer Nature Limited
This article is cited by
-
Soil fauna effects on litter decomposition are better predicted by fauna communities within litterbags than by ambient soil fauna communities
Plant and Soil (2023)
-
Home-field advantage and ability alter labile and recalcitrant litter carbon decomposition in an alpine forest ecotone
Plant and Soil (2023)
-
Soil meso- and micro-fauna community in response to bamboo-fungus agroforestry management
Scientific Reports (2022)
-
Evidence for strong environmental control on bacterial microbiomes of Antarctic springtails
Scientific Reports (2021)
-
Litter decomposition and arthropod composition under different ultraviolet levels following prescribed burn in a subtropical pastureland
Biology and Fertility of Soils (2021)