Abstract
The regulator of G protein signaling (RGS) domain proteins generally attenuate heterotrimeric G protein signaling, thereby fine-tune the duration and strength of signal transduction. In this study, we characterize the functions of RgsD, one of the six RGS domain proteins present in the human pathogenic fungus Aspergillus fumigatus. The deletion (Δ) of rgsD results in enhanced asexual sporulation coupled with increased mRNA levels of key developmental activators. Moreover, ΔrgsD leads to increased spore tolerance to UV and oxidative stress, which might be associated with the enhanced expression of melanin biosynthetic genes and increased amount of melanin. Yeast two-hybrid assays reveal that RgsD can interact with the three Gα proteins GpaB, GanA, and GpaA, showing the highest interaction potential with GpaB. Importantly, the ΔrgsD mutant shows elevated expression of genes in the cAMP-dependent protein kinase A (PKA) pathway and PKA catalytic activity. The ΔrgsD mutant also display increased gliotoxin production and elevated virulence toward Galleria mellonella wax moth larvae. Transcriptomic analyses using RNA-seq reveal the expression changes associated with the diverse phenotypic outcomes caused by ΔrgsD. Collectively, we conclude that RgsD attenuates cAMP-PKA signaling pathway and negatively regulates asexual development, toxigenesis, melanin production, and virulence in A. fumigatus.
Similar content being viewed by others
Introduction
All living cells must adapt or respond to external and internal cues. In cells, environmental cues trigger intrinsic signaling cascades involving a variety of surface receptors and signal transduction elements. Heterotrimeric G proteins (G proteins) play a central role in these processes and G protein mediated signaling is mostly transmitted via cAMP dependent protein kinase A (PKA) pathway, mitogen activated protein kinase (MAPK) pathway, or protein kinase C (PKC) pathway1,2,3. A canonical G protein signaling pathway is activated by interaction of ligands and G protein-coupled receptors (GPCRs), which causes conformation changes in the GDP-bound Gα subunit leading to GDP to GTP exchange, and the subsequent dissociation of Gα -GTP and Gβγ subunit. Free Gα –GTP and Gβγ, or both can activate (or inhibit) a number of downstream signaling pathways. Regulator of G protein signaling (RGS) proteins enhance the intrinsic GTPase activity of Gα thereby accelerate the hydrolysis of GTP bound to Gα, leading the formation of the inactive Gα-GDP::βγ heterotrimer and turn off G protein signaling3,4.
Sst2 of Saccharomyces cerevisiae was the first identified RGS protein. Sst2 interacts with the Gα subunit Gpa1 and functions as a negative regulator of the pheromone response pathway5,6. The second RGS of S. cerevisiae Rgs2 attenuates the Gpa2-mediated signaling for glucose sensing through controlling the cAMP-dependent protein kinase (PKA) pathway7. While only two RGSs have been reported in S. cerevisiae, a plethora of RGSs exists in filamentous fungi8. RGSs of filamentous fungi regulate diverse signals that control vegetative growth, sporulation, stress responses, secondary metabolism, and virulence9. There are six RGS proteins (FlbA, GprK, RgsA, Rax1, RgsC, and RgsD) in the opportunistic human pathogen Aspergillus fumigatus. Among these, we have revealed that FlbA, GprK, and Rax1 play important roles in upstream regulation of G-protein and contribute to proper asexual development, secondary metabolites production, and stress responses10,11 Moreover, our recent study has revealed that RgsC is necessary for proper growth, sporulation, stress response, gliotoxin (GT) production, and external nutrients sensing in A. fumigatus12.
RgsD is known as a putative paralog of RgsA and its transcript is induced by growth under hypoxia condition (http://www.aspergillusgenome.org). RgsA of Aspergillus nidulans was shown to negatively regulate function of the Gα subunit GanB and regulate stress response and asexual sporulation13. As A. fumigatus and A. nidulans are distantly related, it is important to understand the exact functions of this key RGS protein, RgsD, in A. fumigatus. Our thorough characterization of the rgsD function has elucidated its broad repressive roles in asexual development, melanin production, stress response, GT biosynthesis, and virulence. Moreover, RgsD is required to properly control PKA activity. Collectively, understanding the target(s) of RgsD and downstream signaling cascades would provide a development of novel anti-fungal therapeutics against A. fumigatus.
Results
Summary of A. fumigatus RgsD
The ORF of rgsD of A. fumigatus Af293 (Afu5g00900) consists of 867 bp nucleotides with no intron, and is predicted to encode a 288 amino acid length protein. As shown in Supplementary Fig. S1A, the domain architecture of RgsD is very simple, containing just an RGS domain (14 to 147 aa, E-value; 5e-13) and two low complexity regions (218 to 230 aa and 278 to 286 aa). Based on the protein sequences, RgsD was aligned and compared with other putative RgsD-like proteins of Aspergillus (Supplementary Fig. S1B). While RgsD of A. fumigatus Af293 shares amino acid identity ranging from 34.1 to 82.3% with the RgsD-like proteins of A. terreus (ATET_09016), A. clavatus NRRL 1 (ACLA_088640), A. oryzae RIB40 (AO090023000043), and Neosartorya fischeri NRRL 181 (NFIA_041270), it shares low identity with the RgsA protein of A. nidulans (AN5755, 40.7%) and A. niger (An18g06110, 40.9%).
RgsD negatively controls asexual development
Previous studies have revealed that the RGS proteins FlbA, GprK, Rax1 (RgsB), and RgsC are positive regulators of asexual development as the conidia numbers were drastically decreased by the absence of any one of these RGS proteins10,11,12,14. To investigate functions of rgsD, we generated the rgsD null (ΔrgsD) mutant and complemented strains (C′). Although the ΔrgsD mutant demonstrated no significant change in radial growth, it formed very dark colonies with highly increased thallic density and significantly increased formation of mature conidiophores compared to wild type (WT) and C’ strains (Fig. 1A,C). Distinct from the other RGS mutants, the ΔrgsD mutant produced 4 fold more conidia than WT and C′ strains (Fig. 1B). Moreover, the ΔrgsD mutant produced a higher number of mature conidiophores (asexual developmental structures) than WT and C′ strains in liquid medium after 24 hours incubation (Fig. 1C). In WT and C’ strains, mRNA levels of rgsD were high at late stage of development whereas those of other asexual developmental regulators increase at earlier stage of development in WT (Fig. 1D). As shown in Fig. 1E, in the ΔrgsD mutant, mRNA levels of the key asexual developmental regulators abaA, brlA, vosA, and wetA were significantly increased at all time points tested. Conversely, mRNA levels of nsdD and veA, known negative regulators of brlA expression and conidiation, were low in the ΔrgsD mutant compared to WT and C′ (Fig. 1E). These results suggest that RgsD negatively controls conidiation and expression of developmental activators, but positively regulates nsdD and veA. Morevoer, as rgsD mRNA accumulates later phases of conidiation, it is likely that the RgsD protein (but not mRNA) is present and acting at early time points of life cycle and development, and the RgsD protein is re-generated during conidiogenesis.
Negative regulation of asexual development by RgsD. (A) Colony photographs of WT (AF293), ΔrgsD, and complemented (C′) strains inoculated on solid MMY and grown for 3 days. The bottom panel shows the back side of plates. (B) Radial growth rates of three strains and conidia numbers produced by each strain per plate. Student’s t-test: *p < 0.05, **p < 0.01. (C) Photographs of WT, ΔrgsD, and C′ strains observed by liquid-slide culture. Number of total conidiophores per microscopic field were indicated in parenthesis. (D) Levels of rgsD mRNA during the life cycle of A. fumigatus WT. (E) mRNA levels of the asexual developmental regulators in three strains determined by quantitative PCR (qRT-PCR). Cultures were incubated in liquid MMY and mRNA levels were normalized using the ef1α gene, according to the ΔΔCt method. Data are expressed as the mean ± standard deviation from three independent experiments. Student’s t-test: *p < 0.05, **p < 0.01.
RgsD interacts with Gα protein in yeast
As the RgsD protein contains a clear RGS domain, it is conceivable that RgsD might physically interact with one or more of the three Gα proteins (GpaA, GpaB, and GanA) present in A. fumigatus. To test this interaction potential, we carried out the split-ubiquitin yeast two-hybrid interaction assays with RgsD and three Gα proteins. RgsD::Cub plasmid was constructed by fusing Cub to the C-terminus of the full-length RgsD cDNA. Individual Gα protein constructs were generated by fusing full-length GpaA, GpaB, and GanA cDNA with the mutated N-terminal half of ubiquitin (NubG), respectively. As shown in Fig. 2A,B, those yeast transformants co-expressing RgsD::Cub/NubG::GpaB and RgsD::Cub/NubG::GanA grew better than positive control on the medium lacking histidine or adenine, and showed high levels of ß-galactosidase enzyme activity. Somewhat unexpectedly, those yeast transformants co-expressing RgsD::Cub/NubG::GpaA also grew on the medium lacking histidine or adenine, and showed ß-galactosidase enzyme activity equal to the positive control. These results indicate that RgsD may interact GpaB and GanA preferentially, but also has the ability to interact with GpaA.
RgsD interacts with Gα proteins. (A) Interactions between RgsD and Gα proteins in the split-ubiquitin system. The C-terminal half of ubiquitin (Cub) was fused to the full-length RgsD cDNA. The N-terminal half of ubiquitin (NubG) was fused to the C-terminus of full-length GpaA (NubG::GpaA), GpaB (NubG::GpaA), and GanA (NubG::GanA). RgsD::Cub interaction with the control vector pAI-Alg5 served as a correct topology positive control, RgsD::Cub interaction with the empty vector pDL2-Alg5 served as a negative control. The pDHB1-largeT interaction with pAI-Alg5 served as a positive control for the assay. Yeast transformants were grown on the selective medium lacking histidine or adenine after serial dilution. (B) To verify the interaction, ß-galactosidase activity assays were performed. **p < 0.01.
RgsD downregulates cAMP-dependent PKA signaling pathway
To investigate the role of RgsD in governing spore germination, we analyzed the kinetics of germ tube emergence in three strains. Although the germination of the ΔrgsD conidia was started slightly earlier, there were no significant differences between the mutant and WT (Supplementary Fig. S2). The components of the PKA signaling pathway in A. fumigatus, GpaB (Gα), AcyA (adenylyl cyclase), PkaC1 (the major catalytic subunit), and PksP (a polyketide synthase needed for spore pigmentation) are required for the proper growth, asexual sporulation, and conidial pigmentation15,16,17. Previous studies have demonstrated that the cAMP-PKA signaling pathway is attenuated by RgsA in A. nidulans13,18,19. To investigate the relationship between RgsD and cAMP-PKA signaling pathway, we analyzed mRNA levels of gpaB, acyA, pkaC1, and pksP mRNA. As shown in Fig. 3A, gpaB, acyA, pkaC1, and pksP mRNA levels were significantly higher in the ΔrgsD mutant than in WT and C′ strains. To investigate this further, we assessed PKA activity using a peptide substrate, kemptide, which is specifically recognized and phosphorylated by PKA. Proteins were extracted from each strain grown in MMG at indicated time and assayed for PKA activity. The phosphorylated negatively charged kemptide migrates to the anode and the signal is proportional to higher PKA activity in the protein extracts. While WT and C′ strains exhibited very little PKA activity in all tested protein extracts, the ΔrgsD mutant showed 2-fold higher PKA activity at 24 h than other samples (Fig. 3B,C). These results indicate that RgsD is required for proper control of expression of gpaB, acyA, pkaC1, and pksP, and may negatively regulate a cAMP-PKA signaling pathway.
RgsD downregulates a cAMP-PKA signaling pathway. (A) Expression of gpaB, acyA, pkaC1, and pksP mRNA in three strains analyzed by qRT-PCR. Student’s t-test: **p < 0.01. (B) PKA activity of three strains as monitored by gel electrophoresis. A phosphorylated substrate migrates toward the anode (+). Each strain was grown in MMG for indicated time at 37 °C and mycelial extract was analyzed. (C) Kinase activity was determined by the spots (panel B) quantification using spectrophotometer. Note that mRNA levels of cAMP-PKA signaling pathway related genes and PKA activity at 24 hour were significantly increased in the ΔrgsD mutant compared to WT and C′ strains.
Repressive role of RgsD in stress response and melanin biosynthesis
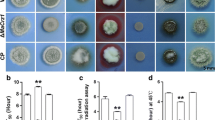
To test a potential role of RgsD in stress response, the ΔrgsD conidia were exposed to UV and H2O2, and the survival rates were determined. As shown in Fig. 4A, the ΔrgsD conidia were more resistant than those of WT and C′ strains against UV irradiation. While the relative survival rates of conidia of WT and C′ strains were about 20%, survival rate of the ΔrgsD conidia was about 40% at 10 mJ/cm2. The difference was clearer at higher dose (20 mJ/cm2), where approximately 18% of the ΔrgsD conidia survived but only about 5% of conidia of WT and C′ strains remained alive (Fig. 4A). As shown in Fig. 4B, the conidia of the ΔrgsD mutant were also more resistant to H2O2. The survival rate of WT, mutant, and C′ conidia was about 65, 95, and 64%, respectively, at 2.5 mM H2O2. Noticeably, the mutant strain formed a strongly pigmented colony. As the proposed functions of melanin are protection against UV irradiation and scavenging reactive oxygen species, we examined mRNA levels of melanin biosynthetic genes, melanin content, and ultrastructure of conidia. The mRNA levels of arp1 and arp2 which function in dihydroxynaphthalene (DHN)-melanin pathway of fungi20 were maximum at 12 hours and gradually decreased in the mutant, whereas those were highest at 24 hours and rapidly decreased in WT and C′ strain (Fig. 4B). Moreover, the amount of melanin in the ΔrgsD conidia was about 1.5-fold higher than that of WT and C′ strains conidia (Fig. 4C). We next examined the cell wall architecture of the WT, ΔrgsD, and C′ conidia with transmission electron microscopy (TEM). The outermost melanized electron dense layer of A. fumigatus determines the conidial color and defends against external stresses21. The ΔrgsD conidia form a darker electron dense layer than those of WT and C′ (Fig. 4D). These indicate RgsD negatively controls stress response and melanin biosynthesis.
Inhibition of stress responses and melanin synthesis mediated by RgsD. (A) Conidial viability of WT, ΔrgsD, and C′ strains in presence of UV irradiation and H2O2 at indicated conditions. Note that the viability of the ΔrgsD conidia was significantly increased compared to WT and C′ strain’s conidia. (B) Expression of melanin biosynthetic genes arp1 and arp 2 mRNA analyzed by qRT-PCR. Student’s t-test: **p < 0.01. (C) Melanin content in three strains’ conidia. Statistical significance was determined by a Student’s t-test: **p < 0.01. (D) TEM photographs of WT, ΔrgsD, and C′ conidia. Note that the ΔrgsD conidia exhibited higher expression of melanin synthesis-related genes’ mRNA, melanin content, and the melanized outmost cell wall than WT and C′ strain.
RgsD negatively controls gliotoxin production
We showed that the key asexual developmental activator BrlA positively regulates gliotoxin (GT) production in A. fumigatus22. Moreover, we also showed that the deletion of RGSs including FlbA, GprK, Rax1, and RgsC resulted in lowered brlA mRNA levels and decreased GT production10,11,12. As the deletion of rgsD resulted in up-regulated brlA expression different from previously reported RGSs, we examined the effect of rgsD deletion in GT production. We assessed levels of GT in WT, ΔrgsD, and C’ strains, and found that the ΔrgsD mutant produced about 5-fold more GT (Fig. 5A). We then checked mRNA levels of key GT biosynthetic genes by qRT-PCR, and found that mRNA levels of gliM, gliP, gliT, and gliZ were significantly (p < 0.01) higher in the ΔrgsD mutant than in WT and C′ strains (Fig. 5B).
The negative role of RgsD in GT production. (A) Determination of GT production in WT, mutant, and C′ strains. The culture supernatant of each strain was extracted with chloroform and subjected to TLC. (B) qRT-PCR analysis of four GT synthetic genes in WT, ΔrgsD, and C′ strains. Statistical differences between WT and mutant strains were evaluated with Student’s unpaired t-test. **p < 0.01.
Nullifying rgsD leads to enhanced virulence
We also tested the effects of RgsD on virulence using the wax moth larvae Galleria mellonella. Conidia (1 × 106) of WT, ΔrgsD, and C′ strains were inoculated to the larvae, and the survival rate of G. mellonella was recorded daily. The most rapid lethality occurred with the ΔrgsD mutant and this strain was significantly more virulent than the other challenge doses (p < 0.0001). As shown in Fig. 6A, larvae inoculated with conidia of either WT or C′ strains began to die at day 3 post-inoculation, with the number of survivors continuing to decrease over the course of the experiment. In the 7 days after infection, about 50% of larvae in both strains died. However, the mortality level of ΔrgsD strain was drastically higher than those of WT and C′ strains, all larvae tested died at 6 days after infection. We also observed that larvae infected with the mutant conidia darkened and shrank at 24 hours post inoculation. In contrast, larvae infected with WT and C′ strains remain in color and did not shrink at same time (Fig. 6B). Typically, infected G. mellonella larvae melanized by forming melanotic capsules surrounding pathogens23,24, and the observed darkening of the infected larvae may be due to the formation of these melanotic capsules. To better understand the fate of A. fumigatus inoculated into G. mellonella, infected larvae were fixed in neutral buffered formalin at 24 hours post infection and sectioned for histopathology. Figure 6C showed Periodic acid-Schiff (PAS) and Grocott methenamine-silver (GMS)-stained sections of uninfected (PBS) and infected larvae. The more fungal cells were observed in ΔrgsD conidia-infected larvae than those of WT and C′ conidia-infected larvae. No fungal cells were detected in the PBS-injected control larvae.
The lack of RgsD leads to enhanced virulence. (A) Log-Rank plots of the survival of G. mellonella after infection with three strains’ conidia. (B) Infection of G. mellonella with the ΔrgsD strain induced melanization of the larva. (C) Histological analysis of larval tissues infected by WT, mutant and C′ strains was performed using PAS and GMS staining 24 hours post inoculation. Arrows indicated fungal hyphae in infected larval tissues. Note that compared to the WT and C′ strain, ΔrgsD strain showed high virulence.
Transcriptome analysis
To investigate the roles of RgsD in A. fumigatus biology, we carried out RNA-sequencing analysis using the ΔrgsD and WT cells collected at 12 hours post asexual-developmental induction as described in the Methods section. Two biological replicates showed a high level of correlation (R = 0.93, Fig. 7A). 9,825 genes were differentially expressed and 7,503 genes expressed with slight fold change (−1 < log2FC < 1) (Fig. 7B). A total of 385 genes showed more than two-fold differential expression (p < 0.05), of which 168 genes exhibited higher transcript levels in ΔrgsD strain than in WT strain and 217 genes were down-regulated. Functional category analysis was carried out by determining Gene Ontology (GO) terms that were enriched in differentially expressed genes (DEGs). The top significant molecular function GO categories are “signal transducer activity”, “transporter activity”, “oxidoreductase activity”, “cofactor binding activity”, and “catalytic activity”. The top significant cellular component GO categories are “fungal-type cell wall”, “cell periphery”, “intrinsic and integral component of membrane”, and “extracellular region”. The top significant biological process GO categories are “secondary metabolic process”, “developmental process”, and “pathogenesis”. The most enriched molecular function, cellular component, and biological process GO categories are “catalytic activity”, “intrinsic component of membrane”, and “metabolic process”, respectively (Fig. 7C). The top 20 DEGs with increased mRNA accumulation levels in ΔrgsD strain compared to WT are listed in Table 1. Notably, mRNA levels of the key regulator of asexual sporulation brlA and the melanin synthesis-related gene abr1 were above 8-fold higher in the ΔrgsD mutant than WT, consistent with our phenotypic data. Numerous genes involved in signal transduction, asexual development, cAMP-dependent PKA signaling pathway, and pigment biosynthesis were also up-regulated in the absence of rgsD (Supplementary Fig. S3). Most of the down-regulated genes were those encoding hypothetical proteins (Table 2), including the MgtC/SapB family membrane protein which are homologous to a bacterial virulence factor but their function was still not known25.
Genome-wide expression analyses of the ΔrgsD mutant and WT. (A) Linear fitted model showing the correlation between overall gene expression for WT and ΔgprK strains. The correlation coefficient R is indicated. (B) Histograms showing general transcriptomic results and columns in whites fall in the −1 < log2FC < 1 fragment count range with low differential expression values. (C) Functional categories of DEGs. The orange bars represent genes whose mRNA levels increased in the ΔrgsD strain, whereas the green bars represent those genes whose mRNA levels decreased in the ΔrgsD strain.
Discussion
RGS proteins are GTPase accelerating proteins (GAPs) that act as negative regulators of multifunctional signaling in many fungi10,11,12,14,26,27. They function by enhancing the intrinsic GTPase activity leading to accelerated hydrolysis of GTP-Gα subunits of heterotrimeric G protein and inactivation of the trimers28,29,30. Since RGS proteins are pathologically and physiologically important regulators of the signaling pathways, elucidating the regulatory mechanisms of RGS proteins will provide an expanded basis for understanding G protein signaling and for controlling human pathogenic fungi.
Among six potential RGS proteins identified in A. fumigatus, previous studies revealed that the RGS proteins FlbA, GprK, Rax1 (RgsB), and RgsC play redundant roles in regulating asexual sporulation, stress response, and virulence10,11,12,14. FlbA (Afu2g11180) regulates GpaA signaling and contributes to proper progression of asexual sporulation10. RgsB (Afu4g12640), the orthologue of Saccharomyces cerevisiae Rax1, also controls growth and asexual development of A. fumigatus. Vegetative growth and conidia number is highly reduced in the absence of rgsB. Moreover, it also modulates the content of trehalose and melanin in conidia, providing resistance against external oxidative stress12. Another RGS protein RgsC (Afu1g09040) is needed for proper growth, conidiation, stress response, and GT production. The deletion of rgsC results in impaired growth and asexual sporulation14. The GPCR-RGS hybrid protein GprK (Afu4g01350) down-regulates the PKA-mediated germination pathway and is responsible for normal asexual development. The ΔgprK mutant shows severely impaired asexual sporulation and reduced tolerance to oxidative stresses11.
Despite having a very simple domain structure (Supplementary Fig. S1A), RgsD may play an opposite role in regulating signaling compared to previously reported RGSs. The deletion of rgsD causes high production of conidia (about 4 fold) coupled with increased expression of key developmental regulators, but reduced the expression of nsdD and veA (Fig. 1). NsdD is known as a major negative regulator of brlA expression and the ΔnsdD mutant produced more conidia than WT in A. fumigatus31. VeA also acts as a negative regulator in conidiation. The absence of veA results in highly increased production of conidiophores and accumulation of brlA mRNA32. These findings indicate that RgsD may function as a negative regulator of conidiation and some developmental regulatory genes.
With the split-ubiquitin system, it appears that RgsD can interact the three Gα proteins in A. fumigatus. However, the data clearly indicate that RgsD might preferentially interact with GpaB, then GanA and GpaA (Fig. 2). Previous studies showed that GpaB stimulates cAMP-PKA signaling15. We demonstrated that the rgsD mutant showed the higher mRNA levels of PKA signaling components and PKA activity, which implicates that RgsD serves as an important negative regulator of cAMP-PKA signaling (Fig. 3). In A. fumigatus, the cAMP-PKA signaling cascade composed G protein α subunit GpaB, adenylate cyclase AcyA, and protein kinase A, regulates growth, development, and secondary metabolites synthesis. Deletion of PKA catalytic subunit pkaC1 showed reduced conidiation, growth, and pigment formation15. PKA activity was not detected in ΔgpaB and ΔacyA strains and conidia of both strains were almost avirulent33. Moreover, elevated PKA activity led to high expression of polyketide synthase gene pkaP which is essential for the production of melanin and pathogenicity15. As expected, the ΔrgsD mutant was more resistant to UV irradiation and oxidative stress, and mRNA of arp1 and arp2 which encode scytalone dehydratase and hydroxynaphthalene reductase, respectively, accumulated more rapidly and at higher levels in the ΔrgsD mutant. Furthermore, the content of melanin in the ΔrgsD conidia was higher and the mutant conidia formed thicker electron dense melanized layer than those of WT and C′ strain (Fig. 4), indicating that RgsD downregulates cAMP-PKA signaling pathway and plays an important negative role in melanin biosynthesis that may modulate fungal response to external stresses.
Synthesis and regulation of GT is done by the gli cluster composed of 13 genes in A. fumigatus34,35. We checked the GT content and mRNA levels of four genes, gliM (o-methyltransferase), gliP (dioxopiperazine synthase), gliT (GT oxidoreductase), and gliZ (zinc finger transcriptional regulator) in three strains. As shown in Fig. 5, both GT production and GT biosynthetic genes mRNA levels increased in the absence of rgsD, suggesting that RgsD serves as a negative regulator of GT synthesis.
It is well-known that both melanin and GT are important virulence determinants of A. fumigatus. DHN-melanin specifically interferes the functions of host phagocytes, leading to survival of the fungus within host cell36. GT inhibits the oxidative burst of neutrophil and increases the ability of the fungus to survive against neutrophil’s attack37. As ΔrgsD strain produces higher amount of melanin and GT, it was conceivable that the mutant strain would be more virulent than WT and C′ strain. To confirm this, we performed virulence studies with insect wax moth larvae. The ΔrgsD conidia were highly virulent to the insect and killed all the larvae within 6 days post inoculation. We readily observed that larvae infected with the mutant conidia darkened at 24 hours inoculation and it seemed to be a positive correlation between the degree of larvae darkening and the level of pathogenicity of the strain. Furthermore, higher density of fungal cell was found in the ΔrgsD mutant infected larvae gut (Fig. 6). From these results we conclude that RgsD modulates (attenuates) virulence likely by downregulating biosynthesis of melanin, GT, and potentially other virulence factors in A. fumigatus.
Comparative transcriptomics of WT and the ΔrgsD mutant was used for the investigation of RgsD regulated target genes and signaling pathways. Notably, the C2H2 type transcription factor BrlA and the conidial pigment biosynthesis oxidase Abr1 were significantly induced in mutant strain (Table 1). BrlA regulates diverse biological processes in A. fumigatus including asexual sporulation and GT production22. Abr1 encodes a multicopper oxidase that plays an important role in the biosynthesis of DHN-melanin20. Biosynthesis of DHN-melanin in A. fumigatus begins with the conversion of acetyl-CoA and malonyl-CoA to 1,3,6,8-tetrahydroxynaphthalene (1,3,6,8-THN) by PksP (polyketide synthase) and Ayg1 (heptaketide hydrolyase). 1,3,6,8-THN is reduced to scytalone by the hydroxynaphthalene (HN) reductase Arp2, followed by dehydration of scytalone to 1,3,8-trihydroxynaphthalene (1,3,8-THN) by Arp1 (scytalone dehydratase). Then, Arp1 and Arp2 catalyze the reduction and dehydration of 1,3,8-THN to 1,8-dihydroxynaphthalene (1,8-DHN). Oxidative polymerization of 1,8-DHN to form DHN-melanin catalyzed by multicopper oxidase Abr1 and laccase Abr220,38,39. The results of the RNA-seq analysis demonstrate the diversity in cellular processes especially, asexual sporulation and DHN-melanin synthesis regulated by RgsD in A. fumigatus. Collectively, we propose a genetic model depicting the regulatory roles of RgsD in A. fumigatus (Fig. 8). In the absence of RgsD, the cAMP-PKA signal transduction pathway may be consistently activated, which might lead to increased melanin and secondary metabolite production, tolerance to environmental stresses, and virulence.
A model depicting the roles of RgsD. The putative RGS domain protein RgsD negatively regulates the cAMP-PKA signaling pathway, which may be activated by the Gα subunit GpaB. Consequence of the lack of RgsD leads to enhanced activation of the cAMP-PKA signaling pathway, which in turn leads to enhanced production of GT and melanin, and hyper-virulence of the fungus. The cAMP-PKA pathway may remove the repressive effects of VosA/VeA/NsdD on brlA and/or may directly activate brlA expression.
Methods
Strains and culture conditions
Glucose minimal medium (MMG) and MMG with 0.1% yeast extract (MMY) with appropriate supplements were used for general culture of A. fumigatus strains40. For liquid submerged culture and phenotypic analyses on air-exposed culture were performed as described previously11. To examine secondary metabolite production, spores of relevant strains were inoculated 50 ml of liquid MMY and incubated at 250 rpm at 37 °C for 4 days.
Generation of the rgsD deletion mutant
The oligonucleotides used in this study are listed in Supplementary Table S1. The rgsD gene was deleted in A. fumigatus AF293.1 (pyrG1) strain41. The deletion construct generated employing double-joint PCR (DJ-PCR)42 containing the A. nidulans selective marker (AnpyrG) with the 5′ and 3′ flanking regions of the rgsD gene was introduced into the recipient strain AF293.143. The selective marker was amplified from A. nidulans FGSC4 genomic DNA with the primer pair oligo 109/oligo 110. The ΔrgsD mutant was isolated and confirmed by PCR, followed by restriction enzyme digestion (Supplementary Fig. S4)42. To complement rgsD null mutant, a single joint PCR (SJ-PCR) method was used42. The ORF of rgsD gene with a promoter and terminator was amplified with primer pairs where the 3′ reverse primer carries overlapping sequences with the hygB gene’s 5′ end. Amplification of the hygB gene was carried out with primer pairs where the 5′ forward primer carries overlapping sequences with hygB gene’s 3′ end. The final amplicon was amplified with the nested primer pair oligo 562/oligo 563 and introduced into a ΔrgsD strain.
Nucleic acid isolation and manipulation
Total RNA isolation and quantitative RT-PCR (qRT-PCR) assays were performed as previously described10,22,44. Expression of target genes mRNA was analyzed with appropriate oligonucleotide pairs (Table S1). For RNA-seq analyses, 12 hours-old culture of WT and mutant strains were harvested from solid MMY. Total RNA was extracted and submitted to eBiogen Inc. (Seoul, Korea) for library preparation and sequencing.
Phenotypic analyses
To examine germination levels, conidia of WT and mutant were inoculated in 5 ml of liquid MMY and incubated at 37 °C. Levels of germination were examined every 2 hours after inoculation under a microscope. Conidia were collected in 0.5% Tween 80 from the entire colony and filtered through Miracloth (Calbiochem, CA), and counted using a hemacytometer. Hydrogen peroxide sensitivity of conidia was examined by incubating 1 ml of spore suspensions containing 105 conidia with varying concentrations (0, 1.25 or 2.50 mM) of H2O2 and incubated for 30 min at room temperature. Each spore suspension was diluted with sterilized H2O, and conidia were inoculated into solid MMY. After incubation at 37 °C for 48 hours, colony numbers were counted and calculated as a survival ratio of the untreated control. UV tolerance test was carried out as follows. Fresh conidia were plated out on solid MMY plates (100 conidia per plate). The plates were irradiated immediately with UV using a UV cross-linker at indicated doses and incubated at 37 °C for 48 hours. The colony numbers were counted and calculated as a survival ratio of the untreated control. The production of gliotoxin (GT) was determined as described previously45. The TLC silica plate (Kiesel gel 60, E. Merck) was developed with toluene:ethyl acetate:formic acid (5:4:1, v/v/v). Melanin content was determined from conidia as described46. Briefly, the conidia were harvested and washed with H2O, then incubated in 1.0 M NaOH at 98 °C and pelleted for 10 min at 17,000 g. Melanin in the supernatants was precipitated for 30 min at pH 1.0 by addition of 12 N HCl, after which it was pelleted at 17,000 g. After washing with H2O, samples dissolved in 0.05 M NaOH and the pH of the melanin solution was set at 7.5 using 1.0 M HCl. Absorbance at 495 nm was used to quantify melanin in biological duplicates.
Protein-protein interaction assay
Yeast two-hybrid interaction assays were performed using DUALhunter Kit (Dualsystem Biotech, Switzerland) according to the manufacturer’s instruction. Briefly, full-length RgsD cDNA was cloned into yeast expression vector pDHB1 and cDNAs of GpaA, GpaB, and GanA were cloned into the pPR3-N vector. All cDNA sequences were confirmed by DNA sequencing. Cub and NubG fusion constructs were co-introduced into host yeast strain NMY51. pAI-Alg5 contains a wild type Nub serves as a positive control and pDL2-Alg5 serves as a negative control. Interaction was determined by the growth of yeast transformants on medium without histidine or adenine, and by measuring the β-galactosidase activity using Yeast ß-Galactosidase Assay Kit (Thermo Scientific, USA).
PKA assay
For determination of PKA activity, Pep Tag Non-Radioactive cAMP-Dependent Protein Kinase Assay kit (Promega, USA) was applied according to the manufacturer’s instructions. Homogenized mycelia of each strain were suspended in extraction buffer33 and incubated on ice for 15 min. After centrifugation, 10 µl of supernatant adjusted to a protein concentration of 3 mg/ml was used in an assay for PKA activity.
TEM
Conidia were fixed in 2.5% glutaraldehyde in 0.1 M phosphate, washed three times with 0.1 M phosphate, post-fixed in 1% osmium tetroxide, incubated for 1 hour in 0.1 M phosphate and dehydrated for 15 min in a graded methanol series from 50% to 100%. Samples were embedded in Epon resin 812. The sections were examined with a Tecnai G2 Spirit Twin Bio-Transmission Electron Microscope (FEI, Hillsboro, USA), with an accelerating voltage of 120 KV.
Insect infection model
The insect survival assay was performed as previously described with some modifications47. Briefly, sixth instar Galleria mellonella were infected by injecting the fresh conidia (1 × 105) into the last left pro-leg and incubated at 37 °C in the dark for the duration of the experiment. Larvae were checked daily for survival and Kaplan-Meier survival curves were analyzed using the Log-Rank (Mantel-Cox) test for significance (p < 0.0001).
RNA-seq experiment
For control and test RNAs, the construction of library was performed using SENSE mRNA-Seq Library Prep Kit (Lexogen, Inc., Austria) according to the manufacturer’s instructions. Briefly, each 2 µg total RNA are prepared and incubated with magnetic beads decorated with oligo-dT and then other RNAs except mRNA was removed. Library production is initiated by the random hybridization of starter/stopper heterodimers to the poly(A) RNA still bound to the magnetic beads. These starter/stopper heterodimers contain Illumina-compatible linker sequences. A single-tube reverse transcription and ligation reaction extends the starter to the next hybridized heterodimer, where the newly-synthesized cDNA insert is ligated to the stopper. Second strand synthesis is performed to release the library from the beads, and the library is then amplified. Barcodes were introduced when the library is amplified. High-throughput sequencing was performed as paired-end 100 sequencing using HiSeq. 2000 (Illumina, Inc., USA). RNA-Seq reads were mapped using TopHat software tool in order to obtain the alignment file. The alignment file was used for assembling transcripts, estimating their abundances and detecting differential expression of genes or isoforms using cufflinks. Gene classification was based on searches done by BioCarta (http://www.biocarta.com/), GenMAPP (http://www.genmapp.org/), DAVID (http://david.abcc.ncifcrf.gov/), and Medline databases (http://www.ncbi.nlm.nih.gov/).
Data Availability
The RNA-seq data are available from NCBI Gene Expression Omnibus (GEO) database (the accession number is GSE100101).
References
Morris, A. J. & Malbon, C. C. Physiological regulation of G protein-linked signaling. Physiol Rev 79, 1373–1430 (1999).
Feldbrugge, M., Kamper, J., Steinberg, G. & Kahmann, R. Regulation of mating and pathogenic development in Ustilago maydis. Curr Opin Microbiol 7, 666–672, https://doi.org/10.1016/j.mib.2004.10.006 (2004).
McCudden, C. R., Hains, M. D., Kimple, R. J., Siderovski, D. P. & Willard, F. S. G-protein signaling: back to the future. Cell Mol Life Sci 62, 551–577, https://doi.org/10.1007/s00018-004-4462-3 (2005).
Yu, J. H. Heterotrimeric G protein signaling and RGSs in Aspergillus nidulans. J Microbiol 44, 145–154 (2006).
Dohlman, H. G., Song, J., Ma, D., Courchesne, W. E. & Thorner, J. Sst2, a negative regulator of pheromone signaling in the yeast Saccharomyces cerevisiae: expression, localization, and genetic interaction and physical association with Gpa1 (the G-protein a subunit). Mol Cell Biol 16, 5194–5209 (1996).
Apanovitch, D. M., Slep, K. C., Sigler, P. B. & Dohlman, H. G. Sst2 is a GTPase-activating protein for Gpa1: purification and characterization of a cognate RGS-Galpha protein pair in yeast. Biochemistry 37, 4815–4822, https://doi.org/10.1021/bi9729965 (1998).
Versele, M., de Winde, J. H. & Thevelein, J. M. A novel regulator of G protein signalling in yeast, Rgs2, downregulates glucose-activation of the cAMP pathway through direct inhibition of Gpa2. EMBO J 18, 5577–5591, https://doi.org/10.1093/emboj/18.20.5577 (1999).
Li, L., Wright, S. J., Krystofova, S., Park, G. & Borkovich, K. A. Heterotrimeric G protein signaling in filamentous fungi. Annu Rev Microbiol 61, 423–452, https://doi.org/10.1146/annurev.micro.61.080706.093432 (2007).
Bayram, O. & Braus, G. H. Coordination of secondary metabolism and development in fungi: the velvet family of regulatory proteins. FEMS Microbiol Rev 36, 1–24, https://doi.org/10.1111/j.1574-6976.2011.00285.x (2012).
Mah, J. H. & Yu, J. H. Upstream and downstream regulation of asexual development in Aspergillus fumigatus. Eukaryot Cell 5, 1585–1595, https://doi.org/10.1128/EC.00192-06 (2006).
Jung, M. G., Kim, S. S., Yu, J. H. & Shin, K. S. Characterization of gprK Encoding a Putative Hybrid G-Protein-Coupled Receptor in Aspergillus fumigatus. PLoS One 11, e0161312, https://doi.org/10.1371/journal.pone.0161312 (2016).
Igbalajobi, O. A., Yu, J. H. & Shin, K. S. Characterization of therax1 gene encoding a putative regulator of G protein signaling in Aspergillus fumigatus. Biochem Biophys Res Commun 487, 426–432, https://doi.org/10.1016/j.bbrc.2017.04.079 (2017).
Han, K. H., Seo, J. A. & Yu, J. H. Regulators of G-protein signalling in Aspergillus nidulans: RgsA downregulates stress response and stimulates asexual sporulation through attenuation of GanB (Gα) signalling. Mol Microbiol 53, 529–540, https://doi.org/10.1111/j.1365-2958.2004.04163.x (2004).
Kim, Y., Heo, I. B., Yu, J. H. & Shin, K. S. Characteristics of a Regulator of G-Protein Signaling (RGS) rgsC. In Aspergillus fumigatus. Front Microbiol 8, 2058, https://doi.org/10.3389/fmicb.2017.02058 (2017).
Grosse, C., Heinekamp, T., Kniemeyer, O., Gehrke, A. & Brakhage, A. A. Protein kinase A regulates growth, sporulation, and pigment formation in Aspergillus fumigatus. Appl Environ Microbiol 74, 4923–4933, https://doi.org/10.1128/AEM.00470-08 (2008).
Liebmann, B., Gattung, S., Jahn, B. & Brakhage, A. A. cAMP signaling in Aspergillus fumigatus is involved in the regulation of the virulence gene pksP and in defense against killing by macrophages. Mol Genet Genomics 269, 420–435, https://doi.org/10.1007/s00438-003-0852-0 (2003).
Jahn, B., Langfelder, K., Schneider, U., Schindel, C. & Brakhage, A. A. PKSP-dependent reduction of phagolysosome fusion and intracellular kill of Aspergillus fumigatus conidia by human monocyte-derived macrophages. Cell Microbiol 4, 793–803 (2002).
Fillinger, S., Chaveroche, M. K., Shimizu, K., Keller, N. & d’Enfert, C. cAMP and ras signalling independently control spore germination in the filamentous fungus Aspergillus nidulans. Mol Microbiol 44, 1001–1016 (2002).
Lafon, A., Seo, J. A., Han, K. H., Yu, J. H. & d’Enfert, C. The heterotrimeric G-protein GanB(α)-SfaD(ß)-GpgA(ɣ) is a carbon source sensor involved in early cAMP-dependent germination in Aspergillus nidulans. Genetics 171, 71–80, https://doi.org/10.1534/genetics.105.040584 (2005).
Tsai, H. F., Wheeler, M. H., Chang, Y. C. & Kwon-Chung, K. J. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J Bacteriol 181, 6469–6477 (1999).
Bernard, M. & Latge, J. P. Aspergillus fumigatus cell wall: composition and biosynthesis. Med Mycol 39(Suppl 1), 9–17 (2001).
Shin, K. S., Kim, Y. H. & Yu, J. H. Proteomic analyses reveal the key roles of BrlA and AbaA in biogenesis of gliotoxin in Aspergillus fumigatus. Biochem Biophys Res Commun 463, 428–433, https://doi.org/10.1016/j.bbrc.2015.05.090 (2015).
Kavanagh, K. & Reeves, E. P. Exploiting the potential of insects for in vivo pathogenicity testing of microbial pathogens. FEMS Microbiol Rev 28, 101–112, https://doi.org/10.1016/j.femsre.2003.09.002 (2004).
Ratcliffe, N. A. Invertebrate immunity–a primer for the non-specialist. Immunol Lett 10, 253–270 (1985).
Gastebois, A. et al. Phylogenetic and functional analysis of Aspergillus fumigatus MGTC, a fungal protein homologous to a bacterial virulence factor. Appl Environ Microbiol 77, 4700–4703, https://doi.org/10.1128/AEM.00243-11 (2011).
Lengeler, K. B. et al. Signal transduction cascades regulating fungal development and virulence. Microbiol Mol Biol Rev 64, 746–785 (2000).
Zhang, P. et al. Regulator of G protein signaling 2 is a functionally important negative regulator of angiotensin II-induced cardiac fibroblast responses. Am J Physiol Heart Circ Physiol 301, H147–156, https://doi.org/10.1152/ajpheart.00026.2011 (2011).
De Vries, L. et al. Activator of G protein signaling 3 is a guanine dissociation inhibitor for Gai subunits. Proc Natl Acad Sci USA 97, 14364–14369, https://doi.org/10.1073/pnas.97.26.14364 (2000).
Dohlman, H. G. & Thorner, J. W. Regulation of G protein-initiated signal transduction in yeast: paradigms and principles. Annu Rev Biochem 70, 703–754, https://doi.org/10.1146/annurev.biochem.70.1.703 (2001).
Siderovski, D. P. & Willard, F. S. The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int J Biol Sci 1, 51–66 (2005).
Lee, M. K. et al. Negative regulation and developmental competence in Aspergillus. Sci Rep 6, 28874, https://doi.org/10.1038/srep28874 (2016).
Park, H. S., Bayram, O., Braus, G. H., Kim, S. C. & Yu, J. H. Characterization of the velvet regulators. In Aspergillus fumigatus. Mol Microbiol 86, 937–953, https://doi.org/10.1111/mmi.12032 (2012).
Liebmann, B., Muller, M., Braun, A. & Brakhage, A. A. The cyclic AMP-dependent protein kinase a network regulates development and virulence in Aspergillus fumigatus. Infect Immun 72, 5193–5203, https://doi.org/10.1128/IAI.72.9.5193-5203.2004 (2004).
Gardiner, D. M., Waring, P. & Howlett, B. J. The epipolythiodioxopiperazine (ETP) class of fungal toxins: distribution, mode of action, functions and biosynthesis. Microbiology 151, 1021–1032, https://doi.org/10.1099/mic.0.27847-0 (2005).
Schrettl, M. et al. Self-protection against gliotoxin–a component of the gliotoxin biosynthetic cluster, GliT, completely protects Aspergillus fumigatus against exogenous gliotoxin. PLoS Pathog 6, e1000952, https://doi.org/10.1371/journal.ppat.1000952 (2010).
Heinekamp, T. et al. Aspergillus fumigatus melanins: interference with the host endocytosis pathway and impact on virulence. Front Microbiol 3, 440, https://doi.org/10.3389/fmicb.2012.00440 (2012).
Sugui, J. A. et al. Gliotoxin is a virulence factor of Aspergillus fumigatus: gliP deletion attenuates virulence in mice immunosuppressed with hydrocortisone. Eukaryot Cell 6, 1562–1569, https://doi.org/10.1128/EC.00141-07 (2007).
Sugareva, V. et al. Characterisation of the laccase-encoding geneabr2 of the dihydroxynaphthalene-like melanin gene cluster of Aspergillus fumigatus. Arch Microbiol 186, 345–355, https://doi.org/10.1007/s00203-006-0144-2 (2006).
Fujii, I. et al. Hydrolytic polyketide shortening by ayg1p, a novel enzyme involved in fungal melanin biosynthesis. J Biol Chem 279, 44613–44620, https://doi.org/10.1074/jbc.M406758200 (2004).
Kafer, E. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv Genet 19, 33–131 (1977).
Xue, T., Nguyen, C. K., Romans, A., Kontoyiannis, D. P. & May, G. S. Isogenic auxotrophic mutant strains in the Aspergillus fumigatus genome reference strain AF293. Arch Microbiol 182, 346–353, https://doi.org/10.1007/s00203-004-0707-z (2004).
Yu, J. H. et al. Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet Biol 41, 973–981, https://doi.org/10.1016/j.fgb.2004.08.001 (2004).
Szewczyk, E. et al. Fusion PCR and gene targeting in Aspergillus nidulans. Nat Protoc 1, 3111–3120, https://doi.org/10.1038/nprot.2006.405 (2006).
Han, K. H., Seo, J. A. & Yu, J. H. A putative G protein-coupled receptor negatively controls sexual development in Aspergillus nidulans. Mol Microbiol 51, 1333–1345, https://doi.org/10.1111/j.1365-2958.2003.03940.x (2004).
Bok, J. W. & Keller, N. P. LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot Cell 3, 527–535 (2004).
Wargenau, A. et al. On the origin of the electrostatic surface potential of Aspergillus niger spores in acidic environments. Res Microbiol 162, 1011–1017, https://doi.org/10.1016/j.resmic.2011.07.006 (2011).
Fuchs, B. B., O’Brien, E., Khoury, J. B. & Mylonakis, E. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence 1, 475–482 (2010).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2017R1A2B4001806) to K.S. Shin. The work at UW was supported by the Intelligent Synthetic Biology Center of Global Frontier Project funded by the Ministry of Education, Science and Technology (No. 2011-0031955) grants to J.H. Yu. We thank all of our lab members for helpful discussion.
Author information
Authors and Affiliations
Contributions
Y.K., M.W.L., S.C.J., Y.H.C. and K.S.S. performed the all experiments. K.S.S. and J.H.Y. designed the experiments and analyzed data. Y.K., M.W.L., J.H.Y. and K.S.S. wrote the manuscript and prepared all data. All authors reviewed and revised the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, Y., Lee, MW., Jun, SC. et al. RgsD negatively controls development, toxigenesis, stress response, and virulence in Aspergillus fumigatus. Sci Rep 9, 811 (2019). https://doi.org/10.1038/s41598-018-37124-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-37124-2
- Springer Nature Limited
This article is cited by
-
RGS4 impacts carbohydrate and siderophore metabolism in Trichoderma reesei
BMC Genomics (2023)
-
In-depth comparative transcriptome analysis of Purpureocillium sp. CB1 under cadmium stress
Applied Microbiology and Biotechnology (2023)