Abstract
A thorough understanding of starch gelatinization is needed to control starch functional properties for food processing and human nutrition. Here, we reveal the mechanism of structural disassembly of rice, maize and wheat starch granules during thermal transitions in which a Rapid Visco Analyzer (RVA) was used to pre-heat the starches to certain transition points in the differential scanning calorimetry (DSC) heating profiles. This was done to generate sufficient material for structural analyses. The results from DSC, Raman, X-ray diffraction and scanning electron microscopy (SEM) analyses all showed that at the conclusion temperature (Tc) of the DSC endotherm rice starch gelatinization was complete, whereas residual structural order remained in maize and wheat starches. Gelatinization of wheat and maize starch was complete at a temperature higher than Tc in the profile, which we define as the end temperature (Te). We propose that Te would be better to define the completion point of starch gelatinization than Tc.
Similar content being viewed by others
Introduction
Starch is a major component in many foods and the main glycemic carbohydrate in the human diet. Native starch granules are insoluble in cold water and are made up of two α-glucans: lightly branched amylose and highly branched amylopectin. Amylose is proposed to be more concentrated at the periphery1,2 or in the amorphous core area of the starch granules3, although some molecules may be interspersed radially among the amylopectin clusters. Starch granules have a complicated hierarchical structure, which is examined at nano to micrometer scales covering: glucosyl links (0.3 to 0.5 nm); alternating crystalline and amorphous lamellae with a periodicity of 9–11 nm; the “blocklet” structures (20 to 500 nm); the semicrystalline growth rings (120 to 550 nm); intact granules (1 to 100 μm)4,5. The multi-scale structures of granules and the changes they undergo during processing and storage are the major determinants of starch functionality and food quality6,7.
When an aqueous suspension of starch granules is heated above a certain temperature, the granules undergo an irreversible order-disorder transition known as gelatinization. Despite extensive studies, there is still a lack of a clear and agreed understanding of the mechanism of this transition8,9,10,11. Many features of gelatinization are incompletely understood, especially the nature of structural changes that give rise to the endo- and exotherms observed in Differential Scanning Calorimetry (DSC) thermograms. DSC is the most widely applied technique to measure heat of gelatinization of starches, but comparisons between studies on the extent of phase transitions are sometimes difficult due to considerable variations in experimental conditions, especially water content, heating rate and time12,13.
A combination of DSC with X-ray Diffraction (XRD) and Small Angle X-ray Scattering (SAXS) has shown that residual crystallinity and lamellar organization remain at the completion of the gelatinization endotherm9,14. Recent studies using DSC and swelling power measurements show that at water:starch ratios of 2:1 or greater, the gelatinization endotherm represents partial swelling of starch granules rather than full gelatinization15,16. To further understand the nature of the gelatinization endotherm obtained at high water contents, pre-heated wheat starch at different stages of gelatinization was generated using the Rapid Visco Analyzer (RVA) to simulate the DSC heating profiles17. The multi-scale structures of these gelatinized starches were characterized by a combination of XRD, Fourier Transform Infrared Spectroscopy (FTIR), Raman, SAXS, DSC and scanning electron microscopy (SEM), which showed that over a wide range of water content (0.5:1~4:1), considerable long- and short-range molecular orders remain at the conclusion temperature (Tc, i.e., the intersection point on the baseline of a tangent to the part of the DSC trace beyond the peak temperature) of the starch gelatinization endotherm18.
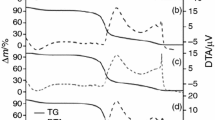
In previous studies, an RVA instrument was used without stirring as a model system to mimic the DSC heating profile and prepare sufficient materials to analyse starch structural changes during gelatinisation and retrogradation17,18. These studies showed that heating starch in the RVA without stirring in different water:starch mixtures of ratios ranging from 0.5 to 4.0 brought about a loss of granular organisation, although some long- and short-range structural order remained at the equivalent of the DSC conclusion temperature. These results, which are consistent with starch gelatinization having been shown to be incomplete after the DSC heating profile15,16, indicate the RVA is useful to model the DSC thermal transitions and provide new insights into structural changes that occur during gelatinisation and retrogradation of starch. In the present study, maize, rice and wheat starches were used to further reveal the nature of DSC endothermic transitions over a wide range of starch:water ratios with the aid of RVA heating. The multi-scale structural changes in these starches were examined after heating in the RVA to Tc and to a higher temperature (Te), which is defined as the point when the DSC trace reaches a flat baseline15,16, as shown in Fig. 1. To the best of our knowledge, this is the first time that the multi-scale structural changes were compared at Tc and Te of DSC endotherm. Our results suggested that Te would be better than Tc to define the point of completion of starch gelatinization.
Materials and Methods
Materials
Starch was isolated from wheat and rice grains as described previously17,18. The amylose contents of wheat and rice starches were 25.0 and 11.5%, respectively, as determined according to the method of Chrastil19. Non-waxy maize starch (27.0% amylose), and amylose (A0512) and amylopectin (A8515) from potato starch, were purchased from Sigma Chemical Co. (St. Louis, Mo., U.S.A.). All other chemical reagents were all of analytical grades.
Differential scanning calorimetry
Thermal properties of starch samples were measured using a Differential Scanning Calorimeter (200F3, Netzsch, Germany) equipped with a thermal analysis data station. Approximately 3.0 mg of native starches were weighed accurately into an aluminum crucible. Distilled water was added to obtain water:starch ratios (v/w) of 0.5, 0.75, 1.0, 1.5, 2.0, 3.0 and 4.0 in the DSC pans, corresponding to water contents of 33.3, 42.9, 50.0, 60.0, 66.7, 75.0 and 80.0%, respectively. The sealed pans were allowed to stand at room temperature for 4 h before analysis. The pans were heated from 20 to 95 °C at a rate of 10 °C/ min. An empty aluminum pan was used as the reference. For the freeze-dried starch samples obtained from RVA, a ratio of water:starch of 5:1 (v/w) was used in the DSC measurements. The thermal transition parameters (onset (To), peak (Tp), conclusion (Tc) temperatures and enthalpy change (ΔH)) were determined from the data recording software. The end temperature (Te), as defined in the introduction, was also determined (Fig. 1).
Preparation of starch samples using a RVA instrument
To obtain sufficient material to characterize structural changes of starch during DSC heating, gelatinized starch samples were prepared by heating starch-water systems in a RVA instrument without stirring under the same heating profile described above for DSC measurements17. Approximately 2.5 g of native starches were weighed accurately into an RVA canister and distilled water was added with a pipette to obtain the water:starch ratios of 0.5, 0.75, 1.0, 1.5, 2.0, 3.0 and 4.0 (v/w). The mixtures in the canister were allowed to stand for 4 h at room temperature before heating. Rice and maize starch-water suspensions were heated separately in the RVA canisters without stirring to To − 10, To, Tp, Tc, Tc + 10, 95 °C. Wheat and maize starch-water suspensions were also heated to Te (Fig. 1). After heating to the designated temperature, the samples were quickly frozen in liquid nitrogen, freeze-dried, ground into a powder, and passed through a 100 μm sieve. A water:starch mixture (4:1, v/w) that was freeze-dried without prior heating was used as a control.
Laser confocal Micro-Raman (LCM-Raman) spectroscopy
Raman spectra of freeze-dried starch samples were measured using a Renishaw Invia confocal Raman microscope system (Renishaw, Gloucestershire, United Kingdom). A 785 nm green diode laser source was used in this study. Spectra were obtained from at least five different positions of each sample in the range of 3200–100 cm−1. The full width at half maximum (FWHM) of the band at 480 cm−1 is used to characterize the short-range molecular order of starch samples20,21.
X-ray diffraction
X-ray diffractograms were obtained using an X-ray diffractometer (XRD-6100, Shimadzu, Tokyo, Japan) with a Cu-Kα source (λ = 0.154 nm) running at 40 kV and 30 mA. All starch samples were stored for one week in a desiccator over a saturated NaCl solution before measurements. The samples were scanned from 4° to 35° (2θ) at a speed of 2°/min and a step size of 0.02°. The relative crystallinity was quantified as the ratio of the crystalline area to the total area between 4° and 35° (2θ) using the Origin software (Version 8.0, Microcal Inc., Northampton, MA, USA).
Scanning electron microscopy (SEM)
The morphology of starch samples was observed using a scanning electron microscope (JSM-IT300LV, JEOL, Japan). Freeze-dried starch samples were mounted on a stub using double-sided adhesive tapes, sputter coated with gold in a sputter coater (JEC-3000FC, Tokyo, Japan). The imaging was performed at an accelerating voltage of 10 kV.
Statistical analysis
Results are reported as the means and standard deviations of at least duplicate measurements. Analyses of variance (ANOVA) by Duncan’s test (p < 0.05) were conducted using the SPSS 19.0 Statistical Software Program (SPSS Inc. Chicago, IL, USA).
Results and Discussion
Thermal properties of native rice and maize starches
The thermal transition parameters of rice and maize starches are presented in Table 1. These two starches displayed gelatinization transitions in the temperature ranges 58.9–72.4 °C (rice) and 64.3–77.2 °C (maize) over a wide range of water contents. With increasing water content, the gelatinization transitions became progressively more pronounced and symmetrical (thermograms not shown). The ∆H of rice starch increased from 2.5 to 12.3 J/g as the water:starch ratio increased from 0.5 to 3, whereas for maize starch ∆H increased from 1.1 to 11.8 J/g with increasing water content from 0.5 to 2.0. The thermal transition temperatures of rice starch did not change greatly with increasing water content. However, Tc of maize starch increased from 72.5 to 77.2 °C as water:starch ratio increased from 0.5 to 2.0, above which it increased slightly. The effect of water content on thermal properties of starch has been studied extensively; the extent of crystallite melting is limited at low water content but increases with increasing water availability11,12,22. From this and other previous studies, it can be seen that the source of starch is a major determinant of its thermal properties9,10,15,23.
Thermal properties of rice and maize starches pre-heated in the RVA
A typical DSC endothermic transition was observed for both starches after they were pre-heated in the RVA at a water:starch ratio of 0.5, with the exception of rice starch pre-heated to 95 °C. However, no endothermic transitions were observed for both starches after pre-heating a water:starch mixture of 0.75:1 in the RVA to 95 °C, nor after pre-heating water:starch mixtures of 1:1 or greater to Tc + 10 or 95 °C. With water-starch mixtures of 2:1, 3:1 and 4:1, endothermic DSC transitions were observed for maize starch after pre-heating to Tc in the RVA, but none were observed for rice starch. The values of Tc for rice and maize starches increased as the temperature of RVA pre-heating was increased (Fig. 2a,c). Similar results were obtained in a previous study on wheat starch17,24.
The enthalpy change (∆H) reflects primarily the disruption of long-range starch crystallinity during DSC heating17. The ∆H of all starch samples decreased markedly with increasing temperature of RVA pre-heating (Fig. 2b,d), indicative of the gradual disruption of starch crystallites during heating in the presence of water. As the water:starch ratio was 2:1 or greater, there was no measurable enthalpy change for the rice starch samples, indicating that starch crystallites were completely disrupted. However, values of ∆H were obtained for the maize starch samples pre-heated to Tc in water-starch mixtures of 2:1 or greater.
Short- and long-range structural order of rice and maize starch
The changes in short- and long-range structural order of the two starches during DSC heating, as modelled in the RVA, were monitored by Raman, and XRD spectra. The intensity of the Raman bands decreased progressively with increasing end temperature of RVA pre-heating (Figure not shown). The full width at half maximum (FWHM) of the band at 480 cm−1 of all rice (Fig. 3a) and maize starch samples (Fig. 3b) increased with increasing end temperature of RVA pre-heating. It is interesting to note that the FWHM for maize starch did not change greatly with RVA pre-heating to Tp, but increased steadily from Tp to 95 °C, indicating that there was some short-range structural order at Tc, the conclusion point of thermal transitions. For rice starch, there was a small increase in FWHM after RVA pre-heating to Tp, and FWHM continued to increase as the temperature of RVA pre-heating was raised to Tc, above which it remained essentially unchanged.
All of the water:starch mixtures presented decreasing relative crystallinity with increasing end temperature of RVA pre-heating (Fig. 3c,d). After pre-heating water:rice starch mixtures of 1:1 or higher to Tc, the relative crystallinity was very low (about 5%), indicating that at Tc of the gelatinization endotherm, crystallites in rice starch were essentially all disrupted. However, with all maize starch samples after pre-heating to Tc in the RVA, the typical A-type X-ray diffraction patterns could still be observed, with residual crystallinity of 9 to 21% (Fig. 3d). The relative crystallinity of maize starch only decreased sharply with pre-heating to temperatures above Tp.
Granular morphology of starch samples
SEM micrographs of rice and maize starch samples at a water:starch ratio of 2:1 after pre-heating to different temperatures are shown in Fig. 4. No significant differences were noted in granular morphology between native starch and starch samples after pre-heating to To (Fig. 4a,A). After pre-heating to Tp, many rice starch granules were deformed and disrupted (Fig. 4b), whereas maize starch showed fewer changes in granular morphology (Fig. 4B). There were still clear granules in maize starch samples after pre-heating to Tc (Fig. 4C), while no intact starch granules were observed in rice starch samples (Fig. 4c). All granules were disrupted after pre-heating to 95 °C (Fig. 4d,D). The morphology changes of both rice and maize starches were generally consistent with DSC, and Raman results.
SEM images of rice and maize starch-water mixtures of 1:2 after pre-heating to different temperatures. (a) rice starch pre-heated to To, (b) rice starch pre-heated to Tp, (c) rice starch pre-heated to Tc, (d) rice starch pre-heated to 95 °C. (A) maize starch pre-heated to To, (B) maize starch pre-heated to Tp, (C) maize starch pre-heated to Tc, (D) maize starch pre-heated to 95 °C. Magnification, 4000× (rice starch), 2000× (maize starch).
From the present study and our previous study17, we found that gelatinization of rice starch at a water:starch ratio of 2:1 or greater was complete at Tc of DSC thermal transition, whereas this was not observed for maize and wheat starches. Hence, further experiments were performed to define when complete gelatinization of these starches occurs. Accordingly, wheat and maize starch samples were prepared by pre-heating water:starch mixtures of 2.0 to 4.0 in the RVA to Tc and Te. Thermal properties of wheat and maize starch samples after pre-heating to Tc and Te were noticeably different (Table 2). After pre-heating to Tc, there were significant enthalpy changes of both starch samples on subsequent heating in the DSC, with typical DSC endothermic transitions being observed (Fig. 5, blue circle). However, enthalpy changes were not detected for both starches after pre-heating to Te.
The short- and long-range ordered structures after pre-heating to Tc and Te were compared. Both starch samples presented similar Raman and XRD spectra (Fig. 6a,c). The intensity of the Raman bands decreased significantly for both starches pre-heated to Te compared with pre-heated to Tc (Fig. 6a), indicating greater disruption of starch crystallites after pre-heating to Te than to Tc. The FWHMs (Fig. 6b) of water-starch mixtures of 2:1 to 4:1 that were preheated to Te was higher than to Tc.
The diffractograms of water-starch mixtures of 2:1 to 4:1 after pre-heating to Tc and Te differed significantly (Fig. 6c). After pre-heating to Tc, all starch samples showed the typical A-type X-ray diffraction patterns, whereas the diffraction peaks almost disappeared after pre-heating to Te. The relative crystallinity was lower for samples pre-heated to Te than for samples pre-heated to Tc (Fig. 6d), consistent with the results of Raman and DSC. These observations indicated that greater disruption of starch ordered structures at Te occurred than at Tc.
Structural disruption of rice starch commenced at the onset temperature of the gelatinization endotherm and was essentially completed at the conclusion temperature. During the gelatinization endotherm, multiscale structures in rice starch were disrupted progressively with increasing temperature from To to Tc. From this result, we can conclude that the gelatinization endotherm of rice starch obtained at a water:starch ratio of 2:1 or greater represents the full gelatinization of rice starch. However, no structural order disruption occurred at To and some structural order in maize starch was still present at the conclusion temperature (Tc) of the DSC endothermic transition obtained when the ratio of water:starch was 2:1 or greater. Further disruption of structural order occurred on heating beyond Tc. This observation was consistent with the previous findings that residual crystallinity still remained at Tc9,14,17. Hence, we can conclude that gelatinization of maize and wheat starches did not commence at To and was not complete at Tc of the gelatinization endotherm. After pre-heating to Te, the multiscale structures of wheat and maize starches were completely disrupted, indicating a complete gelatinization at Te. Hence, we propose that Te of starch gelatinization endotherm can be defined as conclusion temperature of DSC gelatinization endotherm.
The mechanism of the molecular disassembly of starch granules during DSC thermal transition has attracted much research attention11,25. At low to intermediate water content (<67% water; i.e., water starch mixtures of 2:1 or lower), the gelatinization endotherm represents limited swelling of starch granules rather than full gelatinization of starches15,16,17. In water:starch mixtures of 2:1 or higher, the gelatinization endotherm determined by DSC has been considered to represent complete gelatinization of starches since pioneering worked of Donovan22. However, more recent studies have provided evidence that even at a high water content in water:starch mixtures (>67% or water:starch ratio of 2:1), the gelatinization endotherm of wheat and pea starches does not represent full gelatinization16,17,26.
Conclusions
In the present study, the extent of structural changes in starch at temperatures corresponding to key transition points in the DSC endotherm was investigated. To this end, an RVA was used without stirring to mimic DSC heating profiles and obtain sufficient amounts of starch for subsequent analyses by DSC, Raman, XRD and SEM. For rice starch, there was essentially no residual structural order remaining in water-starch mixtures of 2:1 to 4:1 after heating to Tc, indicating the endotherm represents the complete gelatinization. However at Tc, maize and wheat starches still had appreciable amounts of long- and short-range structural order, indicative of incomplete gelatinization. Further disruption of ordered structures occurred when wheat and maize starches were heated beyond Tc to Te, the temperature at which the DSC trace reaches a flat baseline. Hence, we conclude that gelatinization of wheat and maize starches starts after To and is more complete at Te in water:starch mixtures of 2:1 or greater. As starch gelatinization properties are often measured in such mixtures, we propose that Te can be defined as the end point of complete gelatinization.
Change history
16 April 2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
Jane, J. Current understanding on starch granule structures. J. Appl. Glycosci. 53, 205–213 (2006).
Tester, R. F., Karkalas, J. & Qi, X. Starch-composition, fine structure and architecture. J. Cereal Sci. 39, 151–165 (2004).
Wang, S., Blazek, J., Gilbert, E. & Copeland, L. New insights on the mechanism of acid degradation of pea starch. Carbohydr. Polym. 87, 1941–1949 (2012).
Wang, S. & Copeland, L. Effect of acid hydrolysis on starch structure and functionality: a review. Crit. Rev. Food Sci. Nutr. 55, 1081–1097 (2015).
Pérez, S. & Bertoft, E. The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch/Staerke 62, 389–420 (2010).
Wang, S. et al. Molecular order and functional properties of starches from three waxy wheat varieties grown in China. Food Chem. 181, 43–50 (2015).
Copeland, L., Blazek, J., Salman, H. & Tang, M. C. Form and functionality of starch. Food Hydrocolloid. 23, 1527–1534 (2009).
Cooke, D. & Gidley, M. J. Loss of crystalline and molecular order during starch gelatinisation: origin of the enthalpic transition. Carbohydr. Res. 227, 103–112 (1992).
Jenkins, P. J. & Donald, A. M. Gelatinisation of starch: A combined SAXS/WAXS/DSC and SANS study. Carbohydr. Res. 308, 133–147 (1998).
Ratnayake, W. S. & Jackson, D. S. A new insight into the gelatinization process of native starches. Carbohydr. Polym. 67, 511–529 (2007).
Wang, S. & Copeland, L. Molecular disassembly of starch granules during gelatinization and its effect on starch digestibility: a review. Food Funct. 4, 1564–1580 (2013).
Patel, B. K. & Seetharaman, K. Effect of heating rate at different moisture contents on starch retrogradation and starch-water interactions during gelatinization. Starch/Staerke 62, 538–546 (2010).
Schirmer, M., Jekle, M. & Becker, T. Starch gelatinization and its complexity for analysis. Starch/Staerke 67, 30–41 (2015).
Vermeylen, R. et al. Gelatinization of starch in excess water: Beyond the melting of lamellar crystallites. A combined wide- and small-angle X-ray scattering study. Biomacromolecules 7, 2624–2630 (2006).
Wang, S., Li, C., Yu, J., Copeland, L. & Wang, S. Phase transition and swelling behaviour of different starch granules over a wide range of water content. LWT - Food Sci. Technol. 59, 597–604 (2014).
Wang, S. & Copeland, L. Phase transitions of pea starch over a wide range of water content. J. Agric. Food Chem. 60, 6439–6446 (2012).
Wang, S., Zhang, X., Wang, S. & Copeland, L. Changes of multi-scale structure during mimicked DSC heating reveal the nature of starch gelatinization. Sci. Rep. 6, 28271 (2016).
Wang, S., Li, C., Zhang, X., Copeland, L. & Wang, S. Retrogradation enthalpy does not always reflect the retrogradation behavior of gelatinized starch. Sci. Rep. 6, 1–10 (2016).
Chrastil, J. Improved colorimetric determination of amylose in starches or flours. Carbohydr. Res. 159, 154–158 (1987).
Mutungi, C., Passauer, L., Onyango, C., Jaros, D. & Rohm, H. Debranched cassava starch crystallinity determination by Raman spectroscopy: Correlation of features in Raman spectra with X-ray diffraction and 13C CP/MAS NMR spectroscopy. Carbohydr. Polym. 87, 598–606 (2012).
Flores-Morales, A., Jiménez-Estrada, M. & Mora-Escobedo, R. Determination of the structural changes by FT-IR, Raman, and CP/MAS 13C NMR spectroscopy on retrograded starch of maize tortillas. Carbohydr. Polym. 87, 61–68 (2012).
Donovan, J. W. Phase transitions of the starch–water system. Biopolymers 18, 263–275 (1979).
Chen, P. et al. Internal structures and phase-transitions of starch granules during gelatinization. Carbohydr. Polym. 83, 1975–1983 (2011).
Wang, S., Wang, S., Guo, P., Liu, L. & Wang, S. Multiscale structural changes of wheat and yam starches during cooking and their effect on in vitro enzymatic digestibility. J. Agric. Food Chem. 65, 156–166 (2016).
Ratnayake, W. S. & Jackson, D. S. Starch gelatinization. Advances in Food and Nutrition Research 55, 221–268 (2008).
Randzio, S. L., Flis-Kabulska, I. & Grolier, J. P. E. Reexamination of phase transformations in the starch-water system. Macromolecules 35, 8852–8859 (2002).
Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (31522043) and Tianjin Natural Science Foundation for Distinguished Young Scholar (17JCJQJC45600).
Author information
Authors and Affiliations
Contributions
Shujun W. and Shuo W. conceived and designed the study. C.C., F.X. and X.Z. conducted the experiments and wrote the manuscript. Shujun W. and L.C. revised and edited the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, S., Chao, C., Xiang, F. et al. New insights into gelatinization mechanisms of cereal endosperm starches. Sci Rep 8, 3011 (2018). https://doi.org/10.1038/s41598-018-21451-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-21451-5
- Springer Nature Limited
This article is cited by
-
Crystal structure transformations in extruded starch plasticized with glycerol and urea
Polymer Bulletin (2020)
-
Effect of the Addition of Citric Acid and Whey Protein Isolate in Canna indica L. Starch Films Obtained by Solvent Casting
Journal of Polymers and the Environment (2020)