Abstract
Leaf photosynthesis is regulated by multiple factors that help the plant to adapt to fluctuating light conditions. Leaves of sun-light-grown plants are thicker and contain more columnar palisade cells than those of shade-grown plants. Light-induced chloroplast movements are also essential for efficient leaf photosynthesis and facilitate efficient light utilization in leaf cells. Previous studies have demonstrated that leaves of most of the sun-grown plants exhibited no or very weak chloroplast movements and could accomplish efficient photosynthesis under strong light. To examine the relationship between palisade cell shape, chloroplast movement and distribution, and leaf photosynthesis, we used an Arabidopsis thaliana mutant, angustifolia (an), which has thick leaves that contain columnar palisade cells similar to those in the sun-grown plants. In the highly columnar cells of an mutant leaves, chloroplast movements were restricted. Nevertheless, under white light condition (at 120 µmol m−2 s−1), the an mutant plants showed higher chlorophyll content per unit leaf area and, thus, higher light absorption by the leaves than the wild type, which resulted in enhanced photosynthesis per unit leaf area. Our findings indicate that coordinated regulation of leaf cell shape and chloroplast movement according to the light conditions is pivotal for efficient leaf photosynthesis.
Similar content being viewed by others
Introduction
The blue-light (BL) receptor, phototropin (phot), regulates phototropism, chloroplast movement, stomatal opening, and leaf movement and development, all of which ensure efficient light utilization for photosynthesis1,2. Light-induced chloroplast movement (hereafter, referred to as “chloroplast movement”) is found in various plant species, including algae and land plants3. Under low light conditions, chloroplasts move toward light-irradiated area (the “accumulation response”) and are situated on the periclinal cell walls, ensuring the capture of maximum amount of light. Conversely, chloroplasts escape from strong light (the “avoidance response”) and consequently localize on the anticlinal cell walls where light absorption is low. The avoidance response is essential for avoiding the photodamage and for survival under the natural strong light conditions4. The avoidance response is also implicated in facilitating the penetration of light into deeper cells and diffusion of CO2 from air spaces into the mesophyll chloroplasts as a result of increase in the surface area of chloroplasts exposed to intracellular air spaces5.
Chloroplast movement can be detected by measuring the light-induced changes in leaf transmittance (or absorption)6,7. A decrease in leaf transmittance represents the chloroplast accumulation response and an increase reflects the avoidance response. This method of detection is easy and non-invasive and has been used to analyze chloroplast movements in various plant species, including flowering plants, ferns, and mosses8,9,10,11,12,13,14,15,16. Although the magnitude of chloroplast movement varies among the plant species, shade-grown plants tend to exhibit stronger chloroplast movement than sun-grown plants8,9,10,11,13,16. However, some ferns that can grow under a wide range of light conditions have been observed to exhibit stronger chloroplast movement than those that grow under limited light conditions or in a shade12. Notably, no or very subtle chloroplast movements were detected in some sun-grown plants, including climbing plant species8,13,16. In general, the mesophyll cells in palisade cell layers are spherical in the leaves of shade-grown plants. Leaves of sun-grown plants are thicker than those of shade-grown plants. Because the palisade mesophyll cells are more columnar, one or more tiers of columnar palisade cells could facilitate penetration of light into deeper cell layers5,17,18. The more columnar cells in the sun-grown leaves restrict the chloroplast movements and most of the chloroplasts remained aligned on the anticlinal walls regardless of the light conditions5,13,16. The sun-grown leaves should contain more cells and, thus, more chloroplasts per unit leaf area, because total plasma membrane area of the columnar cells per unit leaf area would be larger than that of the spherical cells in the shade-grown plants16. Therefore, the presence of more columnar cells in the sun-grown leaf could contribute to the higher photosynthetic performance per unit leaf area. The constitutive positioning of chloroplasts on the anticlinal walls could be beneficial under strong light for leaf photosynthesis by facilitating the penetration of light into deeper cell layers5,17,18 or CO2 diffusion5,19.
To substantiate the fact that chloroplast movements are restricted in the columnar palisade cells, we analyzed the relationship between the shape of palisade cells and chloroplast movement in the same plant species grown under the same light condition. We used Arabidopsis thaliana angustifolia (an) mutant plants20. The an mutant plants exhibit narrower and thicker leaves although the length of the leaf blade is similar to those of the wild-type (WT) plants. This leaf phenotype in an mutants is caused by the reduction in the size of palisade cells in the direction of leaf width, accompanied with an increase in cell size in the direction of leaf thickness, indicating that the palisade cells in the an mutants are more columnar than those in the WT20. However, the total number of cells in the leaves of WT and an mutant plants is similar20. Thus, the cell structure in the an mutant leaves mimics that of sun-grown leaves, except for the narrow leaf width. As a control for the narrow leaf mutant, we used another narrow leaf mutant, an321. The an3 mutant leaves look like the an mutant leaves, but the narrow leaf phenotype in an3 is attributable to the severe reduction in the number of cells in the leaves21. The size of cells in leaves is larger in an3 mutants compared to that in the WT21. Here, we compared leaf photosynthesis and chloroplast movements between WT, an, and an3 mutant plants.
Results
Leaves of an mutant plants have several characteristics similar to those of sun-grown-leaves
When WT, an, and an3 mutant plants were grown under white light condition (at 120 µmol m−2 s−1), the rosette size was similar in the WT and an3 mutant plants, but was smaller in the an mutant plants (Fig. 1a). Both the an and an3 mutant leaves were narrower than the WT leaves (Fig. 1a and b), as described previously20,21. Consistently, the total leaf area and leaf weight were smaller in the an and an3 mutants compared to that in the WT plants (Fig. 1c and d). The value of the specific leaf area (SLA), which is the ratio of total leaf area to the fresh weight, in both the an and an3 plants was small (Fig. 1e). The lower values of SLA generally mean that the leaves are thicker. Indeed, the leaves of an mutant plants were much thicker than those of WT (Fig. 1f and g), as described previously20. The an3 leaves were also thicker than the WT leaves although they were thinner than the an leaves (Fig. 1f). Compared to the WT leaves, the periclinal cell size of the first palisade cells was slightly smaller, but the anticlinal cell size was much larger in the an leaves (Fig. 1g and Table 1). The an palisade cells were much longer in the direction of leaf thickness (Fig. 1g and Table 1) and, thus, displayed a columnar shape, as described previously20. Consistent with the previous results21, the palisade cells of the an3 leaves had larger cell size at both the periclinal and anticlinal sides (Fig. 1g and Table 1). The length of palisade cells in the an3 leaves in the direction of leaf thickness was intermediate between the lengths in the WT and an leaves (Fig. 1g and Table 1). Under the growth conditions used in this study, the an mutant leaves often contained two layers of the palisade cells, but the WT and an3 leaves contained only one layer (Fig. 1g). Therefore, the an mutant leaves are somewhat similar to the sun-grown leaves in that they are thick and have columnar palisade cells, although the an3 mutant leaves are also thick but have less columnar cells.
Altered leaf morphology in an and an3 mutants. (a) Photograph of 42-day-old plants. Scale bar = 2 cm. (b) Photographs of leaves detached from 42-day-old plants. The left leaf is the youngest and the right is the oldest one (it is one of the cotyledons). Scale bar = 2 cm. (c–e) Total leaf area (c), aboveground fresh weight (d), and specific leaf area (SLA, projected leaf area per unit leaf fresh weight: total leaf area/aboveground fresh weight) (e) of 42-day-old wild-type (WT) and mutant plants. Data show the mean ± SEM (n = 24) of three independent experiments. Significant differences (P < 0.05, Tukey-Kramer) are indicated by different characters. (f) Thickness of leaves in the WT and mutant plants. Data show the mean ± SEM (n = 9) of three independent experiments. Significant differences (P < 0.05, Tukey-Kramer) are indicated by different characters. (g) Leaf cell morphology and chloroplast distribution in an and an3 mutants. Wild type (WT) and mutant plants were grown under white light condition (120 µmol m−2 s−1) for 42 days. Views of the upper surface of palisade tissue cells (upper panel) and cross sections (lower panel) of the leaves from the WT and the mutant plants are shown. Scale bar = 20 µm.
Photosynthesis per unit leaf area is enhanced in an and an3 mutant leaves
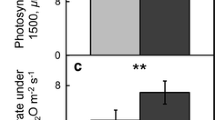
Consistent with the presence of thicker leaves in the an mutant plants, the light absorbance by these leaves was much higher than in the WT (Fig. 2a). However, the light absorbance by the leaves of the an3 mutant plants was slightly lower than that in the WT plants (Fig. 2a). Thus, photosynthetic light utilization could be different between the WT and mutant plants. Under the growth conditions used in this study, the maximum quantum yield of photosystem II (PSII), Fv/Fm, was normal in all the lines (Fig. 2b), indicating that the an and an3 mutant plants had no detectable defects in the electron transport around PSII and there was no damage to the PSII under the experimental conditions. Consistently, the levels of ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (RbcL), PsaA (a core protein of photosystem I), PsbB (CP47 protein of photosystem II), cytochrome f protein of the thylakoid Cyt b6/f-complex (Cyt f), and plastocyanin (PC) did not differ between the WT and mutant plants (Supplemental Figs 1 and 2). However, differences were observed in the chlorophyll content and maximum CO2 assimilation rates between the WT and the mutant plants (Fig. 2c to f). When the chlorophyll a and b contents were measured on the basis of unit leaf area, there was no difference in the chlorophyll a/b ratio (the values of mean ± SEM for WT, an, and an3 were 2.32 ± 0.36, 2.43 ± 0.23, and 2.69 ± 0.10, respectively). However, the values of total chlorophyll content per leaf area were higher in the an mutant plants (Fig. 2c). Concomitant with the higher chlorophyll contents, the CO2 assimilation rate per leaf area was much higher in the an mutant plants (Fig. 2e). Unexpectedly, an3 mutants also exhibited higher chlorophyll contents and CO2 assimilation rate per leaf area although the values were lower than those in the an mutant plants (Fig. 2e). Importantly, the values of total chlorophyll content and CO2 assimilation rate per SLA were comparable between the WT and mutant plants (Fig. 2d and f). Therefore, the enhanced photosynthesis per leaf area in the an and an3 mutants should be attributable to the thicker leaves and/or the altered structure of leaf cells.
Photosynthetic performance of an and an3 mutants. (a) Difference spectra of leaf absorbance between the wild-type (WT) and mutant plants. Leaf absorbance was measured under white light (120 µmol m−2 s−1). The difference in multi-wavelength (350–800 nm) absorbance was calculated by subtracting the absorbance of each mutant from that of the WT. Data show the mean ± SEM of three independent experiments. (b) Maximum photochemical efficiency of PSII (Fv/ Fm) of the leaves in WT, an, and an3 mutant plants. After keeping the leaves in dark for at least 1 h, Fv/Fm was measured. Data show the mean ± SEM of three independent experiments. (c–f) Chlorophyll content and photosynthetic capacity. (c and d) Total chlorophyll content of leaves in the WT and mutant plants. The chlorophyll content of rosette leaves of 42-day-old plants was determined. (e and f) Maximum CO2 assimilation capacity (Amax) in WT, an, and an3 mutant plants. Amax was calculated from each light saturation point. The chlorophyll content and photosynthetic capacity are expressed per leaf area (c and e) or per SLA (d and f). SLA was calculated using detached leaves. Data show the mean ± SEM of three independent experiments.
Light-induced chloroplast movements are restricted in an mutants but not in an3 mutants
The BL-induced chloroplast movements were analyzed by measuring the light-induced changes in leaf transmittance22. In WT plants, weak BL (3 µmol m−2 s−1) induced a decrease in leaf transmittance as a result of the chloroplast accumulation response whereas an increase in the leaf transmittance was induced by strong BL (20 and 50 µmol m−2 s−1) as a result of the avoidance response (Fig. 3a and b). After the strong blue light was turned off, a rapid decrease in leaf transmittance was induced (which is referred to as the “dark recovery response”)23. The chloroplast movements were almost normal in the an3 mutant plants (Fig. 3a). The speed (the average of the changes in transmittance over 1 min for 2–6 min after changes in the light fluence rates) of accumulation, avoidance, and dark recovery responses were not significantly different from those in WT (Fig. 3b; one-way ANOVA followed by Tukey–Kramer multiple comparison post hoc test, P > 0.5 in all the light treatments), although the amplitude of the avoidance response at 20 µmol m−2 s−1 was smaller in the an3 mutant plants (Fig. 3a). Conversely, in the an mutant plants, the light-induced changes in leaf transmittance were severely attenuated (Fig. 3a). The accumulation, avoidance, and dark recovery responses were detectable, but both the speed and amplitude of these responses were strongly suppressed in the an mutant plants (Fig. 3a and b; one-way ANOVA followed by Tukey–Kramer multiple comparison post hoc test, P < 0.01 in all the light treatments). The leaf transmittance before and after the weak BL irradiation was much lower in the an mutant plants than in the WT, consistent with thicker leaves in the an mutant plants (Fig. 3c; one-way ANOVA followed by Tukey–Kramer multiple comparison post hoc test, P < 0.01). Therefore, these results indicate that the shape of palisade cells, but not the cell volume, is an important factor in the restriction of chloroplast movements.
Light-induced chloroplast movements and intracellular distribution in an and an3 mutants. (a) Changes in leaf transmittance caused by light-induced chloroplast movements in wild-type (WT), an, and an3 mutant plants. Coloured boxes on the horizontal axis indicate passage of time (one box denotes 10 min) and light conditions. After 10 min exposure to darkness (indicated by black boxes), the leaves were sequentially irradiated with blue light (BL) at 3, 20, and 50 µmol m−2 s−1 for 60, 40, and 40 min (indicated by white, sky blue, and blue boxes, respectively). The light was turned off at 150 min. (b) The average of the changes in transmittance over 1 min was calculated by measuring the changes in the leaf transmittance rates for 2–6 min after changes in the light fluence rates (3, 20, and 50 µmol m−2 s−1 or dark). (c) Leaf transmittance at 0 and 70 min (i.e., 60 min after weak BL irradiation) after the onset of measurement of leaf transmittance changes. Data in a to c show the mean ± SEM of three independent experiments. (d) Chloroplast distribution in the WT and mutant plants irradiated with weak BL (3 µmol m−2 s−1) and strong BL (50 µmol m−2 s−1) for 3 h. Views of the upper surface of palisade tissue cells (upper panel) and cross sections (lower panel) of leaves from the WT and mutant plants are shown. Scale bar = 20 µm.
To examine the distribution of chloroplasts in the low- or high-light-irradiated WT and mutant plants (Fig. 3d), the number and plan area of chloroplasts on both the periclinal and anticlinal walls was measured and the rate in terms of the number or area that the chloroplasts occupied on the periclinal or anticlinal walls (the “occupancy rates of chloroplast number” or “occupancy rates of chloroplast area”, respectively; see Methods for details) was calculated (Table 2)16. In all the lines, both the accumulation and avoidance responses were induced in the low- and high-light-irradiated plants, respectively (Fig. 3d), but the occupancy rates of chloroplast number and area were different between the WT and mutant plants (Table 2). On the periclinal walls of the an3 palisade cells, the rate of chloroplast number was higher than that in the WT, irrespective of the light conditions, and the rate of chloroplast area was higher in the high light-irradiated plants, but was similar to that of the WT in the low light-irradiated plants. The rate of chloroplast area on the anticlinal walls was also lower in the an3 mutant plants, irrespective of the light conditions. The occupancy rates, based on chloroplast area, on the periclinal walls in the an mutants were significantly lower than those in the WT only under high light conditions. However, under both low and high light conditions, the occupancy rates, according to the number of chloroplasts, on the periclinal walls in the an mutants were much lower than those in the WT, indicating that most of the chloroplasts in the an mutants resided on the anticlinal walls. Therefore, these results suggest that the chloroplast movement and distribution pattern in the an mutant plants are similar to those in the sun-grown plants, including the climbing plant species.
Discussion
Previous studies, using various plant species, have led to an assumption that more columnar cells could restrict the chloroplast movement8,9,10,11,13,16. To test this assumption, we used the Arabidopsis an mutant plants as a model system. Our findings indicate that the cell shape determines how easily chloroplasts can move in response to light.
The present and previous analyses of an leaves indicate that the an leaves are thicker than the WT leaves and the thick leaves result from more columnar cells and more cell layers (Fig. 1 and Table 1)20. The an3 mutant plants are defective in the proliferation of leaf cells and the palisade cells are larger than those of WT21, because of “compensation” mechanism in which cell proliferation and expansion are tightly regulated to ensure appropriate organ size24. In addition to previous analysis of leaf paradermal sections21, our analysis of the leaf cross sections indicates that the an3 palisade cells are longer in all the directions and, thus, does not show columnar shape in contrast to an palisade cells (Fig. 1 and Table 1). Furthermore, the an3 leaves are thicker than the WT leaves although they are thinner than the an leaves. Because there was no increase in the number of cell layers in the an3 mutants (Figs 1g and 3d), the increase in leaf thickness should have resulted from the larger palisade cells.
The an mutant plants showed much higher leaf absorption, chlorophyll content, and photosynthetic performance per unit leaf area (Fig. 2), indicating that thick leaves consisting of columnar cells facilitate photosynthetic light capture and, thus, enhance the photosynthetic performance. In sun-grown plants, especially in climbing plants, most chloroplasts are constitutively localized on the anticlinal walls irrespective of the light conditions16. Because the palisade cells are highly columnar, the periclinal area is very small and, thus, the accumulation response is not effective in these plants. The constitutive localization of chloroplasts on the anticlinal walls should facilitate photoprotection under strong light conditions, in the location where climbing plants are living16. It should also facilitate penetration of light into the deeper cell layers5,17,18. Although it was not prominent, compared to that in the climbing plants, a higher percentage of chloroplasts were localized on the anticlinal walls in the an mutant plants (Table 2). Indeed, consistent with previous analysis in sun-grown plants5,13,16, the light-induced changes in leaf transmittance was severely attenuated in the an mutant plants (Fig. 3). This phenotype in the an mutant plants were similar to those in the plastid movement impaired 1 (pmi1) mutant plants25,26. The chloroplast movement is dependent on actin filaments27 and PMI1 is necessary for the regulation of actin filaments during the light-induced chloroplast movement26. However, unlike in the an mutant leaves, leaf morphology and transmittance are normal in the pmi1 mutants25,26, indicating that defects in the leaf transmittance change between the an and pmi1 mutant plants are caused by different mechanisms. AN is a plant homolog of CtBP/BARS that functions as a transcriptional corepressor or regulator of membrane trafficking in mammals28,29. Although the exact function of plant AN proteins is unknown, the Arabidopsis AN protein is implicated in the vesicle trafficking30 and post-transcriptional regulation31. However, only a small number of genes was derepressed in the non-stressed an mutants28. Consistently, the phototropin protein level was normal in the an mutants (Supplemental Figs 1 and 2). Therefore, it is likely that the reduced light-induced changes in leaf transmittance in an mutants could be caused by the altered leaf cell geometry but not by the defects in the molecular mechanism for chloroplast movements. The an3 mutants exhibited almost normal light-induced changes in leaf transmittance although slightly higher number of chloroplasts still reside on the peliclinal wall under HL conditions (Table 2). The an3 mutant cells are larger and, thus, have more space for chloroplasts to move than WT and an mutant. However, at least in our experimental time scale (i.e., blue light irradiation for 40~60 min), the difference in the light-induced changes in leaf transmittance between WT and an3 might have not been detected. Nevertheless, an3 exhibited normal light-induced changes in leaf transmittance although their leaves are thick and the palisade cells are longer in the direction of leaf thickness as in the case of an mutants. Therefore, restricted chloroplast movement should be attributable to more columnar cells in the an mutants. In more columnar cells, chloroplasts could be appressed to the anticlinal walls, as suggested previously5.
In conclusion, the shape of cells in the leaves strongly affects the movement and distribution of chloroplasts. The coordination between the cell shape and chloroplast distribution is essential for efficient leaf photosynthesis and, thus, for the adaptation to ambient light conditions. The thick an-like leaves, that have long palisade cells and the greater amount of chloroplasts per unit area, are clearly beneficial to plants that are always exposed to strong light, for example the climbing plants. However, under weak light conditions, cells in the deeper layers can not capture light efficiently and perform efficient photosynthesis there because a large part of light could be used only in the first palisade cell layer in the an-like leaves. Importantly, it was shown in multiple plant species, including Arabidopsis32, that strong light makes palisade cells more columnar. In A. thaliana, this light-dependent palisade cell development is regulated in phototropin2- and photosynthesis-dependent manners32. Thus, phototropins enhance leaf photosynthesis by regulating cell development as well as chloroplast positioning in leaves.
Methods
Plant materials and growth conditions
The Arabidopsis thaliana WT, an (an-1)20 and an3 (an3-4)21 plants used in this study were in the Columbia-0 background. For growth analysis, leaf anatomy, and photosynthetic measurements, plants were grown in soil in a controlled growth chamber (at 22 °C, 55% relatively humidity, and 8 h day light conditions) under white light at 120 µmol m−2 s−1. For the measurement of light-induced changes in leaf transmittance, seedlings were cultured on 0.8% agar medium containing 1/3 strength Murashige and Skoog’s salt and 1% sucrose, and grown under white light at ca. ~100 µmol m−2 s−1 (16 h)/dark (8 h) cycle at 23 °C in an incubator.
Analysis of spectral light absorbance
For measurements of the spectral light absorbance in a whole leaf, detached leaves from 3-week-old plants were placed on the surface of 1% gellan gum and irradiated with white light at 120 µmol m−2 s−1 for 3 h. The absorbance at wavelengths ranging from 300 to 800 nm was measured at every 1 nm with a microplate reader (Multiskan GO, ThermoFisher).
Quantification of plant biomass and leaf thickness
Immediately after the fresh weights of all the rosette leaves of 6-week-old plants were measured, their photographs were taken. Leaf thickness was measured from microscopic images (TCS SP8, Leica) of sections of leaves from the 6-week-old plants. The measurements of leaf area and thickness were carried out with Image J (National Institutes of Health).
Analysis of CO2 assimilation
CO2 assimilation in intact leaves was analyzed with an open gas exchange system (Li-6400, LI-COR) attached to a normal chamber (LI-COR). After the plants were dark-adapted for at least 3 h, the measurements were performed under a controlled atmospheric conditions (temperature 22 °C, relative humidity 50–60%, and a CO2 concentration of 400 µl L−1). The light response curve of photosynthesis was obtained according to the protocol provided by the manufacturer, and was used for determining the saturation value of CO2 assimilation. The value of Amax was calculated as the average maximum net photosynthesis.
Estimation of chlorophyll content
Chlorophylls were extracted from four or five full-expanding leaves with 80% acetone. The OD of the clarified chlorophyll extracts were measured at 645 nm and 663 nm and the chlorophyll content was calculated as described in a previous report33.
Immunoblot analysis
Total protein was extracted from the rosette leaves of 6-week-old plants in a protein extraction buffer that contained 50 mM Tris⋅HCl (pH 7.5), 100 mM NaCl, 5 mM EDTA, 0.5% Triton X-100, 1 mM DTT, and 1 mM PMSF. After SDS-PAGE on 12% acrylamide gels, the proteins were transferred to polyvinylidene fluoride membranes. Antibodies against phot134, phot235, RbcL, PsaA, PsbB, Cyt f, and PC (Agrisera, Vännäs, Sweden) were used for western blotting experiments.
Analyses of chloroplast photorelocation movements
Chloroplast photorelocation movements were analyzed by measuring the light-induced changes in leaf transmittance, as described previously22. The third leaves were excised from 16-day-old seedlings and placed on 1% (w/v) gellan gum in a 96-well plate. The leaves were dark-adapted for at least 1 h and were used for the measurement of transmittance.
Observation of chloroplast distribution patterns
Three-week-old plants were irradiated with weak BL and strong BL for 3 h. The cross-sections of leaves that were fixed with 2.5% glutaraldehyde (WAKO) were made with a vibrating microtome (VT1200 S, Leica). Intracellular chloroplast distribution on the upper cell surface of the palisade cells and in the cross-sections was observed under a laser scanning confocal microscope (TCS SP8, Leica). For confocal microscopic imaging (Figs 1g and 3d), the projection images were constructed from z-stacks using the software supplied by the manufacturer. The number of chloroplasts at the periclinal walls was counted after the weak- or strong-BL irradiation, and was used for calculation of the number of chloroplasts at the anticlinal walls as the difference from the total chloroplast number in a cell shown in Table 1. Data for chloroplast distribution pattern in Table 2 was taken as described previously16. Under weak BL irradiation, the occupancy rates of chloroplast number were calculated as the percentage of chloroplasts accumulated to the periclinal walls or remaining at the anticlinal walls compared to the total number of chloroplasts in a cell. The occupancy rates of chloroplast number were calculated as the percentage of chloroplasts that moved toward the anticlinal walls or remained at the periclinal walls compared to the total number of chloroplasts in a cell under strong BL exposure. The occupancy rate of chloroplast area is the percentage of periclinal or anticlinal wall area, which calculated as the projection or surface area and shown in Table 1, occupied by the chloroplast area and multiplied by the number of chloroplasts at the periclinal or anticlinal walls.
Statistical analysis
Comparisons among the groups were performed by using one-way ANOVA followed by Tukey–Kramer multiple comparison post hoc test. The differences were considered to be significant at P < 0.05. Statistical analysis was performed using Excel 2011 (Microsoft, USA) with the add-in software Statcel 336.
References
Christie, J. M. Phototropin blue-light receptors. Annu. Rev. Plant Biol. 58, 21–45 (2007).
Suetsugu, N. & Wada, M. Evolution of three LOV blue light receptor families in green plants and photosynthetic stramenopiles: Phototropin, ZTL/FKF1/LKP2 and aureochrome. Plant Cell Physiol. 54, 8–23 (2013).
Suetsugu, N. & Wada, M. Chloroplast photorelocation movement: A sophisticated strategy for chloroplasts to perform efficient photosynthesis in Advances in photosynthesis-Fundamental aspects (ed. Najafpour, M. M.) 215–234 (InTech, 2012).
Kasahara, M. et al. Chloroplast avoidance movement reduces photodamage in plants. Nature 420, 829–832 (2002).
Terashima, I. & Hikosaka, K. Comparative ecophysiology of leaf and canopy photosynthesis. Plant Cell Environ. 18, 1111–1128 (1995).
Inoue, Y. & Shibata, K. Light-induced chloroplast rearrangements and their action spectra as measured by absorption spectrophotometry. Planta 114, 341–358 (1973).
Walczak, T. & Gabryś, H. New type of photometer for measurements of transmission changes corresponding to chloroplast movements in leaves. Photosynthetica. 14, 65–72 (1980).
Inoue, Y. & Shibata, K. Comparative examination of terrestrial plant leaves in terms of light-induced absorption changes due to chloroplast rearrangement. Plant Cell Physiol. 15, 717–721 (1974).
Brugnoli, E. & Björkman, O. Chloroplast movements in leaves: Influence on chlorophyll fluorescence and measurements of light-induced absorbance changes related to ∆pH and zeaxanthin formation. Photosynth. Res. 32, 23–35 (1992).
Park, Y.-I., Chow, W. S. & Anderson, J. M. Chloroplast movement in the shade plant Tradescantia albiflora helps protect photosystem II against light stress. Plant Physiol. 111, 867–875 (1996).
Trojan, A. & Gabryś, H. Chloroplast distribution in Arabidopsis thaliana (L.) depends on light conditions during growth. Plant Physiol. 111, 419–425 (1996).
Augustynowicz, J. & Gabryś, H. Chloroplast movements in fern leaves: correlation of movement dynamics and environmental flexibility of the species. Plant Cell Environ. 22, 1239–1248 (1999).
Davis, P. A., Caylor, S., Whippo, C. W. & Hangarter, R. P. Changes in leaf optical properties associated with light-dependent chloroplast movements. Plant Cell Environ. 34, 2047–2059 (2011).
Königer, M. & Bollinger, N. Chloroplast movement behavior varies widely among species and does not correlate with high light stress tolerance. Planta 236, 411–426 (2012).
Königer, M. Chloroplast movement in higher plants, ferns and bryophytes: A comparative point of view in Photosynthesis in Bryophytes and Early Land Plants, Advances in Photosynthesis and Respiration 37 (eds Hanson, D.T. and Rice, S.K.) 131–150 (Springer Science, 2014).
Higa, T. & Wada, M. Chloroplast avoidance movement is not functional in plants grown under strong sunlight. Plant Cell Environ. 39, 871–882 (2016).
Vogelmann, T. C. Plant tissue optics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 44, 231–251 (1993).
Kume, A. Importance of the green color, absorption gradient, and spectral absorption of chloroplasts for the radiative energy balance of leaves. J. Plant Res. 130, 501–514 (2017).
Terashima, I., Hanba, Y. T., Tazoe, Y., Vyas, P. & Yano, S. Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J. Exo. Bot. 57, 343–354 (2006).
Tsuge, T., Tsukaya, H. & Uchimiya, H. Two independent and polarized processes of cell elongation regulate leaf blade expansion in Arabidopsis thaliana (L.) Heynh. Development 122, 1589–1600 (1996).
Horiguchi, G., Kim, G.-T. & Tsukaya, H. The transcription factor AtGRF5 and the transcription coactivator AN3 regulate cell proliferation in leaf primordia of Arabidopsis thaliana. Plant J. 43, 68–78 (2005).
Wada, M. & Kong, S.-G. Analysis of chloroplast movement and relocation in. Arabidopsis. Methods Mol. Biol. 774, 215–234 (2011).
Suetsugu, N. et al. RPT2/NCH1 subfamily of NPH3-like proteins is essential for the chloroplast accumulation response in land plants. Proc. Natl. Acad. Sci. USA 113, 10424–10429 (2016).
Horiguchi, G. & Tsukaya, H. Organ size regulation in plants: insights from compensation. Front. Plant Sci. 2, 24 (2011).
DeBlasio, S. L., Luesse, D. L. & Hangarter, R. P. A plant-specific protein essential for blue-light-induced chloroplast movements. Plant Physiol. 139, 101–114 (2005).
Suetsugu, N., Higa, T., Kong, S.-G. & Wada, M. PLASTID MOVEMENT IMPAIRED-RELATED1 mediate photorelocation movements of both chloroplasts and nuclei. Plant Physiol. 169, 1155–1167 (2015).
Kadota, A. et al. Short actin-based mechanism for light-directed chloroplast movement in Arabidopsis. Proc. Natl. Acad. Sci. USA 106, 13106–13111 (2009).
Kim, G.-T. et al. The ANGUSTIFOLIA gene of Arabidopsis, a plant CtBP gene, regulates leaf-cell expansion, the arrangement of cortical microtubules in leaf cells and expression of a gene involved in cell-wall formation. EMBO J. 21, 1267–1279 (2002).
Folkers, U. et al. The cell morphogenesis gene ANGUSTIFOLIA encodes a CtBP/BARS-like protein and is involved in the control of the microtubule cytoskeleton. EMBO J. 21, 1280–1288 (2002).
Minamisawa, N. et al. ANGUSTIFOLIA, a plant homolog of CtBP/BARS, functions outside the nucleus. Plant J. 68, 788–799 (2011).
Bhasin, H. & Hülskamp, M. ANGUSTIFOLIA, a plant homolog of CtBP/BARS localizes to stress granules and regulates their formation. Front. Plant Sci. 8, 1004 (2017).
Kozuka, T., Kong, S.-G., Doi, M., Shimazaki, K. & Nagatani, A. Tissue-autonomous promotion of palisade cell development by phototropin 2 in Arabidopsis. Plant Cell 23, 3684–3695 (2011).
Arnon, D. I. Copper enzymes in isloated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24, 1–15 (1949).
Inoue, S. et al. Blue light-induced autophosphorylatiuon of phototropin is a primary step for signaling. Proc. Natl. Acad. Sci. USA 105, 5626–5631 (2008).
Inoue, S. et al. Functional analyses of the activation loop of phototropin2 in Arabidopsis. Plant Physiol. 156, 117–128 (2011).
Yanai, H. Statcel 3 – The useful add-in software forms on Excel (3rd ed). OMS, Tokyo, Japan. (2011).
Acknowledgements
We thank Shin-ichiro Inoue (Nagoya University, Japan) and Ken-ichiro Shimazaki (Kyushu University, Japan) for anti-PHOT1 and anti-PHOT2 antibodies. We appreciate Center for Advanced Instrumental and Educational Supports, Faculty of Agriculture, Kyushu University for using confocal microscopy. This work was supported by JSPS KAKENHI Grant Numbers JP15K18713 (to E.G.), JP26840097 and JP15KK0254 (to N.S.), JP20227001, JP23120523, JP25120721, and JP25251033 (to M.W.); and Grant-in-Aid for JSPS Research Fellow Number 17J06717 (to T.H.); and the Research Grant for Young Investigators of Faculty of Agriculture, Kyushu University (to E.G.); and the Japan Prize Foundation award (to E.G.).
Author information
Authors and Affiliations
Contributions
E.G. and N.S. conceived and designed the research. E.G., N.S., and T.H. performed the experiments. E.G., N.S., T.H., H.T., and M.W. analyzed the data. H.T. contributed to plant materials. T.M. assisted the experiments by E.G. N.S. and E.G. wrote the paper. All authors read and edited the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gotoh, E., Suetsugu, N., Higa, T. et al. Palisade cell shape affects the light-induced chloroplast movements and leaf photosynthesis. Sci Rep 8, 1472 (2018). https://doi.org/10.1038/s41598-018-19896-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-19896-9
- Springer Nature Limited
This article is cited by
-
SPAD: potential phenotyping method for characterization of blueberry
Molecular Biology Reports (2022)
-
Living between land and water – structural and functional adaptations in vegetative organs of bladderworts
Plant and Soil (2021)
-
Leaf structure and seed histochemistry analyses provided structural insights into the improved yield and quality of tree peony seed under light shading conditions
Scientific Reports (2020)
-
Chloroplast avoidance movement: a novel paradigm of ROS signalling
Photosynthesis Research (2020)
-
Light-induced chloroplast movements in Oryza species
Journal of Plant Research (2020)