Abstract
Comparative syntheses of key demographic parameters are critical not only for identifying data gaps, but also for evaluating sources of heterogeneity among estimates. Because demographic studies frequently exhibit heterogeneity, evaluating sources of heterogeneity among estimates can inform biological patterns and conservation actions more broadly. To better understand adult survival in marine turtles and avoid drawing inaccurate conclusions from current estimates, we conducted a comprehensive meta-analysis to test how heterogeneity among estimates was partitioned among phylogenetic, biogeographic and methodological factors. Fifty-nine studies from five marine turtle species met the minimum selection criteria for inclusion in our meta-analysis. Heterogeneity among survival estimates was first partitioned between differences in ocean basin (Indo-Pacific versus Atlantic), then by differences among family/tribe within the Indo-Pacific (Chelonini versus Carettini and Dermochelidae). However, apparent differences attributed to biogeography (ocean basin effect) and phylogeny (family/tribe effect) were highly correlated with methodological differences in tag type, model type, habitat type and study duration, thereby confounding biological interpretations and complicating efforts to use many current survival estimates in population assessments. Our results highlight the importance of evaluating sources of heterogeneity when interpreting patterns among similar demographic studies and directly inform efforts to identify research priorities for marine turtles globally.
Similar content being viewed by others
Introduction
Demographic studies in ecology and conservation biology form the basis for assessing population viability and managing ecological risk1. However, estimates of key demographic parameters, such as survival and recruitment, viewed in isolation often provide limited and/or potentially biased inferences. In this regard, comparative syntheses of similar demographic studies are critical not only for identifying data gaps, but also for evaluating sources of heterogeneity among estimates. Because demographic studies frequently exhibit heterogeneity due to system-specific nature of biological phenomena2 and study-specific differences in methodology, identifying important sources of heterogeneity can inform biological patterns and conservation actions more broadly2,3,4. Conversely, comparative studies that fail to evaluate sources of heterogeneity risk drawing inaccurate conclusions and misleading management decisions.
We conducted a systematic review and comprehensive meta-analysis5,6 of annual survival rates for adult marine turtles to generate precision-weighted, species-specific estimates and prediction intervals from existing data7 and to explicitly model sources of heterogeneity among estimates2. In theory, because all marine turtles exhibit conserved life history patterns, including slow growth and delayed sexual maturity8, adult survival rates should be high (>0.90) and exhibit limited natural heterogeneity among estimates. Our goal was to quantify heterogeneity among survival estimates and test how heterogeneity is influenced by (a) phylogeny, (b) biogeography and (c) methodology. If heterogeneity is influenced by phylogeny or biogeography, then biological differences in survival among species/populations may exist. In this case, survival estimates that are lower than expected would highlight important species- or region-specific hotspots in adult mortality – areas of conservation concern. However, if heterogeneity is also strongly influenced by methodological differences, then statistical biases related to certain methodologies introduce artificial variation and mask important biological differences. In this case, the use of survival estimates that are lower than expected may mislead management decisions if methodological biases are not accounted for. We expect the findings of this synthesis to highlight the importance of evaluating sources of heterogeneity when interpreting demographic estimates2 and directly inform efforts to identify research priorities for marine turtles globally9,10,11,12.
Methods
Literature review and selection criteria
We followed the PRISMA protocols for assembling a dataset suitable for meta-analytic evaluation5,6. Specifically, we conducted a two-tiered literature search to compile annual survival probability estimates for adult marine turtles. A structured search was conducted in Google Scholar, Sea Turtle Document Library (seaturtle.org) and Sea Turtle Online Bibliography (Archie Carr Center for Sea Turtle Research, University of Florida) using the following Boolean search terms: survival, survivorship, mortality, and the names of the seven marine turtle genera. Then, an unstructured literature search was conducted by reviewing the reference lists of all the relevant publications and reports from the structured search. References compiled from the structured and unstructured search were reviewed and only those that estimated annual survival probabilities for adult turtles were retained (i.e., multiyear or stage-based estimates and estimates for juveniles were excluded). Seventy-eight survival estimates were found in the global literature review with 59 meeting the minimum selection criteria for inclusion in the meta-analysis (Supplementary Table S1). Marine turtle taxonomy/phylogeny follows Duchene et al.13. Dermochelidae (leatherbacks) is sister to Chelonidae, which includes Chelonini (flatback and green turtles) and Carettini (hawksbill, loggerhead and ridley turtles). The measure of effect size and precision of each estimate was the study-specific inverse-precision weighted annual survival probability and the associated standard error, respectively.
Informative covariates
The following potentially informative covariates (or moderators) were compiled for all survival estimates: specific publication ID, research group (based on common authors), type of publication (journal or report), publication year, taxonomic group (species, tribe and family), study site, geographic region with subgrouping for two oceans (Indo-Pacific and Atlantic), study duration (years), habitat type (nesting or foraging), tag type and statistical estimation type with subgrouping for modeling procedures versus enumeration calculations. Each estimate was categorized by whether tag loss, imperfect detection and temporary emigration were explicitly accounted for or not. The population of interest in each study was also characterized in terms of population size, population trend, direct harvest history and fisheries bycatch impact using primarily Wallace et al.14,15. Details on covariate characterization can be found in the Supplementary Methods. Study duration and publication year were also used to account for various forms of publication bias16,17.
Statistical modeling approach
We used a precision-weighted random-effects model approach to summarize the 59 survival estimates into species-specific subgroups before accounting for informative covariates17,18,19. In this 2-level analysis, the random effect was the specific study. Each study-specific survival estimate was a response variable soft-bound between 0–1. Response variables were therefore logit-transformed prior to fitting the random-effects models to ensure the predicted estimates were also between 0–120 and then back-transformed for any predicted summaries. Species-specific random-effects models were fitted using the metafor package for R21 and displayed in a subgroup forest plot that was augmented with the species-specific random effects estimates and the prediction intervals for those estimates7.
Because many of the potentially informative covariates in our meta-analysis were highly correlated, we could not isolate individual effects or explicitly model interactions among covariates simultaneously. Therefore, we first used a recursive partitioning or conditional inference regression tree approach22,23 to explore underlying patterns among the potential effects of 12 correlated covariates on the 59 survival estimates. The conditional inference tree model was fitted and then displayed using the partykit package for R24 with a minimum split criterion of 0.95 (so P-value < 0.05). Because the conditional inference tree approach does not account for the precision of survival estimates, we also tested a series of meta-regression models using metafor to explore other potentially informative covariates when estimate precision was explicitly considered. Models with different predictors were compared based on Akaike Information Criterion (AICc).
We then used a mixed-effects meta-regression approach19,25 to model annual survival rates conditioned on the main interaction effect – ‘ocean by family/tribe’ – identified within the conditional inference tree and four additional moderators identified by exploratory meta-regression modeling – tag type, model type, habitat type and study duration. We also included a 3-level hierarchical structure26,27 to test for potential non-independence between studies conducted by the same research group (based on common authors), an important assumption to account for in meta-analytic studies17,28. In this 3-level hierarchical analysis, the random effects were study within research group. The mixed effects meta-regression models were fitted using a multivariate parameterization to accommodate assessment of random effect structures29. The model-predicted study-specific inverse-precision weighted annual survival estimates and covariate effects were summarized as covariate-specific boxplots. Continuous variables, like study duration, were modeled using B-splines (splines package)30 to account for potential nonlinear functional forms29 and predicted estimates were summarized using a cubic regression spline GAM-based smoother31 implemented within the ggplots2 package for R32.
The I2 statistic2,33 was used to assess the level of unexplained heterogeneity estimated in the 3-level hierarchical meta-regression model fit to the 59 studies, and a simple R2 measure appropriate for the multivariate parameterization of a meta-regression model was used as a metric of the overall model fit. The Cochrane QE test was used as a formal test of residual heterogeneity34, while an omnibus F-test was used to test for significance of the set of all covariates included in the meta-regression model35. Lastly, we explored potential publication bias16,17 using a contour-enhanced funnel plot36 of the model predicted study-specific survival estimates using metafor.
Data availability
The dataset supporting this article has been uploaded as part of the electronic supplementary material.
Results
Study- and species-specific survival estimates
The 59 species-specific survival rate estimates derived from the random-effects model are summarized in Fig. 1. Here the random-effect was the specific study. It is apparent that there was considerable species-specific heterogeneity in adult survival rate estimates. This was especially apparent for green and leatherback turtles that might be attributable to geographic and methodological factors. This apparent heterogeneity was explored further using hierarchical or multilevel meta-regression modeling to account for potentially informative phylogenetic4, geographic and methodological covariates. We have also included the species-specific prediction intervals in the subgroup forest plot, which shows for instance that a new flatback adult survival rate study would be expected to provide an annual estimate ca. 0.93 (95% prediction interval: 0.77–0.98). On the other hand, for leatherbacks it would be 0.85 (95% prediction interval: 0.45–0.97).
Random-effects forest plot of the inverse-variance weighted annual survival estimates for the 59 marine turtle studies (letters after reference year indicate different estimates from the same reference)37,40,41,–42,51,52,53,54,55,55,56,57,58,59,60,61,52,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,–88. The species-specific pooled or random-effect survival rate estimates (RE diamonds) are shown in addition to the prediction intervals (horizontal bar through each RE diamond). Plot ordered by effect size within each species and solid square = survival rate and size of symbol reflects relative weighting, horizontal bars = 95% confidence interval of each study-specific survival rate. Colored icons show potentially informative study-specific covariates.
Informative covariates
The conditional inference tree approach identified two significant nodes at a minimum split criterion of 0.95: (Node 1) Atlantic versus Indo-Pacific oceans and (Node 2) Chelonini versus Carettini and Dermochelidae nested within the Indo-Pacific Ocean (Fig. 2). No other covariates remained in the final tree model at 0.95. The three terminal nodes are boxplot summaries of the model-derived survival estimates (n = sample size within a terminal node). In addition to ocean and species, the best-fitting models from the exploratory meta-regression approach (estimates weighted by precision) included tag type (and tag loss), model type, habitat type and study duration, suggesting the methodology as well as phylogeny and biogeography might have important effects on adult marine turtle survival rates.
Covariate-specific model-predicted survival estimates
The 3-level hierarchical meta-regression model (random effects = study within research group) was a better fit than a 2-level model (random effect = study) (log-likelihood ratio test = 58.1, df = 2, P < 0.001). The 3-level model with a nonlinear functional form for study duration was a better fit than the same model with a linear functional form (log-likelihood ratio test = 19.7, df = 2, P < 0.001). The inclusion of the six moderators in the accepted 3-level, nonlinear regression model led to a significant improvement in overall model fit (QE = 121.4, df = 14, P < 0.001). However, ca. 97% of unaccounted variance was due to residual heterogeneity (tau2 = 0.005, 95% CI: 0.003–0.011; I2 = 97.2), and only ca. 37% of the residual heterogeneity in the accepted model was accounted for by inclusion of the six moderators (R2 = 0.37). This high level of residual heterogeneity was significant (QE = 508.6, df = 44, P < 0.001) and apparently typical of ecological studies2,28.
Covariate-specific survival estimates were derived from the 3-level hierarchical meta-regression model fit to the 59 studies conditioned on family/tribe (3 levels), ocean (2 levels), tag type (4 levels), method type (5 levels), habitat type (2 levels) and the study duration by tag type interaction term (Fig. 3). General patterns among model-predicted survival estimates include: (1) Chelonini > Carettini ~ Dermochelidae (Fig. 3a), (2) Indo-Pacific > Atlantic (Fig. 3b), (3) titanium > inconel ~ PIT > monel (Fig. 3c), (4) statistical modeling > enumeration (Fig. 3d) and (5) foraging areas > nesting beaches (Fig. 3e). While tag type was mostly ocean-specific, study duration appears to have no effect or a negative effect on survival estimates from metal tags and a positive effect on survival estimates from PIT tags, though most are of relatively short duration (Fig. 3f).
Multi-panel display summarizing the covariate-specific model-predicted estimates derived from the 3-level hierarchical meta-regression model fit to the 59 studies conditioned on the following covariates: (a) ocean (2 levels), (b) family (3 levels), (c) tag type (4 levels), (d) model type (5 levels), (e) habitat type (2 levels) and (f) study-duration by tag-type interaction term. Closed blue dots in the boxplots shows the predicted mean, horizontal bar = predicted median. The underlying trend in the tag-specific study-duration subplots (bottom right panel) shown by a cubic regression spline smooth with an inverse-precision weighted 95% confidence polygon.
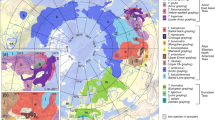
Global geographic distribution of the 59 survival estimates included in the meta-analysis. Icon shapes indicate different marine turtle species and colors indicate different habitats (foraging grounds versus nesting beaches). The map was generated using the Maptool function at www.seaturtle.org89.
Publication bias
A contour-enhanced funnel plot of the predicted study-specific survival estimates derived from the 3-level hierarchical meta-regression model fit to the 59 studies showed no evidence of any form of publication bias (Supplementary Fig. S1). There was also no temporal effect of publication year in the conditional inference tree or the exploratory meta-regression approach. Therefore, we found no evidence of publication bias in the marine turtle survival rates for which we could test for using a range of approaches.
Discussion
Annual survival estimates for adult marine turtles exhibit considerable heterogeneity. Survival estimates were generated from five turtle species, three major oceans and five decades of research involving numerous methodological techniques. For this reason, heterogeneity among estimates might be expected. However, because adult survival is a key demographic parameter and marine turtles exhibit conserved life-history patterns, including slow growth and delayed sexual maturity8, the extent of heterogeneity found among survival estimates complicates accurate biological interpretations. To better understand heterogeneity in adult marine turtle survival rates, we conducted a comprehensive meta-analysis to test how heterogeneity among estimates was partitioned among phylogenetic, biogeographic and methodological factors. Results from this study represent an important step towards identifying the key factors that drive differences among survival estimates and setting research and management priorities for marine turtles globally11,12.
Evaluating sources of heterogeneity
Results from the recursive partitioning or conditional inference regression tree approach22,23 indicate that heterogeneity among survival estimates was first partitioned between differences in ocean basin (Indo-Pacific versus Atlantic), then by differences among family/tribe within the Indo-Pacific (Chelonini versus Carettini and Dermochelidae). Though often ignored, phylogenetic non-independence can change the results of ecological meta-analyses4. However, in this case, differences were not associated with phylogenetic similarity – Carettini is more closely related to Chelonini (both in Chelonidae) than to Dermochelidae13. Therefore, survival estimates in Carettini and Dermochelidae are likely similar and lower than Chelonini due to other biological or methodological factors, not phylogenetic history. Estimates for Indo-Pacific Chelonini might be higher because this group includes flatback turtles, which are endemic to the Indo-Pacific, exhibit different and potentially less vulnerable habitat-use patterns (exclusively neritic versus both neritic and oceanic), and have been consistently monitored with robust methodologies (see below)37. Estimates specifically from the Northwest Atlantic (as there are no estimates from the Northeast or South Atlantic) are lower than the Indo-Pacific. However, this is not consistent with any apparent differences in natural or anthropogenic mortality rates or any known differences in life-history behavior among populations in different oceans. Predictors that might be indicative of differences in adult mortality among turtle populations such as harvest history and fisheries bycatch impact14,15 were not found to contribute to heterogeneity among survival estimates (Fig. 2). Therefore, our results suggest that while heterogeneity is connected to biogeography (ocean effect) and phylogeny (family/tribe effect), differences cannot be attributed to phylogenetic similarity or apparent regional differences in adult mortality. Instead, region- and species-specific differences appear to be tightly linked to the use of different methodologies applied in different oceans and therefore to a different composition of turtle species/populations.
The ocean and family/tribe predictors were highly correlated with methodological predictors, including tag type, method/model type, habitat type and study duration. Results from exploratory meta-regression modeling showed that these four factors, in addition to ocean and species, were consistently found within the best-fitting models. Direct monitoring of marine turtles in the wild is inherently difficult and CMR studies remain the most reliable tool for estimating annual survival rates. However, such efforts are clearly vulnerable to statistical biases when methodologies cannot account for complexities inherent to the biology and study of marine turtles such as imperfect detection, tag loss, and temporary and permanent emigration. First, our results highlight the importance of tag type and its ramifications on tag loss on survival estimates. We found that estimates generated from monel tags were consistently lower than estimates generated primarily from inconel, titanium and PIT tags. Estimates generated from monel tags are likely biased low and effected by study duration (Fig. 3f) due to factors related to higher rates of tag loss and subsequent individual misidentification: monel tags have lower retention rates38, tend to corrode in seawater39 and were often applied only singly in early studies (e.g.40,41). Similar concerns with respect to tag loss may apply to inconel tags, although to a lesser extent than monel. Second, our results show that early attempts to estimate survival via enumeration methods that rely on time-series counts of individuals violate far more CMR assumptions than statistical modeling procedures such as CJSRE and MSORD, which are designed to estimate and account for biases including imperfect detection and temporary emigration42,43. Although robust statistical modeling procedures are well accepted and almost universally used, the accuracy of estimates will always depend on the quality of CMR data to which the models are applied. Third, our results suggest that CMR data collected in foraging areas may be less prone to statistical biases than data collected on nesting beaches. Because marine turtles spend considerably more time in foraging areas, estimates from foraging areas may be more robust to statistical biases associated with temporary, as well as permanent, emigration. Methodological differences clearly contribute to heterogeneity among survival estimates.
Patterns among different methodologies and their associated biases are highly correlated with biological patterns: more low estimates come from the Atlantic Ocean, Carettini and Dermochelidae, monel tags, enumeration calculations and nesting beaches, while more high estimates come from the Indo-Pacific ocean, Chelonini, titanium tags, statistical modeling procedures and foraging areas (Figs 1 and 4). We proposed that if heterogeneity was influenced by phylogeny or biogeography, then biological differences in adult survival may exist and management action should be directed towards species/populations associated with survival estimates that are relatively low. However, this interpretation was contingent on whether or not heterogeneity was also influenced by methodology. Because heterogeneity among survival estimates was strongly influenced by different methodologies that were highly correlated with phylogenetic and biogeographic differences, we cannot make accurate biological interpretations to confidently direct management actions. Whether estimates that are lower than expected indicate elevated rates of adult mortality or simply highlight the use less robust methodologies is currently undecipherable. For this reason, efforts to apply many current survival estimates to model population viability and interpret long-term trends risk drawing inaccurate conclusions and misleading management actions. This highlights the need to critically evaluate current survival estimates and attempt to correct or account for statistical biases when possible. Additionally, future estimates should strive to apply robust methodologies that eliminate or minimize statistical biases, so that the detection of important region- or species-specific differences in adult mortality are not masked by heterogeneity associated with differences in methodology. Because adult mortality from over-harvesting and fisheries bycatch remains a significant conservation concern for marine turtles14,15, more work is needed to generate new, more comparable estimates that will improve the accuracy of assessments of the status and trends in marine turtle populations9,10,44 and help guide conservation measures and management in the future.
Identifying data gaps
Our systematic review of annual survival estimates for adult marine turtles revealed important region- and species-specific data gaps that should be high priorities for research to support conservation11,12. At the region-level for all applicable species, there is a noticeable lack of estimates from the eastern North Atlantic (including the Mediterranean Sea), South Atlantic, western and northern Indian Ocean (including the Red Sea and Persian Gulf), western North Pacific and eastern South Pacific (Fig. 4), all areas that support globally important marine turtle populations14 and some that also host long-term research and monitoring projects capable of generating estimates of adult survival rates. At the species-level, we found estimates for all seven marine turtle species. However, all estimates for Kemp’s and olive ridley turtles (n = 4 and 1, respectively) were excluded for not meeting the minimum selection criteria for inclusion in the meta-analysis. Because these estimates were not deemed sufficiently robust and all are at least two decades old, new estimates for ridleys should be a research priority, especially given their susceptibility to overexploitation from human threats such as fisheries bycatch45. Globally, estimates from nesting beaches are far more frequent than estimates from foraging habitats, which are primarily from eastern Australia (Fig. 4). Although logistically more challenging, priority should be given to estimating survival rates in foraging habitats, especially in the well-studied northwest Atlantic where estimates from nesting beaches overwhelmingly predominate.
Survival estimates from unstudied regions and species will not only improve assessments of specific populations, but will also improve our understanding of the important factors that affect survival rates and allow us to better decipher biological versus methodological sources of heterogeneity among estimates. New estimates generated from traditional CMR data should avoid methodologies that may generate artificially low estimates of survival rates if possible and, if used, should acknowledge and attempt to account for statistical biases associated with those methods. Future studies may strive to implement standardized robust methodologies that eliminate or minimize potential biases and isolate the biological signal resulting from actual sources of mortality. Though not yet practically implementable in most cases, the standardized use of population-wide genetic “tagging” applied in both nesting and foraging areas holds great potential for future studies. Estimates using this approach generated across many regions and species would be directly comparable, allowing researchers and managers to identify regional hotspots in marine turtle mortality and implement more effective management strategies at a global scale.
The value of meta-analysis
Meta-analysis is a powerful tool ideally suited for combining the results of demographic studies. Because demographic studies in ecology and conservation biology often exhibit considerable heterogeneity2, estimates of key parameters, such as adult survival rates, viewed in isolation may provide limited and/or potentially biased inferences. Our results show the value of meta-analysis in generating robust species-specific demographic estimates and identifying data gaps, but also highlight the importance of evaluating sources of heterogeneity when interpreting patterns among similar demographic studies. Because demographic studies frequently exhibit heterogeneity due to system-specific nature of biological phenomena2 and study-specific differences in methodology, identifying important sources of heterogeneity can inform biological patterns and conservation actions more broadly2,3,4. Synthesized data are essential for modelling wildlife population dynamics exposed to various anthropogenic hazards46 and for testing ecological hypotheses such as the importance or not of dispersal for species exposed to habitat loss47. Because there are currently few meta-analyses of wildlife demographic rates3,48, this approach represents a productive and important area for future research.
Global research on marine turtles is poised to benefit from the application of meta-analysis. The accumulation of demographic and biological information from decades of research across all species and biogeographic regions has elicited global assessments and numerous review articles in recent years (e.g.14,15,44,49,50). Meta-analytic comparisons of key parameters and threats associated with the biology and conservation of marine turtles − such as breeding and recruitment rates, population and individual growth rates, and fisheries bycatch and plastic ingestion rates − will reveal novel patterns and valuable insights, while accounting for important sources of heterogeneity that may obscure important interpretations2,3,4,25,28. Such analyses serve to guide research and conservation priorities for marine turtles into the future.
References
Burgman, M. A., Ferson, S. & Akçakaya, H. R. Risk assessment in conservation biology. (Chapman & Hall, 1993).
Senior, A. M. et al. Heterogeneity in ecological and evolutionary meta-analyses: its magnitude and implications. Ecology 97, 3293–3299, https://doi.org/10.1002/ecy.1591 (2016).
Boyce, M. S., Irwin, L. L. & Barker, R. Demographic meta-analysis: synthesizing vital rates for spotted owls. J. Appl. Ecol. 42, 38–49, https://doi.org/10.1111/j.1365-2664.2004.00986.x (2005).
Chamberlain, S. A. et al. Does phylogeny matter? Assessing the impact of phylogenetic information in ecological meta-analysis. Ecol. Lett. 15, 627–636, https://doi.org/10.1111/j.1461-0248.2012.01776.x (2012).
Liberati, A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 6 doi: e1000100 (2009).
Vetter, D., Rücker, G. & Storch, I. Meta-analysis: a need for well-defined usage in ecology and conservation biology. Ecosphere 4 https://doi.org/10.1890/ES13-00062.1 (2013).
IntHout, J., Ioannidis, J. P., Rovers, M. M. & Goeman, J. J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 6, https://doi.org/10.1136/bmjopen-2015-010247 (2016).
Stearns, S. The Evolution of Life Histories. (Oxford University Press, 1992).
National Research Council. Assessment of sea-turtle status and trends: integrating demography and abundance. (National Academies Press, 2010).
Bjorndal, K. A. et al. Better science needed for restoration in the Gulf of Mexico. Science 331, 537–538, https://doi.org/10.1126/science.1199935 (2011).
Hamman, M. et al. Global research priorities for sea turtles: informing management and conservation in the 21st century. Endang. Species Res. 11, 245–269, https://doi.org/10.3354/esr00279 (2010).
Rees, A. F. et al. Are we working towards global research priorities for management and conservation of sea turtles? Endang. Species Res. 31, 337–382, https://doi.org/10.3354/esr00801 (2017).
Duchene, S. et al. Marine turtle mitogenome phylogenetics and evolution. Mol. Phylogenet. Evol. 65, 241–250, https://doi.org/10.1016/j.ympev.2012.06.010 (2012).
Wallace, B. P. et al. Global conservation priorities for marine turtles. PLoS One 6, e24510, https://doi.org/10.1371/journal.pone.0024510 (2011).
Wallace, B. P. et al. Impacts of fisheries bycatch on marine turtle populations worldwide: toward conservation and research priorities. Ecosphere 4, https://doi.org/10.1890/ES12-00388.1 (2013).
Murtaugh, P. A. Journal quality, effect size, and publication bias in meta-analysis. Ecology 83, 1162–1166, https://doi.org/10.1890/0012-9658(2002)083[1162:JQESAP]2.0.CO;2 (2002).
Nakagawa, S. & Santos, E. S. A. Methodological issues and advances in biological meta-analysis. Evol. Ecol. 26, 1253–1274, https://doi.org/10.1007/s10682-012-9555-5 (2012).
Gurevitch, J., Curtis, P. S. & Jones, M. H. Meta-analysis in ecology. Adv. Ecol. Res. 32, 199–247, https://doi.org/10.1016/S0065-2504(01)32013-5 (2001).
Sutton, A. J. & Higgins, J. Recent developments in meta-analysis. Stat. Med. 27, 625–650, https://doi.org/10.1002/sim.2934 (2008).
Kieschnick, R. & McCullough, B. D. Regression analysis of variates observed on (0, 1): Percentages, proportions and fractions. Stat. Model. 3, 193–213, https://doi.org/10.1191/1471082X03st053oa (2003).
Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48, https://doi.org/10.18637/jss.v036.i03 (2010).
Strobl, C., Malley, J. & Tutz, G. An introduction to recursive partitioning: rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol. Methods 14, 323–348, https://doi.org/10.1037/a0016973 (2009).
Seibold, H., Zeileis, A. & Hothorn, T. Model-based recursive partitioning for subgroup analyses. Int. J. Biostat. 12, 45–63, https://doi.org/10.1515/ijb-2015-0032 (2016).
Hothorn, T. & Zeileis, A. Partykit: A modular toolkit for recursive partitioning in R. J. Mach. Learn. Res. 16, 3905–3909 (2015).
van Houwelingen, H. C., Arends, L. R. & Stijnen, T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat. Med. 21, 589–624, https://doi.org/10.1002/sim.1040 (2002).
Konstantopoulos, S. Fixed effects and variance components estimation in three-level meta-analysis. Res. Synth. Methods 2, 61–76, https://doi.org/10.1002/jrsm.35 (2011).
Tuck, S. L. et al. Land-use intensity and the effects of organic farming on biodiversity: a hierarchical meta-analysis. J. Appl. Ecol. 51, 746–755, https://doi.org/10.1111/1365-2664.12219 (2014).
Gilman, E., Chaloupka, M., Swimmer, Y. & Piovano, S. A cross-taxa assessment of pelagic longline by-catch mitigation measures: conflicts and mutual benefits to elasmobranchs. Fish Fish. 17, 748–784, https://doi.org/10.1111/faf.12143 (2016).
Gasparrini, A., Armstrong, B. & Kenward, M. G. Multivariate meta-analysis for non-linear and other multi-parameter associations. Stat. Med. 31, 3821–3839, https://doi.org/10.1002/sim.5471 (2012).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/ (2017).
Wood, S. N. Generalized additive models: an introduction with R. (CRC Press, 2017).
Wickham, H. ggplot2: Elegant graphics for data analysis. (Springer, 2016).
Higgins, J. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–58, https://doi.org/10.1002/sim.1186 (2002).
Viechtbauer, W. & Cheung, M. W-L. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 1 https://doi.org/10.1002/jrsm.11 (2010).
Viechtbauer, W., López-López, J. A., Sánchez-Meca, J. & Marín-Martínez, F. A comparison of procedures to test for moderators in mixed-effects meta-regression models. Psychol. Methods 20, 360–374, https://doi.org/10.1037/met0000023 (2015).
Peters, J. L., Sutton, A. J., Jones, D. R., Abrams, K. R. & Rushton, L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J. Clin. Epidemiol. 61, 991–996, https://doi.org/10.1016/j.jclinepi.2007.11.010 (2008).
Groom, R., Griffiths, A. & Chaloupka, M. Estimating long-term trends in abundance and survival for nesting flatback turtles in Kakadu National Park, Australia. Endang. Species Res. 32, 203–211, https://doi.org/10.3354/esr00795 (2017).
Limpus, C. J. Estimation of tag loss in marine turtle research. Wildlife Res. 19, 457–469, https://doi.org/10.1071/WR9920457 (1992).
Balazs, G. H. Factors affecting the retention of metal tags on sea turtles. Marine Turtle Newsletter 20, 11–14 (1982).
Bjorndal, K. A. Demography of the breeding population of the green turtle, Chelonia mydas, at Tortuguero, Costa Rica. Copeia 1980, 525–530, https://doi.org/10.2307/1444530 (1980).
Chua, T. H. Nesting population and frequency of visits in Dermochelys coriacea in Malaysia. J. Herpetol. 22, 192–207, https://doi.org/10.2307/1563998 (1988).
Kendall, W. L. & Bjorkland, R. Using open robust design models to estimate temporary emigration from capture-recapture data. Biometrics 57, 1113–1122, https://doi.org/10.1111/j.0006-341X.2001.01113.x (2001).
Converse, S. J., Kendall, W. L., Doherty, P. F. Jr. & Ryan, P. G. Multistate models for estimation of survival and reproduction in grey-headed albatross (Thalassarche chrysoma). Auk 126, 77–88, https://doi.org/10.1525/auk.2009.07189 (2009).
Mazaris, A. D., Schofield, G., Gkazinou, C., Almpanidou, V. & Hays, G. C. Global sea turtle conservation successes. Sci. Adv. 3, e1600730, https://doi.org/10.1126/sciadv.1600730 (2017).
Plotkin, P. T. Biology and Conservation of Ridley sea turtles (The Johns Hopkins University Press, 2007).
Chaloupka, M. & Balazs, G. H. Using Bayesian state-space modelling to assess the recovery and harvest potential of the Hawaiian green sea turtle stock. Ecol. Model. 207, 93–109, https://doi.org/10.1016/j.ecolmodel.2007.02.010 (2007).
Quesnelle, P. E., Lindsay, K. E. & Fahrig, L. Low reproductive rate predicts species sensitivity to habitat loss: a meta-analysis of wetland vertebrates. PLoS One 9, e90926, https://doi.org/10.1371/journal.pone.0090926 (2014).
Benítez-López, A. et al. The impact of hunting on tropical mammal and bird populations. Science 356, 180–183, https://doi.org/10.1126/science.aaj1891 (2017).
Hays, G. C., Mazaris, A. D. & Schofield, G. Different male vs. female breeding periodicity helps mitigate offspring sex ratio skews in sea turtles. Front. Mar. Sci. 1, 43, https://doi.org/10.3389/fmars.2014.00043 (2014).
Lee, P. L. M., Schofield, G., Haughey, R. I., Mazaris, A. D. & Hays, G. C. A review of patterns of multiple paternity across sea turtle rookeries. Adv. Mar. Biol. (in press) https://doi.org/10.1016/bs.amb.2017.09.004 (2017).
Limpus, C. J., Parmenter, C. J. & Chaloupka, M. Monitoring of Coastal Sea Turtles: GapAnalysis 5. Flatback turtles, Natator depressus, in the Port Curtis and Port Alma Region. Report produced for the Ecosystem Research and Monitoring Program Advisory Panel as part of Gladstone Ports Corporation’s Ecosystem Research and Monitoring Program http://gpcl.com.au/EnvironmentDocuments/EHPMonitoringofCoastalSeaTurtlesGapAnalysis5Flatback%20turtles.pdf (2013).
Guinea, M., Chaloupka, M. & Limpus, C. Bare Sand Island flatback turtle capture-mark-recapture program (1996–2014). Report prepared for use in Department of Environment and Energy (Commonwealth of Australia) recovery plan for marine turtles in Australia (Canberra, Australia) (2015).
Chaloupka, M. Using multistate open robust design statistical modeling approach for estimating key demographic parameters for the flatback nesting population on Barrow Island, Western Australia (2005/06–2013/14). Society of Petroleum Engineers International Conference on Health, Safety and Environment SPE 156747 (2014).
Chaloupka, M. Using multistate open robust design statistical modeling approach for estimating key demographic parameters for the flatback nesting population at Mundabullangana, Western Australia (2009/10–2013/14). Society of Petroleum Engineers International Conference on Health, Safety and Environment SPE 156747 (2015).
Seminoff, J., Jones, T. T., Resendiz, A., Nichols, W. J. & Chaloupka, M. Y. Monitoring green turtles (Chelonia mydas) at a coastal foraging area in Baja California, Mexico: multiple indices describe population status. J. Mar. Biol. Assoc. UK 83, 1355–1362, https://doi.org/10.1017/S0025315403008816 (2003).
Chaloupka, M. Phase 1- Assessment of suitability of Queensland Parks & Wildlife Service Sea Turtle Data for use in models of the population dynamics of the Southern Great Barrier Reef Green Turtle Stock. Great Barrier Reef Marine Park Authority Research Publication 74, 1–49 (2002).
Limpus, C., Jones, K. & Chaloupka, M. Fibropapilloma disease in marine turtles in eastern Indian Ocean - South Western Pacific Ocean. NOAA Tech Memo NOAA-TM-NMFS-PIFSC-054 (2016).
Chaloupka, M. & Limpus, C. Estimates of sex- and age-class-specific survival probabilities for a southern Great Barrier Reef green sea turtle population. Mar. Biol. 146, 1251–1261, https://doi.org/10.1007/s00227-004-1512-6 (2005).
Piacenza, S. E., Balazs, G. H., Hargrove, S. K., Richards, P. M. & Heppell, S. S. Trends and variability in demographic indicators of a recovering population of green sea turtles. Endang. Species Res. 31, 103–117, https://doi.org/10.3354/esr00753 (2016).
Troëng, S. & Chaloupka, M. Variation in adult annual survival probability and remigration intervals of sea turtles. Mar. Biol. 151, 1721–1730, https://doi.org/10.1007/s00227-007-0611-6 (2007).
Campbell, C. L. & Lagueux, C. J. Survival probability estimates for large juvenile and adult green turtles (Chelonia mydas) exposed to an artisanal marine turtle fishery in the western Caribbean. Herpetologica 61, 91–103, https://doi.org/10.1655/04-26 (2005).
Solow, A. R., Bjorndal, K. A. & Bolten, A. B. Annual variation in nesting numbers of marine turtles: the effect of sea surface temperature on re-migration intervals. Ecol. Lett. 5, 742–746, https://doi.org/10.1046/j.1461-0248.2002.00374.x (2002).
García-Cruz, M. A. et al. Population trends and survival of nesting green sea turtles Chelonia mydas on Aves Island, Venezuela. Endang. Species Res. 29, 103–116, https://doi.org/10.3354/esr00695 (2015).
Penaloza, C. Demografía y viabilidad de las población de tortuga verde, Chelonia mydas, en Isla deAves. Universidad Simón Bolívar (2000).
Richardson, J. I., Bell, R. & Richardson, T. H. Population ecology and demographic implications drawn from an 11-year study of nesting hawksbill turtles, Eretmochelys imbricata, at Jumby Bay, Long Island, Antigua, West Indies. Chelonian Conserv. Biol. 3, 244–250 (1999).
Prince, R. I. T. & Chaloupka, M. Estimating demographic parameters for a critically endangered marine species with frequent reproductive omission: hawksbill turtles nesting at Varanus Island, Western Australia. Mar. Biol. 159, 355–363, https://doi.org/10.1007/s00227-011-1813-5 (2012).
Bell, I., Schwarzkopf, L. & Manicom, C. High survivorship of an annually decreasing aggregation of hawksbill turtles, Eretmochelys imbricata, found foraging in the northern Great Barrier Reef. Aquat. Conserv. 22, 673–682, https://doi.org/10.1002/aqc.2245 (2012).
Limpus, C. & Chaloupka, M. Moreton Bay loggerhead turtle capture-mark-recapture program (1990–2008). Report produced for the Queensland Department of Environment and Heritage (2008).
Heppell, S. S., Limpus, C. J., Crouse, D. T., Frazer, N. B. & Crowder, L. B. Population model analysis for the loggerhead sea turtle, Caretta caretta, in Queensland. Wildlife Res. 23, 143–159, https://doi.org/10.1071/WR9960143 (1996).
Chaloupka, M. & Limpus, C. Survival probability estimates for the endangered loggerhead sea turtle resident in southern Great Barrier Reef waters. Mar. Biol. 140, 267–277, https://doi.org/10.1007/s002270100697 (2002).
Richardson, J. I. & Richardson, T. H. An experimental population model for the loggerhead sea turtle (Caretta caretta) in Biology and conservation of sea turtles (ed. Bjorndal, K. A.) 165–176 (Smithsonian Institution Press, 1995).
Pfaller, J. B. et al. Accounting for imperfect detection is critical for inferring marine turtle nesting population trends. PLoS One 8, e62326, https://doi.org/10.1371/journal.pone.0062326 (2013).
Lamont, M. M., Fujisaki, I. & Carthy, R. R. Estimates of vital rates for a declining loggerhead turtle (Caretta caretta) subpopulation: implications for management. Mar. Biol. 161, 2659–2668, https://doi.org/10.1007/s00227-014-2537-0 (2014).
Monk, M. H., Berkson, J. & Rivalan, P. Estimating demographic parameters for loggerhead sea turtles using mark-recapture data and a multistate model. Popul. Ecol. 53, 165–174, https://doi.org/10.1007/s10144-010-0205-x (2011).
TEWG (Turtle Expert Working Group). An assessment of the loggerhead turtle population in the western North Atlantic Ocean. NOAA Tech Memo NMFS-SEFSC-575 (2009).
Frazer, N. B. Survivorship of adult female loggerhead sea turtles, Caretta caretta, nesting on Little Cumberland Island, Georgia, USA. Herpetologica 39, 436–447 (1983).
Thorson, J. T., Punt, A. E. & Nel, R. Evaluating population recovery for sea turtles under nesting beach protection while accounting for nesting behaviors and changes in availability. J. Appl. Ecol. 49, 601–610, https://doi.org/10.1111/j.1365-2664.2012.02143.x (2012).
Taylor, B. L. Population viability analysis for the Cumberland Island loggerhead turtle population. NOAA Tech Memo NMFS-SEFSC-361 (1995).
Henry, J. D., Phaneuf, P. J. & Sprayberry, T. H. Survivorship estimates for female loggerhead sea turtles, Caretta caretta, nesting on Wassaw and Pine Islands, Georgia. NOAA Tech Memo NMFS-SEFSC-415 (1998).
Phillips, K., Mansfield, K. L., Die, D. J. & Addison, D. S. Survival and remigration probabilities for loggerhead turtles (Caretta caretta) nesting in the Eastern Gulf of Mexico. Mar. Biol. 161, 863–870, https://doi.org/10.1007/s00227-013-2386-2 (2014).
Rivalan, P., Prévolt-Julliard, A. C. & Girondot, M. First estimation of the annual female survival rate based on tagging data from French Guiana. NOAA Tech Memo NMFS-SEFSC-567 (2008).
Rivalan, P. et al. Trade-off between current effort and delay to next reproduction in the leatherback turtle. Oecologia 145, 564–574, https://doi.org/10.1007/s00442-005-0159-4 (2005).
Stewart, K. R. et al. Increased nesting, good survival and variable site fidelity for leatherback turtles in Florida, USA. Biol. Conserv. 176, 117–125, https://doi.org/10.1016/j.biocon.2014.05.008 (2014).
Dutton, D. L., Dutton, P. H., Chaloupka, M. & Boulon, R. H. Increase of a Caribbean leatherback turtle Dermochelys coriacea nesting population linked to long-term nest protection. Biol. Conserv. 126, 186–194, https://doi.org/10.1016/j.biocon.2005.05.013 (2005).
Pilcher, N. & Chaloupka, M. Using community-based monitoring to estimate demographic parameters for a remote nesting population of the Critically Endangered leatherback turtle. Endang. Species Res. 20, 49–57, https://doi.org/10.3354/esr00480 (2013).
Tomillo, M. P. S. Factors affecting population dynamics of eastern Pacific leatherback turtles (Dermochelys coriacea). Drexel University (2007).
Dutton, D. L., Dutton, P. H. & Boulon, R. Recruitment and mortality estimates for female leatherbacks nesting in St. Croix, U.S. Virgin Islands. NOAA Tech Memo NMFS-SEFSC-443 (2000).
Spotila, J. R., Reina, R. D., Steyermark, A. C., Plotkin, P. T. & Paladino, F. V. Pacific leatherback turtles face extinction. Nature 405, 529−530, https://doi.org/10.1038/35014729 (2000).
SEATURTLE.ORG Maptool. SEATURTLE.ORG, Inc. http://www.seaturtle.org/maptool/ (9 November 2017).
Acknowledgements
We are grateful to the dedicated research teams that have undertaken the long-term studies from which we have derived demographic data. Without these long-term studies, any analyses such as ours would not be possible. We thank the compliers and maintainers of the Sea Turtle Document Library (seaturtle.org) and the Sea Turtle Online Bibliography (Archie Carr Center for Sea Turtle Research, University of Florida). We thank Chevron Australia for use of the Barrow Island and Mundabullangana data. Publication of this article was funded in part by the University of Florida Open Access Publishing Fund.
Author information
Authors and Affiliations
Contributions
J.B.P. and M.C. collected and managed data. All authors conceived and designed the study, analyzed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pfaller, J.B., Chaloupka, M., Bolten, A.B. et al. Phylogeny, biogeography and methodology: a meta-analytic perspective on heterogeneity in adult marine turtle survival rates. Sci Rep 8, 5852 (2018). https://doi.org/10.1038/s41598-018-24262-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-24262-w
- Springer Nature Limited
This article is cited by
-
Anthropogenic impacts on threatened species erode functional diversity in chelonians and crocodilians
Nature Communications (2023)
-
Phylogeny explains capture mortality of sharks and rays in pelagic longline fisheries: a global meta-analytic synthesis
Scientific Reports (2022)
-
A decision support tool for integrated fisheries bycatch management
Reviews in Fish Biology and Fisheries (2022)
-
One beach amongst many: how weak fidelity to a focal nesting site can bias demographic rates in marine turtles
Marine Biology (2022)
-
Applying a sequential evidence hierarchy, with caveats, to support prudent fisheries bycatch policy
Reviews in Fish Biology and Fisheries (2022)