Abstract
Caragana korshinskii Kom. has a significant function in desert-grassland revegetation in arid regions. Plant reproduction in arid regions can be restricted due to inadequate pollen receipt and reduced pollen transfer. An assessment of pollination success as a result of pollen limitation and pollinator visitation in various C. korshinskii populations is presently lacking. We thus tested three different treatments (pollen addition, control, and procedural control) to elucidate how pollen limitation affects seed numbers per flower in C. korshinskii. We also determined the effect of pollinator visit frequency on seeds per flower. Our results demonstrated that there was a higher proportion of open flowers and mature fruits in the managed population than in the natural population. Pollen addition significantly increased seed number per flower, and pollen limitation was determined to be a significant limiting factor in seed production. Furthermore, Apis mellifera was determined to be the principal pollinator, and pollinator visitation frequency was significantly correlated with open flower number. Our findings also demonstrated that pollinator visitation rate and seed production were positively correlated. Management and pollinator visitation could affect seed production, which may explain the higher seeds per flower in the managed population compared with the natural population.
Similar content being viewed by others
Introduction
As plants are immobile, they therefore depend on abiotic or biotic vectors to facilitate pollen transfer for sexual reproduction1, which has shaped floral attraction and plant mating systems1. Many studies have indicated that pollinator visits and behavior could affect the pollination success of plants2,3. Pollinator limitation can occur because flower visitors are either erratic or exhibit a preference for more attractive flowers1,2.
The pollination success of a plant can be hampered by an inadequate or insufficient supply of pollen, called “pollen limitation”4. There are both ecological and evolutionary determinants and consequences for pollen limitation1. In addition, many studies have measured the scale of pollen limitation based on the pollen limitation index (PL index) for each reproductive component4,5. There has been particular focus on pollen limitation because low pollen transfer and resource availability can impact seed production1. Pollen limitation is a widely-observed phenomenon that is typically interpreted as an indication of insufficient pollinator visitation in arid areas6,7. There is abundant evidence of pollen limitation due to insufficient pollinator services, particularly in animal-pollinated plants8,9.
Changes in habitat that cause increases or decreases in plant density may consequently alter pollinator availability and thus the pollination success of plants10. Human impacts on landscapes as well as grazing can also negatively influence pollinator visitor frequency6. Numerous plant species that rely on less effective pollinators may be subject to significant decreases in pollination success if pollinator activity is influenced by severe environmental conditions or climate change11.
Caragana korshinskii Kom. (Leguminosae: Ammopiptanthus) has an important function in the establishment of arid vegetation12. The aims of the present study were to (1) assess the floral trait differences between natural and managed populations, (2) establish the potential impact of pollen limitation on seeds per flower in both natural and managed populations and evaluate the correlation between pollinator visitation frequency and open flower number, and (3) determine how the seed production of C. korshinskii is impacted by pollinator visitation.
Results
Grazing effects on vegetation
The vegetation cover (VC), vegetation density (VD), vegetation height (VH), and aboveground plant biomass (AGB) of C. korshinskii are indicated in Fig. 1. The findings show that the VD, VH, and AGB of the managed patch were significantly increased in comparison to those in the natural patch (df = 1, P < 0.05). However, no difference in VC was observed between the two studied patches (df = 1, P > 0.05).
Floral traits
Flowering generally took place from May until late June in both populations. At approximately 08:30, the flowers started to open and were completely open by 09:00. At approximately 14:00, the flowers started closing, and pollinator activity had ceased by 19:00 (Table 1). However, we observed that the managed population experienced a longer flower production period and flowering peak than the natural population (P < 0.05; Fig. 2).
In the natural patch, the production peak of the mature fruits occurred in the third week of July. While generally similar patterns in mature fruit production were detected in the natural and managed populations, there were significant differences observed during the first week of August, with an increased number of mature fruits observed in the managed population (F = 18.36; P < 0.05; Fig. 2).
Pollen limitation
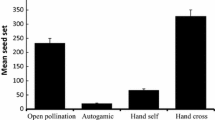
In the natural population, compared with the control and procedural control flowers, the mean number of seeds per flower was not significantly different in the natural population (Fig. 3), with the control values of 2.61 ± 0.8 (mean ± SE) and procedural control values of 2.49 ± 0.7 [Generalized linear Model (GLM), treatments effect, likelihood ratio χ2 = 0.89, df = 1, P > 0.05)]. In the managed patch, the mean seeds per flower, 2.90 ± 0.6 (control) and 3.97 ± 1.1 (pollen added), were significantly different between the control and pollen added treatment based on the GLM model (GLM, treatments effect, likelihood ratio χ2 = 31.24, df = 1, P < 0.001). Pollen supplementation was associated with a significant increase in mean seed production between the control and pollen added treatment in 2016–2018 in both patches (GLM, treatments effect, df = 1, P < 0.05; Table 2). Our findings demonstrated that the pollen limitation index in the natural population (0.29 ± 0.03) was more intense than in the managed population (0.27 ± 0.03).
Pollinator visitation and activity
Apis mellifera ligustica Spin was the principal pollinator in the tested patched, pushing the petals of unopened flowers out to enter the flower. In addition, A. mellifera had successful visits because its hairy body could easily deposit more pollen per visit. Pollen release peaked between 10:00 and 14:00. Apis mellifera ligustica is active from 08:00 until 18:00, and the majority of the activity of A. mellifera ligustica overlapped with the open flowers of C. korshinskii. Of the 261 observed pollinator visits, A. mellifera accounted for 66.3% of the total visits. Other occasional visitors included Bombus lucorum L., Serica orientalis Motschulsky, Anthophora fulvitarsis Brulle, and Eristalis cerealis Fabricius, but these species only assisted in pollinator visits as they rarely touched the stigma and only visited infrequently.
The flowers of C. korshinskii have evolved a tripping mechanism. Pollinator activity is initiated with open flowers, with the pollinator acting as a tripping agent. We found that there was a significant correlation between the frequency of pollinator visits and the number of flowers that were open in both populations (natural, r = 0.96; managed, r = 0.86, P < 0.05; Fig. 4). Furthermore, our findings demonstrated that the Vf of A. mellifera ligustica in the managed population (5.26 ± 0.6 visits/hour) was significantly higher than Vf in the natural population (3.89 ± 0.5 visits/hour; df = 1, P < 0.05).
Pollinator visitation effects on seed production
Of the flowers in the natural patch, effective pollinators visited (V) 43.62% at least once, and 25.13% generated seeds (S), which resulted in the visited flowers (S/V × 100%) having a seed production percentage of 57.61%. Our findings demonstrated that in the managed patch, 49.36% of the flowers had been visited and 30.80% produced seeds, which resulted in the visited flowers having a seed production of 62.39%. These findings illustrated that there was a significant correlation between the seed production percentages among the visited flowers and the pollinator visitation rates both in the natural patch (the percentage of seeds: r = 0.72, P < 0.01) and the managed patch (the percentage of seeds: r = 0.79, P < 0.01; Fig. 5).
Discussion
Floral traits influencing pollinator visitation and activity
Floral traits impact pollinator activity and visitation and also affect pollination efficiency13,14. Generalist pollinators are subject to a high degree of environmental variation, and thus pollinator learning can potentially have a significant function in plant-insect coevolution12. Ortíz et al. (2010) demonstrated that flower resource density could be associated with the variation in visiting frequency and pollinator behavior15. In particular, nectar and pollen are the targets of pollinators16,17. We discovered that the proportion of open flowers could be significantly impacted by management, with the flowers in the managed population possessing more floral resources than those in the natural population. These findings might provide an explanation as to why a positive relationship exists between pollinator visiting frequency and the number of flowers that are open.
The likelihood of successful pollen vectors visiting a plant increases with pollinator activity18. The convergent evolution of floral traits with the traits of their common pollinators is widespread in plants and constitutes one of the most visual demonstrations of natural selection19. In arid regions, a high frequency of pollinator visits is more efficient because the filaments of flowers dry easily. In addition, pollinator visits are sometimes erratic and less persistent partly as a result of strong winds and high temperatures, which could severely impact pollinator activity11. In the present analysis, complete opening of the flowers was observed between 08:00 and 14:00, which enabled pollen release and represented a significant period for successful pollinating of C. korshinskii. Furthermore, the greatest A. mellifera activity overlapped with this period. Therefore, A. mellifera had the highest visiting frequency in comparison to the other pollinators.
Pollen limitation and pollinator visitation in different populations
Plant life-history and the mating system may be correlated with the likelihood or degree of pollen limitation20. In many flowering plants, pollen limitation affects processes from pollination to seed production1,19 (Ashman et al. 2004; Ryan & David 2013). Pollen limitation is believed to have occurred if pollen supplementation increases fruit or seed production20,21. We discovered that pollen addition significantly increased the seeds per flower in the present study, indicating that seed production was pollen-limited in this species.
Earlier reports suggested that pollen quantity limitation is associated with both the pollinator frequency and pollinating effectiveness1. The foraging patterns of pollinators can be altered by management and human impacts on habitats, affecting pollinator behavior16,22. In the majority of angiosperms, pollinator activity is declining as a result of reduced floral rewards (nectar or pollen) and a lack of nesting requirements for pollinator species23,24. The results of the current study showed that grazing significantly influences vegetation density, vegetation height, and aboveground plant biomass in natural patches. In addition, management could significantly affect the peak flowering period and the proportion of open flowers.
Pollen limitation is significantly associated with pollinator activity and visitation, and pollen limitation typically takes place when incompatible pollen is deposited when pollinators are infrequent due to decreased floral resources5,9,25. Elucidating the association between pollen limitation and pollinator visitation across populations could be necessary for improving our ability to predict decreased pollinators and seed production in conserved plant populations26,27. In addition, a positive association between pollinator visitation frequency and open flower numbers was detected in both populations. Additionally, the flower density was greater in the managed than in the natural population. Therefore, the managed plants could attract more pollinator visits than the natural plants as a result of increased floral resources in the former.
Pollinator visitation affects the pollination success of C. korshinskii
The sexual reproduction of seed plants is dependent on pollination, and pollination by insects appears to be dominant in angiosperms28. Reproductive success or failure is greatly dependent on pollinator activity and frequency29. Pollination has recently been shown to be crucial for the sexual reproduction of seed plants, with pollinator visiting frequency constituting an important predictor of pollination success30,31. The main biotic factors influencing pollination success include pollinator visitation, pollen quality, and reward systems32.
In arid regions, overgrazing is considered as a significant contributor to grassland degradation33. Furthermore, trampling by livestock erodes the crust and soil aggregate stability, while livestock feeding decreases vegetation cover34. Management and habitat changes also can affect pollinator visitation, which could further alter the outcrossing success of plants35. An earlier study indicated a reduction in pollinators is associated with a decreased quantity of pollen supplied to the stigmas and a reduction in cross-pollen transfer probability, thus causing decreased seed production2. Arid environments are associated with an increased frequency of unreliable and persistently less abundant pollinators partly as a result of grazing and habitat fragmentation, which severely hamper pollinator visits16. Decreased numbers of pollinators limit cross-pollen transfer probability, which might disturb the pollination process of plants, thus altering the foraging patterns of pollinators and contributing to the decline in plant populations22,36. When moderate grazing management was performed, we found that grazing had a significant impact on plant resources in the process of pollination.
In C. korshinskii, insect pollination plays a dominant role in the breeding system37. Moreover, A. mellifera tended to visit regions with more resources and stayed longer in the managed population. The findings of the present study also confirm that increased pollinator visitation rates are associated with increased seed production.
Methods
Species
Caragana korshinskii is a shrub that typically ranges between 0.4 and 2.0 m in height and occurs primarily in the provinces of western Inner Mongolia and Gansu. The root system of C. korshinskii is well-developed and drought resistant. This species is also valued for its medicinal properties38.
Study area and experimental design
The study site was situated in the Urat Desert grassland (Fig. 6) to the north of Yinshan Mountain (41°06′–41°25′ N and 106°59′–107°05′ E). Based on the meteorological data obtained from the Urat Desert Grassland Research Station, the average annual temperature, average annual potential evapotranspiration, and average annual rainfall is 5.6 °C, about 2200 mm, and about 153.9 mm, respectively.
The experimental design comprised two patches and 12 plots, six natural plots, and six managed plots (20 × 20 m). In the natural patch, grazing experiments were performed. In addition, natural plots were arranged symmetrically and enclosed by undisturbed vegetation (gray area). Species other than C. korshinskii were removed from the managed patch (white area). Water was provided in the managed patch.
This experiment included two patches with six natural plots and six managed plots of 20 m × 20 m each. The studied plots were originally desert-grassland, with C. Korshinskii being the naturally dominant species in the six plots without artificial management. Reaumuria songarica (Pall.) Maxim was an associated species in the natural patches. In the natural patches, grazing experiments were performed from 2016 to 2018. Based on the grazing capacity of the desert steppe in Inner Mongolia, a moderate grazing intensity (0.25 sheep per ha) was established. Grazing ensued daily from May to August during the grazing period (from 07:00 to 19:00), and the sheep were placed in an enclosure during nighttime. In the managed patches, C. korshinskii was also the dominant species, and other species were repeatedly cleared (five times per year). In addition, we protected these plots from livestock grazing and also provided water. The natural and managed patches were separated by approximately 1 km to avoid the mutual interference of pollinators. In both patches, the average plant density was 15 individuals per 100 m2 (Fig. 1).
Floral traits
In the studied patches, we randomly labeled 50 flowers to observe the anthers and buds. Floral phenology (anthesis and pollen dehiscence) was investigated with bagged flower buds and observed from 06:00 until senescence. In each population, the phenological characters of 10 individual C. korshinskii plants per plot were given labels and studied. On each branch, the flowers in anthesis and fruits on branches were enumerated during the entirety of the reproductive season (from May to August) for each plant. The proportion of flowers in anthesis and the numbers of mature fruits were then calculated.
Pollen limitation
In order to calculate the degree of pollen limitation, a pollen supplementation experiment was set-up during flowering. Three treatments were established to assess the impact of pollen limitation on seeds per flower: control, procedural control, and pollen addition.
Eighteen healthy plants were labeled in each patch, and one inflorescence on each labeled plant was marked. Fresh pollen from plants situated 15 m distant or farther from the 18 experimental plants was obtained for pollen supplementation (six plants per plot) until sufficient pollen grains were obtained. For 12 labeled plants, we labeled eight flowers on each plant separate from the marked inflorescence and added outcrossed pollen to 4 flowers as the pollen-added treatment. Another 4 flowers in the same inflorescence were left untouched as controls. In the remaining six plants, four flowers from the center of the inflorescence of each plant were also marked and constituted the procedural control (CC treatment).
In August, we collected and counted all seeds per flower from control and pollen-added treatments. In this study, we used the seeds per flower to calculate pollen limitation. The following equation was used to calculate the pollen limitation index for the controls:
Where RSC and RSPA are seeds per flower in the control and pollen-added treatment, respectively. Positive values of pollen limitation indicate a high intensity of pollen limitation, whereas negative values indicate the opposite39.
Visitation frequency and pollinator activity
To conduct surveys of the pollinators in the 12 plots (six plots per patch), we selected 72 healthy plants in the studied patches. For each selected plant, 10 flowers were labeled with tags at the bud stage. These marked flowers were observed daily between 07:00 and 19:00. Pollinators collecting nectar and pollen were recorded, and pollinator visit duration was measured using digital audio tape recorders. The quantity and identity of floral visitors from May to August were determined, and pollinators were captured with insect nets. In addition, we determined that effective pollination had occurred when pollinators collected pollen or delivered it to the stigmas37. Pollinators that visited the flowers were sampled for identification in the laboratory. We recorded the visiting frequency of the pollinators (Vf), which was calculated based on the following equation40:
Pollinator visitation affect seed production
To assess the impact of pollinator visitation on seed production, 18 plants in the natural patch and 18 plants in the managed patch were labeled during flowering. Ten flowers from each plant were randomly selected and tagged. The flowering stage and growth development of the labeled flowers were determined from the film recordings. Furthermore, the proportions of flowers that were open and the pollinator visits in the period May–August were recorded. We observed that the effective pollinators carried large quantities of pollen grains during their visits. In addition, these species had a higher visiting frequency than the other pollinators. The number of visited flowers and seed production were recorded when all seeds were mature. Additionally, the visited flower and mature seed percentages were calculated in accordance with the following equation24:
Where S and V represent the proportion of flowers producing seeds and the proportion of visited flowers, respectively.
Data analyses
A GLM was used to assess the impacts of pollination treatments (C, CC and PA), population types and years (from 2016 to 2018) on seeds per flower41. A gamma distribution and a logit link function were implemented in the model. The pollination treatments, population types, and years constituted the fixed factors, and the mean seeds per flower represented the dependent variable in the model. The likelihood ratio test was applied in the model, and treatment differences were assessed with Tukey’s multiple comparisons.
Analysis of variance (ANOVA) was used to evaluate the production of open flowers and mature fruits in both populations. Regression analyses were performed in SPSS 21.042,43. In addition, regression analyses using the number of open flowers as the independent variable were also performed, with pollinator visitation frequency as the dependent variable in this model.
References
Ashman, T. L. et al. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85, 2408–21 (2004).
Lennartsson, T. Extinction thresholds and disrupted plant-pollinator interactions in fragmented plant populations. Ecology 83, 3060–3072 (2002).
Hadley, A. S. & Betts, M. G. The effects of landscape fragmentation on pollination dynamics: absence of evidence not evidence of absence. Biol Rev 87, 526–544 (2012).
Gómez, J. M. et al. Changes in pollinator fauna cause spatial variation in pollen limitation. J Ecol 98, 1243–1252 (2010).
Wesselingh, R. A. Pollen limitation meets resource allocation: towards a comprehensive methodology. New Phytol 174, 26–37 (2007).
Nayak, K. G. & Davidar, P. Pollinator limitation and the effect of breeding systems on plant reproduction in forest fragments. Acta Oecol 36, 191–196 (2010).
Wagenius, S. & Lyon, S. P. Reproduction of Echinacea angustifolia in fragmented prairie is pollen-limited but not pollinator-limited. Ecology 91, 733–742 (2010).
Ishii, J. & Kadono, Y. Factors influencing seed production of Phragmites australis. Aquat Bot 72, 129–141 (2002).
Suzuki, N. Pollinator limitation and resource limitation of seed production in the Scotch broom, Cytisus scoparius (Leguminosae). Plant Spec Biol 15, 187–93 (2000).
Hadley, A. S. & Betts, M. G. The effects of landscape fragmentation on pollination dynamics: absence of evidence not evidence of absence. Biol Rev 87, 526–544 (2012).
Michael, A. W., Margaret, T. E. & Robert, S. B. Conservation impact of climatic variability on pollination of the federally endangered plant, Clematis socialis (Ranunculaceae). Southeast Nat 2, 11–24 (2003).
Johnson, S. D. & Steiner, K. E. Generalization versus specialization in plant pollination systems. Trends Ecol Evol 15, 140–143 (2000).
Muchhala, N. Adaptive trade-off in floral morphology mediates specialization for flowers pollinated by bats and hummingbirds. Am Nat 169, 494–504 (2007).
Sletvold, N., Grindeland, J. M. & Ågren, J. Pollinator-mediated selection on floral display, spur length and flowering phenology in the deceptive orchid Dactylorhiza lapponica. New Phytol 188, 385–392 (2010).
Ortíz, F. E. et al. Pollination biology of Myrtillocactus schenckii (Cactaceae) in wild and managed populations of the Tehuacán Valley, México. J Arid Environ 74, 897–904 (2010).
Rodríguez-Cabal, M. A., Aizen, M. A. & Novaro, A. J. Habitat fragmentation disrupts a plant-disperser mutualism in the temperate forest of South America. Biol Conserv 139, 195–202 (2007).
Pauw, A., Stofberg, J. & Waterman, R. J. Flies and flowers in Darwin’s race. Evolution 63, 268–279 (2009).
Ashman, T. L. & Morgan, M. T. Explaining phenotypic selection on plant attractive characters: male function, gender balance or ecological context? Proc R Soc Lon B 271, 553–59 (2004).
Ryan, D. B. R. & David, A. M. Resource reallocation does not influence estimates of pollen limitation or reproductive assurance in Clarkia Xantiana Subsp. Parviflora (Onagraceae). Am J Bot 100, 1916–1921 (2013).
Burd, M. Bateman’s principle and plant reproduction—the role of pollen limitation in fruit and seed set. Bot Rev 60, 83–139 (1994).
Knight, T. M. et al. Pollen limitation of plant reproduction, ecological and evolutionary causes and consequences. Annu Rev Ecol Syst 36, 467–497 (2005).
Cresswell, J. E. Spatial heterogeneity, pollinator behaviour and pollinator-mediated gene flow: bumblebee movements in variously aggregated rows of oil-seed rape. Oikos 78, 546–556 (1997).
Bierzychudek, P. Pollinator limitation of plant reproductive effort. Am Nat 117, 838–40 (1981).
Amparo, L., Rebekka, L. & Ørjan, T. Pollen limitation, species’ floral traits and pollinator visitation: different relationships in contrasting communities. Oikos 124, 174–186 (2015).
Wagenius, S. Scale dependence of reproductive failure in fragmented Echinacea populations. Ecology 87, 931–941 (2006).
Eckert, C. G. et al. Plant mating systems in a changing world. Trends Ecol Evol 25, 35–43 (2010).
Fernández, J. D., Bosch, J., Nieto-Ariza, B. & Gómez, J. M. Pollen limitation in a narrow endemic plant: geographical variation and driving factors. Oecologia 170, 421–431 (2012).
Tamura, S. & Kudo, G. Wind pollination and insect pollination of two temperate willow species, Salix miyabeana and Salix sachalinensis. Plant Ecol 147, 185–192 (2000).
Revel, N. et al. Investigating the relationship between pollination strategies and the size-advantage model in zoophilous plants using the reproductive biology of Arum cylindraceum and other European Arum species as case studies. Arthropod-Plant Inte 6, 35–44 (2012).
Tewksbury, J. J. et al. Corridors affect plants. animals, and their interactions in fragmented landscapes. P Natl Acad Sci Usa 99, 12923–12926 (2002).
Vázquez, D. P., Morris, W. F. & Jordano, P. Interaction frequency as a surrogate for the total effect of animal mutualists on plants. Ecol Lett 8, 1088–1094 (2005).
Corbett, S. A. Nectar sugar content: estimating standing crop and secretion rate in the field. Apidologie 34, 1–10 (2003).
Li, Y. Q., Zhao, H. L., Zhao, X. Y., Zhang, T. H., Li, Y. L. & Cui, J. Y. Effects of grazing and livestock exclusion on soil physical and chemical properties in desertified sandy grassland, Inner Mongolia, northern China. Environ Earth Sci 63, 771–783 (2011).
Du, H. Q., Zuo, X. A., Li, S., Wang, T. & Xue, X. Wind erosion changes induced by different grazing intensities in the desert steppe, Northern China. Agr Ecosyst Environ 274, 1–13 (2019).
VanDyck, H. & Matthysen, E. Habitat fragmentation and insect flight: a changing ‘design’ in a changing landscape? Trends Ecol Evol 14, 172–174 (1999).
Melissa, F., Linley, K. J., Kyle, B. & Daniel, J. S. Mitigation of pollen limitation in the lowbush blueberry agroecosystem: effect of augmenting natural pollinators. Ecosphere 6, 1–19 (2015).
Chen, M. & Zhao, X. Y. Effect of fragmented habitats on pollen limitation and pollinator behavior in Caragana korshinskii Kom. Sci Total Environ 654, 1056–1063 (2019).
Zeng, J. F., Huo, C. Y. &Han, X. Z. The Flora of China. Science Press. Beijing, pp. 13-67 (199i3).
Cosacov, A., Nattero, J. & Cocucci, A. A. Variation of pollinator assemblages and pollen limitation in a locally specialized system: the oil–producing Nierembergia linariifolia (Solanaceae). Ann Bot 102, 723–734 (2008).
Goverde, M. et al. Small-scale habitat fragmentation effects on pollinator behaviour: experimental evidence from the bumblebee Bombus veteranus on calcareous grasslands. Biol Conserv 104, 293–29
Chen, M. & Zuo, X. A. Effect of Pollen Limitation and Pollinator Visitation on Pollination Success of Haloxylon ammodendron (Mey, C. A.) Bunge in Fragmented Habitats. Front Plant Sci (2019).
Zhang, D. Y., Shi, X., Wang, J. C., Liu, H. L. & Gaskin, J. F. Breeding system and its consequence on fruit set of a rare sand dune shrub Eremosparton songoricum (Fabaceae: Papilionoideae): implications for conservation. J Arid Land 3, 231–239 (2011).
Chen, M., Zhao, X. Y. & Zuo, X. A. Pollinator activity and pollination success of Medicago sativa L. in a natural and a managed population. Ecol Evol 8, 9007–9016 (2018).
Acknowledgements
This research work was supported by National basic resource survey “Survey on the desert major plant communities in China” (2017FY100200), National Natural Science Foundation of China (31600252; 41771117) plays the important role in the design of the study and collection, analysis. In addition, China National Key Research and Development Plan (2016YFC0500506) and CAS “Light of West China” Program (Y729821001) play the important role in interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
M.C. and X.Y.Z. conceived and designed the experiments. M.C. performed the experiments. M.C. and X.Y.Z. analyzed the data. M.C. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, M., Zhao, Xy. Impact of floral characters, pollen limitation, and pollinator visitation on pollination success in different populations of Caragana korshinskii Kom. Sci Rep 9, 9741 (2019). https://doi.org/10.1038/s41598-019-46271-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-46271-z
- Springer Nature Limited