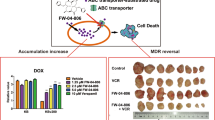
Abstract
Cytotoxic activities of acetylshikonin and acetoxyisovalerylshikonin alone and in combination with chemotherapeutic agents against parental and drug resistant cell lines were determined using the MTT assay. Effects of Shikonin derivatives on BCRP, MDR1 and MRP transcript and protein levels were relatively measured. Finally, accumulation and efflux kinetics were conducted. The results revealed cell- and concentration-dependency of the cell cytotoxicity. Acetylshikonin and acetoxyisovalerylshikonin transiently made the mRNA ocean turbulent, but FACS analyses using fluorescent-labeled antibodies showed no significant change in the MDR-protein levels. Functional kinetics revealed significant block of MDR1, BCRP and MRP transporter in the presence of shikonin derivatives. Maximum accumulation fold changes was quantified to be 4.4 and consequently, acetoxyisovalerylshikonin pretreated EPG85.257RDB cells was chemosensitized to daunorubicin tension 3.1-fold. Although, the MDR blockage was reported to follow time- and cell-dependent patterns, MDR1, BCRP and MRP2 responses to the shikonins are concentration-independent. These data suggest uncompetitive transporter blockage behavior of these agents. The results indicated that shikonin derivatives stimulate uptake and reduce efflux of chemotherapeutic agents in the malignant cancer cells, suggesting that chemotherapy in combination with shikonin compounds may be beneficial to cancer cells that overexpress multidrug resistance transporters.
Similar content being viewed by others
Introduction
Drug resistance is one of the main challenges in cancer chemotherapy and is influenced by several important factors which include drug potency, cancer cell response to the treatments, tumor microenvironment and heterogeneity of cancer cells1,2. Multidrug resistance (MDR) or chemo-resistance represents an event whereby cancer cells exhibit tolerance to a specific chemotherapeutic agent or a class of pharmaceutical drugs3. Target-dependent, drug dependent, and drug/target-independent are the most common MDR phenotypes4,5. Overexpression of ATP-binding cassette (ABC) transporter superfamilies are the most significant mechanisms underlying MDR phenotype, which consume ATP and efflux either cytotoxic drugs or targeted anticancer agents6. The most prevalent ABC superfamily members are including P-glycoprotein (P-gp/ABCB1), multidrug resistance-associated protein (MRP/ABCC), and breast cancer resistance protein (BCRP/ABCG2), which may over-activated or up-regulated in malignant cancers, and develop a distinctive defense contradictory to chemotherapeutics. These pumps clearly reduce the intracellular concentration of frequent endotoxin and exotoxins, therefore causing MDR-phenotype7,8.
Recently, many investigators explored herbal bioproducts such as the MDR cell chemosensitizers with least cytotoxicity. Some naturally active ingredients like carotenoid, coumarin, flavonoid, naphthoquinone, stilbenoid, and terpenoid derivatives obtained from plants and fungi have been previously reported as MDR modulators/inhibitors9,10. Various studies have revealed that herbal extracts synergistically increased chemotherapy potency, reduced the chemotherapeutics doses, and increased therapeutic window of cytotoxic agents, especially via inverse MDR in cancer cells. These natural MDR-modulators mainly act as antagonists, reverse agonists, transcript down-regulators, protein down-regulators, metabolism modulators, or ATP binding interaction interferers which reduce chemotherapeutics efflux from the cancer cells10,11. Naphthazarins and their derivatives have been investigated for a variety of biological functions, such as antibacterial, antimalarial, antifungal and anticancer properties12,13.
Shikonin is one of the natural naphthazarins which is the major constituent of red extracts of the roots of Lithospermum erythrorhizon and Alkanna frigida and has been traditionally used for the treatment of sore throat, injuries, infections the and gastrointestinal disorders14,15. Shikonin derivatives including isobutyrylshikonin, acetylshikonin, dimethylacrylshikonin and isovalerylshikonin have also demonstrated selective anticancerous, tyrosine kinase inhibitory, ROS generation, anti-angiogenesis, proteasome inhibitory, anti-inflammatory and anti-glycolysis activities16,17. Earlier, shikonin was reported to downregulate MDR1 protein and chemosensitized cells resistance to chemotherapy18. There is little information on the modulatory mechanism of alkylated naphthazarin moieties on ABC-transporters, although, higher cancer cytotoxicity via acceleration of apoptotic protein production and triggering of death signaling receptors has been reported for some chemically modified shikonin derivatives16,19. Therefore, the present study mainly focused on the restrictive effects of two novel alkylated shikonin derivatives: acetylshikonin and β-acetoxyisovalerylshikonin, on ABC-transporter expression level and function in human cancer cell lines. These results present well-known compounds helpful in the management of malignant multidrug resistant cancers.
Results
In vitro cytotoxic behaviors of shikonins
Sensitivity of the parental cells and their MDR-resistant lines to shikonin derivatives and standard chemotherapeutic agents were measured. After a 5-day treatment with serial dilutions of chemical agents, a dose-response curve was fitted to the normalized MTT data (Supp. Fig. S1). Table 1 shows the IC10 and IC50 values for acetylshikonin and acetoxyisovalerylshikonin and IC50 data for the chemotherapeutic agents. Acetylshikonin presented higher toxicity than the acetoxyisovalerylshikonin for EPG85.257RDB and MCF7MX cell lines. Primary investigations revealed that shikonin derivatives inhibited cell growth time-, dose- and cell-dependently. In addition, the combination of shikonins with standard chemotherapeutics resulted in a statistical significant improvement of daunorubicin, mitoxantrone or cisplatin toxicity and confirmed synergistic toxicity (P < 0.001, Tables 1 and 2). Acetylshikonin combination therapy mostly showed more prominent toxic effects on both parental and resistant cells. Dose-response curves illustrated significant decreases of viable cell fractions against higher concentrations of the combination therapy (Supp. Fig. S2).
MDR pump transcripts level and relative protein quantification
Relative BCRP, MDR1 and MRP transcript and protein quantities were measured using real time PCR and flow cytometer instruments according to the validated standard protocols. Real time based transcript quantifications was optimized on Pfaffl method using high effective primer pairs (Supp. Table S1). Initial experiments showed that MCF7MX, EPG85.257RDB and A2780RCIS cells express BCRP, MDR1 and MRP2 approximately 800-, 310- and 13-fold more than their corresponding parents, respectively. Totally, MDR1 and MRP1 transcripts were up-regulated in the resistant cells time-dependently and BCRP and MRP2 represented down-regulation patterns over time in these cells after shikonin treatments; however, this could not be generalized to all cells, treatments or times (Supp. Fig. S3). Parent cells generally follow the same expression patterns after treatments just like their resistant counterparts, even though they do not express sophisticated MDR pumps (Supp. Fig. S3). Relative BCRP protein quantifications showed up-regulation after 48 h of acetoxyisovalerylshikonin treatment (P < 0.05) and down-regulation after 72 h of acetylshikonin treatment (P < 0.05) in MCF7 cells; however, the resistant EPG85.257RDB and MCF7MX efflux protein fluctuations are statistically insignificant (P > 0.05) over a 3-day of shikonin exposure (Fig. 1).
Relative quantification of MDR proteins using flow cytometer. MDR1 and BCRP protein levels of EPG85.257 and MCF7 parental and their resistant counterparts were quantified in the presence of acetylshikonin and acetoxyisovalerylshikonin. ACS, acetylshikonin; AVS, acetoxyisovalerylshikonin; Con, control.
Accumulation and efflux kinetic
Effects of naphthazarin on the chemotherapeutics accumulation and efflux in the sensitive and resistant cell lines were flow cytometric evaluated. BCRP, MDR1 and MRP2 pump activity was significantly reduced in the acetylshikonin and acetoxyisovalerylshikonin treated MCF7/MX, EPG85.257/RDB and A2780/RCIS cells, respectively (Fig. 2, Supp. Figs S4 and S5). Inconsequence, the shikonins led to a time-dependent accumulation of mitoxantrone and daunorubicin in the respective cells during the incubation periods (P < 0.001). Maximum daunorubicin and mitoxantrone accumulation was detected after 24 and 48 h of acetoxyisovalerylshikonin treatment of EPG85.257RDB and MCF7MX, respectively (P < 0.001). Although, both treatments showed a dynamic reduction in pumps efflux activity, acetoxyisovalerylshikonin resulted in more potent inhibitory activity (P < 0.001). Cells accumulation and efflux pattern of acetylshikonin treated EPG85.257RDB returned to the control levels after 72 h of drug exposure. MRP2 pump activity in A2780RCIS cells was less affected by both treatments in comparison with the other resistant cells (Fig. 2, Supp. Fig. S5).
Accumulation and efflux patterns of daunorubicin and mitoxantrone in MDR-resistant cells. Cells were incubated with IC10 values of acetylshikonin and acetoxyisovalerylshikonin for 24, 48 and 72 h and then treated with IC50 concentration of daunorubicin or mitoxantrone in the presence or absence of a specific inhibitor (indomethacin for MRP2 pumps, verapamil for MDR1 pumps or novobiocin for BCRP pumps). Average fluorescent intensities were recorded. Efflux and accumulation were calculated from corresponding equations. The symbols (***), (**)and (*) represent pump activity differences between shikonin treated and untreated cells as P < 0.001, P < 0.05 and P < 0.01, respectively. ACS, acetylshikonin; AVS, acetoxyisovalerylshikonin; Con, control.
Discussion
The main reason for numerous types of chemotherapy failure is overexpressing of MDR pumps in cancer cells20. Such cells frequently present increased ATP-dependent efflux pumps feature and demonstrate prominent ability of increased efflux and reduced influx21. Numerous herbal-derived extracts and pure constituents are known to increase the chemotherapeutic effects of anticancer agents by modulating the ABC-transporters’ transcription, translation, and activity as well as regulating cell survival, apoptotic signaling pathways, drug distribution, and cellular metabolisms which make them less toxic and promising candidates for targeting MDR resistance22,23,24.
It has been confirmed in the literature that shikonin derivatives exhibit antineoplastic properties mainly by cancer cell growth inhibition, apoptotic pathway induction, DNA topoisomerase interference, antimitogenic action, angiogenesis reduction, and proteasome inhibition25,26,27. Alkylated shikonin derivatives on C2 and C7 showed stronger cytotoxicity on cancer cells rather than normal cells (Supp. Fig. S6). Reports showed that introduction of oxygen-containing alkyls or cycloalkyls on C2 position enhanced the cytotoxicity effects against multiple drug resistant cancer cells18,28,29,30. Table 1 shows results consistent with previous studies and confirms that acetylshikonin and acetoxyisovalerylshikonin with higher levels of oxygen atoms exert remarkable toxic effects on both parental and resistant cancer cells. Although, presence of shorter alkyl groups (2–6 carbons) has been reported to have stronger antitumor activity than longer chains (7–20 carbons) in few researches31,32; such patterns could not be extended to A2780/RCIS cells in the present study and Table 1 showed that shikonin toxicity is most likely cell specific. Table 2 presents the interaction of the new derivatives with normal metabolic pathways of MDR-resistant cells which is overall referred to as “Warburg effect”. The mechanisms that enhance this susceptibility may include direct MDR-pump interference, cellular transcription and translation alteration, alteration of oxidoreductase enzymes, ATPase blockage, interruption of gaining energy in tumor cells through the glycolysis pathway, or enhancement of bioavailability of chemotherapeutics which has not been fully investigated yet33,34. Though Tables 1 and 2 shows attracting cytotoxic effects of two shikonin analogs on the multidrug resistant cells, evidences on the association between the structural relationship and desired functions of the derivatives remain unclear.
Preliminary results in this study showed up-regulation of MDR1 and MRP1, and down-regulation of BCRP and MRP2 pumps after naphthazarin treatments in the transcriptional levels as primary adaptation and xenobiotic detoxification responses; however, the translational pump levels remained unchanged, and such inconsistency has been reported previously which could be attributed to post-transcriptional changes, the protein half-life and environmental conditions35,36,37. Besides, flow cytometric analyses showed that BCRP, MDR1 and MRP2 efflux function were significantly disrupted during the 72 h treatments (Fig. 2). Acetoxyisovalerylshikonin led to maximum 4-fold daunorubicin accumulation in EPG85.257RDB cells and the corresponding cell was sensitized to daunorubicin toxicity 3-fold. It can be concluded that increased drug accumulation inside these cells is possibly due to inhibition of pump activity after the treatment and resulted in chemotherapy sensitization. Interestingly, an appropriate balance was observed between efflux decrease and accumulation increase responses in the most resistant cells. Mitoxantrone and daunorubicin accumulation in respective MCF7MX and A2780RCIS were less affected by shikonin derivatives and the chemosensitization to the chemotherapeutics was also less observed. MCF7MX cells presented chemotoleration features for mitoxantrone cytotoxicity during both shikonin treatments even though BCRP pump activity was significantly (P < 0.001) blocked during the experiment time. Such behaviors may be attributed to alteration of metabolism pathways of cancer cells associated with energy consumption, chemotherapeutics metabolism and physicochemical inactivation of the hydroxyl naphthoquinones in this cell36,38. Our initial investigations demonstrated that chemosensitization and MDR-associated efflux blockage are less likely dependent on shikonin dosage volumes; such separate activities support the hypothesis that shikonins are uncompetitive pump blockers. This phenomenon may due to shikonin derivatives interference in ATP production, distribution or consumption36,39. Moreover, ATP transporters that actively efflux xenobiotics gain their energy in tumor cells through the glycolytic pathway. Maybe, inhibiting glycolysis in these cells may also increase the concentrations of chemotherapeutic agents in the cell34,40,41.
Conclusion
The results of the present study indicate that oxygenated shikonin derivatives have potently chemosensitized MDR1 overexpressing cancer cells to the daunorubicin treatment. ABC-transporter blockage follows time- and cell-dependent patterns but MDR1, BCRP and MRP2 responses to the shikonins are concentration-independent. Data suggest that naphthazarin derivatives may be effective uncompetitive transporter blockers. These findings introduced acetylshikonin and acetoxyisovalerylshikonin as attractive candidates for multidrug resistant cancers therapy in clinical practice. However, further investigations are required to uncover unique mechanism of actions and structural activity relationships underlying their effects on efflux transporter.
Materials and Methods
Reagents
The chemical were obtained from commercial resources. MTT, chemotherapeutic agents and MDR pump inhibitors were purchased from Sigma-Aldrich (Sigma-Aldrich, Deisenhofen, Germany). Cell culture media and components were purchased from Gibco (Grand Island, NY, USA). BCRP and MDR1, primary antibodies (mouse monoclonal IgG), goat anti-mouse secondary IgG1 and IgG2a conjugated with FITC, and the appropriate isotype controls were obtained from Abcam (Cambridge, USA). Shikonin analogous were generously provided by Professor Alireza Yazdinezhad (Zanjan University of Medical Sciences). All other compounds and solvents were of analytical grade and obtained from Merck Company (Darmstadt, Germany).
Cell culture and cell viability assays
A2780RCIS (MRP2 overexpressing human epithelial ovarian cancer), EPG85.257RDB (MDR1 overexpressing human gastric adenocarcinoma), MCF7MX (BCRP overexpressing human epithelial breast cancer), and their parental counterparts were generously provided by Professor Herman Lage (Molecular Pathology Department, University Medicine Berlin). Cells were cultured in RPMI-1640 supplemented with 2 mM L-glutamine, 10% FBS, 100 IU/ml penicillin and 100 μg/ml streptomycin in a pre-equilibrated humidified incubator at 37 °C and 5% CO2. Media for multidrug resistant A2780RCIS, EPG85.257RDB and MCF7MX cell lines was also supplemented with 33.21 μM cisplatin, 4.74 μM daunorubicin or 0.10 μM mitoxantrone, respectively. Shikonins cytotoxicity was determined after a 5-day incubation of the treated cells by MTT assay at 570 nm using microplate reader. IC10 and IC50 values were calculated from the scatter plot of the percentage viability versus drug concentrations and defined as the drug concentrations that reduced the surviving cells in the wells by 10 and 50% as compared to the control, respectively. Combination treatment of the cells with defined IC10 values of acetylshikonin and acetoxyisovalerylshikonin in the presence of serially diluted cisplatin, daunorubicin or mitoxantrone was also reported as synergistic cytotoxicity. SIC50 value was defined as the chemotherapeutic agent concentration that was co-treated with any of shikonins and reduced the surviving fraction of the cells by 50% when compared with the untreated cells. SIC50 was measured from scatter plot of relative cell viability versus any of the chemotherapeutic agent concentrations. All the experiments were accomplished three independent times in pentaplicate42.
Relative quantification of the MDR pump transcripts
Cells were seeded at 3 × 105 cells/well and treated with IC10 concentrations of acetylshikonin and acetoxyisovalerylshikonin for 24, 48, and 72 h before RNA extraction. High quality total RNA was isolated (RNeasy Mini Kit, Qiagen) and cDNA was generated (QuantiTect Reverse Transcription Kit, Qiagen) from 1 μg RNA following the manufacturer’s instruction. Quantitative transcript analyses were conducted using the QuantiTect SYBR Green PCR kit (Qiagen, Germany). Real time amplification was set to an initial denaturation step for 10 min at 94 C, followed by 40 cycles of amplification (94 °C for 15 s, annealing temperature for 20 s and 72 °C for 25 min) and finally, a normal melting analysis. BCRP, MDR1, MRP1, MRP2 and β-actin primer sequences and properties are provided in the supplementary materials. All the amplification reactions were performed in triplicate and results were normalized to β-actin, and relative fold changes in the expression were quantified according to Pfaffl method43.
Flow cytometry analyses of relative MDR pump levels
Resistant and parent cancer cells were treated with IC10 concentrations of acetylshikonin or acetoxyisovalerylshikonin for 24, 48, and 72 h in 6-well plates. Thereafter, the monolayer cells were trypsinized, harvested and finally fixed with formaldehyde (10% v/v) and cold methanol (90% v/v) for 10 min, respectively. Then, the cells were resuspended in blocking suspension of 10% w/v bovine serum albumin for 1 h at room temperature to minimize nonspecific antibody binding. EPG85.257, A2780, MCF7 resistant and parent cells were independently incubated in 0.01 v/v PBS-diluted primary antibody of either human BCRP, MDR1 or MRP2 supplemented with 2% BSA and 0.01% Tween-20 for 1 h at 4 °C. Subsequently, the cells were incubated in PBS buffer containing 0.01 v/v FITC-conjugated goat anti-mouse secondary immunoglobulin, 2% BSA, and 0.01% Tween-20 for 20 min on ice in the dark. PBS-washing was included twice between all steps. Subsequently, the protein levels were analyzed using a FACSCaliburTM flow cytometer with proper negative controls (autofluorescent, secondary antibody and isotype controls). Intact cells were separated from cellular debris using forward/side scatter parameters. FITC-labeled proteins were excited with the 488 nm laser and the emission fluorescence intensity was recorded using photomultiplier tube with a 530/30 nm band-pass filter (FL1). Finally, data were processed using WinMDI (version 2.8) and FlowJo (version 7.6.1) for 3 independent experiments as compared to the corresponding untreated controls42,43.
Effects of shikonin on chemotherapeutics accumulation and efflux kinetic
Briefly, the MDR-resistant cancerous cells and their parental counterparts were seeded at a density of 5 × 105/well in 6-well plates and incubated with IC10 values of acetylshikonin and acetoxyisovalerylshikonin for 24, 48 and 72 h. Then, the cells were harvested and divided into two individual groups. First one was treated with only fluorescent chemotherapeutic substrate (1 μM daunorubicin for MDR1 and MRP or 3 μM mitoxantrone for BCRP efflux pumps); the second one received a corresponding specific pump inhibitor also (10 μM verapamil for MDR1, 100 μM indomethacin for MRP or 200 μM novobiocin for BCRP) for 30 min at room temperature. Cells were harvested, PBS-washed and divided into portions. The first fractions were placed on ice and immediately, FACS analyzed for accumulation kinetics. The second fraction was treated with RPMI-1640 containing 10% FBS either with (for inhibitor-treated cells in the accumulation step) or without (for inhibitor-untreated cells in the accumulation step) pump inhibitor for another 1 h in the dark at room 37 °C. After PBS washing for efflux assay, these cells were subjected to FACS analyses. IC10 values of shikonin derivatives were presented in all the steps of kinetic assays. Forward/side scatter gating included the viable single cells of the study and the fluorophores were excited at 488 nm. Daunorubicin and mitoxantrone emission was recorded at FL2 (585 nm) and FL3 (670 nm), respectively. 105 events were recorded for samples and the following equations were used for the mathematical models where, MFI, modulator, sample and control are mean fluorescent intensity, pump inhibitors, shikonin treated cells and untreated cells, respectively. Appropriate negative controls were also included in the study and the experiments were repeated at least three independent times42,44.
Statistical analyses
All the experiments were carried out in triplicates and means ± standard errors were reported. The obtained results were subjected to statistical analyses using Student’s t-test or one-way ANOVA with SPSS-22 software. Results that showed P-values less than 0.05 were considered to be statistically significant.
Data availability
The datasets generated and/or analyzed during the current study are available on request from the corresponding author.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
Gottesman, M. M., Fojo, T. & Bates, S. E. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2, 48–58 (2002).
Han, W. et al. Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Mol Cancer Ther 6, 1641–1649 (2007).
Zinzi, L. et al. Small and Innovative Molecules as New Strategy to Revert MDR. Front Oncol 4, 2 (2014).
Anreddy, N. et al. Tyrosine kinase inhibitors as reversal agents for ABC transporter mediated drug resistance. Molecules 19, 13848–13877 (2014).
Li, Y., Atkinson, K. & Zhang, T. Combination of chemotherapy and cancer stem cell targeting agents: Preclinical and clinical studies. Cancer Lett 28, 103–109 (2017).
Levatić, J. et al. Accurate models for P-gp drug recognition induced from a cancer cell line cytotoxicity screen. J Med Chem 56, 5691–5708 (2013).
Chen, Z. et al. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: a review of the past decade. Cancer lett 370, 153–164 (2016).
Bachas, S., Eginton, C., Gunio, D. & Wade, H. Structural contributions to multidrug recognition in the multidrug resistance (MDR) gene regulator, BmrR. Proc Natl Acad Sci USA 108 (2011).
Schnabel, C. et al. Total synthesis of natural and non-natural Delta(5,6)Delta(12,13)-jatrophane diterpenes and their evaluation as MDR modulators. J Org Chem 76, 512–522 (2011).
Szakacs, G., Paterson, J. K., Ludwig, J. A., Booth-Genthe, C. & Gottesman, M. M. Targeting multidrug resistance in cancer. Nat Rev Drug Discov 5, 219–234 (2006).
Zahreddine, H. & Borden, K. L. B. Mechanisms and insights into drug resistance in cancer. Front Pharmacol 4, 28 (2013).
Papageorgiou, V. P., Assimopoulou, A. N., Couladouros, E. A., Hepworth, D. & Nicolaou, K. C. The chemistry and biology of alkannin, shikonin, and related naphthazarin natural products. Angew. Chem. Int. Ed. 38, 270–301 (1999).
Chen, X., Yang, L., Oppenheim, J. J. & Howard, M. Z. Cellular pharmacology studies of shikonin derivatives. Phytother Res 16, 199–209 (2002).
Staniforth, V., Wang, S.-Y., Shyur, L.-F. & Yang, N.-S. Shikonins, phytocompounds from Lithospermum erythrorhizon, inhibit the transcriptional activation of human tumor necrosis factor α promoter in vivo. J Biol Chem 279, 5877–5885 (2004).
Mao, X., Yu, C. R., Li, W. H. & Li, W. X. Induction of apoptosis by shikonin through a ROS/JNK-mediated process in Bcr/Abl-positive chronic myelogenous leukemia (CML) cells. Cell Res 18, 879–888 (2008).
Wang, R., Yin, R., Zhou, W., Xu, D. & Li, S. Shikonin and its derivatives: a patent review. Exp Opin Ther Pat 22, 977–997 (2012).
Malik, S., Bhushan, S., Sharma, M. & Ahuja, P. S. Biotechnological approaches to the production of shikonins: a critical review with recent updates. Crit Rev Biotechnol 36, 327–340 (2016).
Jin, Y. D., Ren, Y., Wu, M. W., Chen, P. & Lu, J. Effect of shikonin on multidrug resistance in HepG2: The role of SIRT1. Pharm Biol 53, 1016–1021, https://doi.org/10.3109/13880209.2014.952836 (2015).
Liu, J. et al. Modulation of orphan nuclear receptor Nur77-mediated apoptotic pathway by acetylshikonin and analogues. Cancer Res 68, 8871–8880 (2008).
Wu, C. P., Calcagno, A. M. & Ambudkar, S. V. Reversal of ABC drug transporter-mediated multidrug resistance in cancer cells: evaluation of current strategies. Curr Mol Pharmacol 1, 93–105 (2008).
Sharom, F. J. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics 9, 105–127 (2008).
Elahian, F., Sepehrizadeh, Z., Moghimi, B. & Mirzaei, S. A. Human cytochrome b5 reductase: structure, function, and potential applications. Crit Rev Biotechnol 34, 134–143 (2014).
Elahian, F., Reiisi, S., Shahidi, A. & Mirzaei, S. A. High-throughput bioaccumulation, biotransformation, and production of silver and selenium nanoparticles using genetically engineered Pichia pastoris. Nanomedicine 13, 853–861 (2017).
Molnar, J. et al. Reversal of multidrug resistance by natural substances from plants. Curr Top Med Chem 10, 1757–1768 (2010).
Chen, J. et al. Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene 30, 4297 (2011).
Pyrko, P., Schönthal, A. H., Hofman, F. M., Chen, T. C. & Lee, A. S. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res 67, 9809–9816 (2007).
Duan, D., Zhang, B., Yao, J., Liu, Y. & Fang, J. Shikonin targets cytosolic thioredoxin reductase to induce ROS-mediated apoptosis in human promyelocytic leukemia HL-60 cells. Free Radic Biol Med 70, 182–193 (2014).
Yang, F. et al. SH-7, a new synthesized shikonin derivative, exerting its potent antitumor activities as a topoisomerase inhibitor. Int J Cancer 119, 1184–1193 (2006).
Rao, Z., Liu, X., Zhou, W., Yi, J. & Li, S. S. Synthesis and antitumour activity of beta-hydroxyisovalerylshikonin analogues. Eur J Med Chem 46, 3934–3941 (2011).
Cui, X. R. et al. Comparison of the cytotoxic activities of naturally occurring hydroxyanthraquinones and hydroxynaphthoquinones. Eur J Med Chem 43, 1206–1215 (2008).
Lu, Q., Liu, W., Ding, J., Cai, J. & Duan, W. Shikonin derivatives: synthesis and inhibition of human telomerase. Bioorganic & medicinal chemistry letters 12, 1375–1378 (2002).
Ahn, B. Z., Baik, K. U., Kweon, G. R., Lim, K. & Hwang, B. D. Acylshikonin analogues: synthesis and inhibition of DNA topoisomerase-I. J Med Chem 38, 1044–1047 (1995).
Ponizovskiy, M. R. Warburg effect mechanism as the target for theoretical substantiation of new possibility cancer disease treatment. Crit Rev Eukaryot Gene Expr 21 (2011).
Kovalev, A. A., Tsvetaeva, D. A. & Grudinskaja, T. V. Role of ABC-cassette transporters (MDR1, MRP1, BCRP) in the development of primary and acquired multiple drug resistance in patients with early and metastatic breast cancer. Exp Oncol 35, 287–290 (2013).
Liu, X. & Sun, G. Shikonin enhances Adriamycin antitumor effects by inhibiting efflux pumps in A549 cells. Oncol Lett 14, 4270–4276 (2017).
Wu, H. et al. Anticancer agent shikonin is an incompetent inducer of cancer drug resistance. PloS one 8, e52706 (2013).
Du, Hw et al. Multiple correlations of mRNA expression and protein abundance in human cytokine profile. Mol Biol Rep 41, 6985–6993 (2014).
Spyrelli, E. D. et al. Metabolic profiling study of shikonin’s cytotoxic activity in the Huh7 human hepatoma cell line. Mol Biosyst 13, 841–851 (2017).
Xuan, Y. & Hu, X. Naturally-occurring shikonin analogues–a class of necroptotic inducers that circumvent cancer drug resistance. Cancer Lett 274, 233–242 (2009).
Hou, X. et al. Drug efflux by breast cancer resistance protein is a mechanism of resistance to the benzimidazole insulin-like growth factor receptor/insulin receptor inhibitor, BMS-536924. Mol Cancer Ther 10, 117–125 (2011).
Nambaru, P. K. et al. Drug efflux transporter multidrug resistance-associated protein 5 affects sensitivity of pancreatic cancer cell lines to the nucleoside anticancer drug 5-fluorouracil. Drug Metab Dispos 39, 132–139 (2011).
Elahian, F. et al. The anticancer agent prodigiosin is not a multidrug resistance protein substrate. DNA and cell biology 32, 90–97 (2013).
Elahian, F., Kalalinia, F. & Behravan, J. Dexamethasone downregulates BCRP mRNA and protein expression in breast cancer cell lines. Oncol Res 18, 9–15 (2009).
Mirzaei, S. A. et al. ABC-transporter blockage mediated by xanthotoxin and bergapten is the major pathway for chemosensitization of multidrug-resistant cancer cells. Toxicol Appl Pharmacol 337, 22–29 (2017).
Acknowledgements
The authors are grateful to Shahrekord University of Medical Sciences for the financial support (grant number: 1393–01–74–2377). They also thank the anonymous reviewers for their thoughtful comments, which have helped to improve the quality of the article.
Author information
Authors and Affiliations
Contributions
F. Elahian and S.A. Mirzaei coordinated the study, designed the experiments and wrote the manuscript. S. Reiisi performed the statistical analyses and participated in intellectual discussions of the data and manuscript writing. A. Shekari (Master degree), P. Ghiasi (Master degree), F. Aliakbari (PharmD degree), and E. Azadfallah (PharmD degree) are students involved in the molecular experiments as part of their theses.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mirzaei, S.A., Reiisi, S., Ghiasi Tabari, P. et al. Broad blocking of MDR efflux pumps by acetylshikonin and acetoxyisovalerylshikonin to generate hypersensitive phenotype of malignant carcinoma cells. Sci Rep 8, 3446 (2018). https://doi.org/10.1038/s41598-018-21710-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-21710-5
- Springer Nature Limited