Abstract
Excessive accumulation of β-amyloid (Aβ) is thought to be a major causative factor in the pathogenesis of Alzheimer’s disease (AD). Pretreating Aβ-induced neurotoxicity is a potential therapeutic approach to ameliorate the progression and development of AD. The present study aimed to investigate the neuroprotective effect of shikonin, a naphthoquinone pigment isolated from the roots of the traditional Chinese herb Lithospermum erythrorhizon, on Aβ1–42-treated neurotoxicity in PC12 cells. Pretreating cells with shikonin strongly improved cell viability, decreased the malondialdehyde and reactive oxygen species (ROS) content, and stabilized the mitochondrial membrane potential in Aβ1–42-induced PC12 cells. In addition, shikonin strongly improved the response of the antioxidant system to ROS by increasing the levels of superoxidedismutase, catalase and glutathione peroxidase. Furthermore, shikonin has the ability to reduce proapoptotic signaling by reducing the activity of caspase-3 and moderating the ratio of Bcl-2/Bax. These observations indicate that shikonin holds great potential for neuroprotection via inhibition of oxidative stress and cell apoptosis.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disease, and it is typified by progressive brain degeneration and deterioration of cognitive function in the elderly people1,2,3. Due to a lack of effective treatments, AD has become one of the most devastating diseases in the world. The neuropathological characteristics of AD consist of deposition of extracellular senile plaques formed by excessive aggregation of β-amyloid protein (Aβ), neurofibrillary tangles, and neuron loss induced by neuronal apoptosis. Deposition of Aβ in the brain has been considered to be a critical effect in the progression of AD1,4. Aβ is the main component of senile plaques that has been implicated in kinds of physiological processes, such as cell survival and synaptic activity5,6. However, studies have suggested that changes in Aβ1–42 physicochemical properties and concentration potentially trigger its transition from physiological to pathological7.
The exact mechanisms of Aβ-induced neurotoxicity are still remain obscure, it has been reported that pathological deposition of Aβ leads to oxidative stress and a series of cascade reactions of apoptosis, inducing the progressive degeneration of cognition functions in AD patients. Thus, several researches have explored the activity of antioxidant and antiapoptotic drugs to ameliorate AD. Currently, the pharmacological treatment used to delay cognitive dysfunction in AD patients principally includes two types of drugs in the clinic, acetylcholinesterase inhibitors and glutamate modulators8. In addition, it has been reported that several alternative approaches have preventative effects on the progression of AD, including anti-Aβ antibodies, inhibitors of β- and γ-secretases, antioxidants and anti-inflammatory agents9,10,11,12. However, the stability, validity, cost, safety and development time restrict the use of these treatments for preventing AD13. Thus, searching for a safer and more effective drug for the treatment of AD remains an important challenge in drug discovery.
Shikonin, a naphthoquinone pigment, is the chief active component extracted from the root of Lithospermum erythrorhizon, which has long been used in traditional oriental medicine for wound healing, urticaria and other allergic diseases (Fig. 1)14. It has been reported that shikonin possesses several pharmacological properties, such as antioxidant, anti-platelet activation, antiatherosclerosis, antithrombotic, anti-inflammatory, antitumor and antimicrobial properties15,16,17,18,19,20. These effects of shikonin are considered to be associated with its oxygen radical scavenging function21,22. According to reports, shikonin has the ability to protect against several types of reactive oxygen species (ROS)22,23, indicating that shikonin could protect against neurodegeneration from oxidative damage. Furthermore, it is suggested that shikonin plays a significant protective role in brain and hepatic ischemia/reperfusion injury by reducing ROS22,24,25. In this study, we examined the pro-survival activity of shikonin against oxidative damage induced by Aβ1–42 toxicity in PC12 cells. We determined the protective activity of shikonin on Aβ-induced damage and cell apoptosis in PC12 cells and completed initial research to investigate the underlying mechanism.
Results
Effect of Shikonin on Aβ1–42-Induced Cytotoxicity in PC12 cells
We applied 100 µM Aβ1–42 aggregates to all PC12 cell groups throughout our experiments (Figs S1 and S2, Supporting Information). As indicated in 3-(4, 5-dimethyldiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay of cell viability, treatment of PC12 cells with 100 µM Aβ1–42 for 12 h induced cytotoxicity, as demonstrated by the cell viability reduction to 35.27% compared with control group (Fig. 2A). When the cells were pretreated with serial concentrations of shikonin (3.47, 10.42, 34.72 µM) for 12 h, the cell viability was significantly increased (54.01, 60.29 and 71.51% of the control value, respectively) in a concentration-dependent manner. Furthermore, in MTT assay and flow cytometric detection of Aβ-induced SH-SY5Y cells, we found that viability of cells pretreated with shikonin was also strongly improved in a concentration-dependent manner (Figs S3, S4 and S5, Supporting Information).
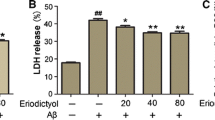
Protective effect of shikonin to against Aβ1–42-induced cytotoxicity in PC12 cells. Cell viability was measured with MTT (A) and LDH release (B) assays. Data are shown as the percent of values in control value, and the values are given as mean ± SD (n = 5). # P < 0.01 compared with the control group (no Aβ1−42); *P < 0.05 and **P < 0.01 compared with the Aβ1–42-induced group.
As indicated in lactate dehydrogenase (LDH) release rate test of cell viability, treatment of PC12 cells with 100 µM Aβ1–42 for 12 h caused a 238.66% increase in LDH release rate compared to control group (Fig. 2B). When the cells were pretreated with serial concentrations of shikonin (3.47, 10.42, 34.72 µM) for 12 h, the LDH release rate was significantly decreased (234.35, 202.44 and 175.66% of the control value, respectively) in a concentration-dependent manner.
The effects of shikonin pretreatment on Aβ1–42-induced apoptosis in PC12 cells were detected using a TUNEL assay (Fig. 3). PC12 cells treated with Aβ1–42 showed obviously different staining activity than untreated cells, signifying apoptotic behavior. However, after pretreatment of PC12 cells with 34.72 µM shikonin, the Aβ1–42 resulted in significantly less staining, suggesting lower level of apoptosis. These results demonstrate that shikonin protects PC12 cells and SH-SY5Y cells from Aβ1–42-induced cytotoxicity and improves cell viability.
Protective effect of shikonin against Aβ1–42-induced apoptosis in PC12 cells analyzed with TUNEL staining (400×). Blue: PC12 cell nuclei counterstained with DAPI. Green: TUNEL staining of nuclei exhibiting DNA fragmentation. PC12 cells were pretreated with shikonin for 12 h, and then Aβ1–42 was added to the culture for 12 h. (A) control; (B) 100 µM Aβ1–42 treated alone; (C) 100 µM Aβ1–42 with 34.72 µM shikonin.
Effect of Shikonin on Oxidative Stress in PC12 cells
Oxidative stress was detected by testing the level of intracellular ROS (Fig. 4A,B) and malondialdehyde (MDA) (Fig. 4C). After exposure to 100 µM Aβ1–42 for 12 h, ROS production and the MDA level were increased to 144.94% and 110.61% of the control value respectively. When the PC12 cells were pretreated with different concentrations of shikonin (3.47, 10.42, 34.72 µM) for 12 h, the intracellular ROS level (130.53, 127.51 and 120.95% of the control value, respectively) and the MDA level (108.71, 104.25 and 101.15% of the control value, respectively) were significantly decreased in a concentration-dependent manner.
Effect of shikonin on Aβ1–42-induced oxidative stress in PC12 cells. Cells were induced with no Aβ1−42 (control) or with 100 μM Aβ1−42 following pretreatment with serial concentrations of shikonin. (A) ROS formation was detected with a fluorescence microscope (200×) and flow cytometry (insets): (a) control, (b) 100 µM Aβ1–42 treated alone, (c) 100 µM Aβ1–42 + 3.47 µM shikonin, (d) 100 µM Aβ1–42 + 10.42 µM shikonin, (e) 100 µM Aβ1–42 + 34.72 µM shikonin. (B) Detected fluorescence levels from the ROS assay are shown as the percentage of values in the untreated control group (mean ± SD; n = 3). (C) Results of MDA assay are shown as the percentage of values in the untreated control group (mean ± SD; n = 5). # P < 0.05 and ## P < 0.01 compared with the control group (no Aβ1−42); *P < 0.05 and **P < 0.01 compared with the Aβ1–42-induced group.
Effects of Shikonin on Aβ1–42- induced anti-oxidative enzymes in PC12 Cells
To determine whether the cell protection effect of shikonin is associated with the activity of anti-oxidative enzymes, the levels of superoxidedismutase (SOD) (Fig. 5A), catalase (CAT) (Fig. 5B) and glutathione peroxidase (GSH-Px) (Fig. 5C) were assessed. After exposure to 100 µM Aβ1–42 for 12 h, the levels of SOD, CAT and GSH-Px were decreased to 43.67%, 89.83% and 46.55% that of the control values respectively. When the PC12 cells were pretreated with different concentrations of shikonin (3.47, 10.42, 34.72 µM) for 12 h, the intracellular SOD level (57.24, 56.47 and 85.14% of the control value, respectively), CAT level (93.22, 95.61 and 96.95% of the control value, respectively) and GSH-Px level (62.07, 82.15 and 93.44% of the control value, respectively) were significantly ameliorated in a concentration-dependent manner.
Effect of shikonin on the activity of anti-oxidative enzymes in PC12 cells. PC12 cells were induced with no Aβ1−42 (control) or with 100 μM Aβ1−42 following pretreatment with serial concentrations of shikonin. (A) Effect of shikonin on Aβ1−42-induced SOD levels. (B) Effect of shikonin on Aβ1−42-induced CAT levels. (C) Effect of shikonin on Aβ1−42-induced GSH-Px levels. Data are shown as the percentage of control group (mean ± SD; n = 5). # P < 0.05 and ## P < 0.01 compared with the control group (no Aβ1−42); *P < 0.05 and **P < 0.01 compared with the Aβ1–42-induced group.
Effect of Shikonin on Aβ1–42-induced Mitochondrial Membrane Potential in PC12 cells
The mitochondrial membrane potential was assessed using a JC-1 assay (Fig. 6). After PC12 cells were treated with 100 µM Aβ1–42 for 12 h, the mitochondrial membrane potential was decreased to 62.75% compared with the control group. When PC12 cells were pretreated with serial concentrations of shikonin for 12 h, the mitochondrial membrane potential was significantly improved (75.53, 79.86 and 87.67% of the control value) in a concentration-dependent manner.
Effect of shikonin on changes in the mitochondrial membrane potential induced by Aβ1–42. PC12 cells were induced with no Aβ1−42 (control) or with 100 μM Aβ1−42 following pretreatment with serial concentrations of shikonin. The alteration of mitochondrial membrane potential was detected with JC-1 staining. Data are shown as the percentage of values in the untreated control group (mean ± SD; n = 3). # P < 0.01 compared with the control group (no Aβ1−42); *P < 0.05 and **P < 0.01 compared with the Aβ1–42-induced group.
Effects of Shikonin on Aβ1–42-induced Apoptotic Protein Expression and Activation in PC12 Cells
The ratio of antiapoptotic protein Bcl-2 to proapoptotic protein Bax and the relative levels of activation of caspase-3 have been reported to be correlated with apoptosis. To investigate the protective mechanisms of shikonin, the expression level of activated caspase-3 and the ratio of Bcl-2/Bax in PC12 cells were measured by immunofluorescence analysis (Fig. 7). Aβ1–42 strongly improved the content of cleaved caspase-3 to 136.33% compared with the control group and ameliorated the ratio of Bcl-2 to Bax to 67.63% compared with the control group. Shikonin at a concentration of 34.72 µM partially inhibited these changes in comparison to the Aβ1–42-induced group.
Inhibitory effect of shikonin on Aβ1−42-induced changes in activated caspase-3 and the Bcl-2/Bax ratio in PC12 cells. Cells were induced with no Aβ1−42 (control) or with 100 μM Aβ1−42 following pretreatment with shikonin. (A) Levels of Bax, Bcl-2 and cleaved caspase-3 were measured with a fluorescence microscope (400×). (B) Semiquantitative image analysis of cleaved caspase-3. Data are shown as the percentage of control group (mean ± SD; n = 3). (C) Semiquantitative image analysis of Bcl-2/Bax ratio. Data are shown as the percentage of control group (mean ± SD; n = 3). # P < 0.01 compared with the control group (no Aβ1−42); *P < 0.05 compared with the Aβ1–42-induced group.
Discussion
In this study, we have demonstrated that shikonin can partially protect PC12 cells from injury caused by Aβ-induced toxicity, oxidative stress and mitochondrial membrane depolarization. Although the precise mechanism of Aβ-induced neurotoxicity has not been completely elucidated, increasing evidence suggests that oxidative stress is closely involved in AD pathogenesis and ROS overproduction causes neuronal death in AD26,27. To assess the clinical potential of shikonin, we investigated its ability to ameliorate Aβ1–42-induced alterations in PC12 cells. The PC12 cell line has been widely employed as a general in vitro model to evaluate neuronal damage and neurotoxicity in AD. Furthermore, PC12 cells are able to provide high throughput and retain of a mature neuron phenotype28. The biochemistry and morphology of neuron growth factor (NGF)-induced PC12 cells are similar to neurons, and PC12 cells are particularly sensitive to Aβ peptides. In addition, several reports have suggested that Aβ1–42 not only leads to cytotoxicity and cell death but also induces ROS overproduction and mitochondrial dysfunction in PC12 cells29. We also observed these effects in our study. Therefore, the PC12 cells used in our experiments provide a reliable approach to determine whether shikonin affords protection against Aβ-induced cytotoxicity.
In our study, both MTT and LDH release tests were used to assess the neuroprotective effect of shikonin against the Aβ1–42-treated decrease in cell viability. PC12 cells apoptosis induced by Aβ1–42 was observed directly using TUNEL staining with and without shikonin pretreatment. Additionally, human neuroblastoma SH-SY5Y cells were used to further investigate the neuroprotective effect of shikonin against Aβ1–42-induced neurotoxicity. In the MTT assay, shikonin lowered Aβ1–42-induced SH-SY5Y cell death. Also the protective effects of shikonin noted in cell viability corresponded to the findings seen in cellular apoptotic. These experimental results indicate that shikonin has the ability to prevent PC12 and SH-SY5Y cells against the cytotoxicity induced by Aβ1–42. Further studies to investigate the mechanism of the neuroprotective activities indicated that shikonin pretreatment improved Aβ1–42-mediated oxidative stress, mitochondrial dysfunction and cellular apoptosis.
Oxidative stress is a disturbance in the balance between ROS production and the antioxidant defense system, which plays a major effect in the cell injury and neuronal degeneration in AD30. Several in vitro and in vivo studies have demonstrated that Aβ1–42 treatment causes ROS overproduction. Excessive ROS production is known to damage biomacromolecules in cells, consisting of proteins, lipids and DNA, eventually leading to neurodegeneration and depression31,32. In our study, we also found that Aβ1–42 treatment of PC12 cells not only increased ROS production but also increased the MDA content, and these results are in agreement with previous studies27,33. Furthermore, pretreating cells with shikonin attenuated these alterations in a concentration-dependent manner, indicating that the antioxidant effect of shikonin can mitigate apoptosis in AD.
Neurons depend on oxidative metabolism for survival. Under conditions of oxidative stress, there is an increase in ROS production and can result in cell damage or apoptosis. Under physiological conditions, there is a balance between the ROS production and the endogenous cellular antioxidant systems, including the cooperative action of SOD, CAT and GSH-Px34,35,36. It is known that SOD and GSH-Px serve as the primary line of intracellular defense against ROS by catalyzing their conversion to less reactive species. SOD is an important antioxidant enzyme in mitochondria against oxidative stress, and the activity of GSH-Px is to reduce the lipid hydroperoxides and free hydrogen to alcohols and water34,35. Additionally, CATs are commonly regarded as the second line of defense against dismutating peroxide into water and molecular oxygen36. When cells are treated with Aβ, sudden bursts of ROS cannot be eliminated by the cellular antioxidant enzymes, and the accumulation of ROS induces cellular membrane, protein and DNA oxidative damage. In our study, we discovered that Aβ1–42 significantly increased the levels of SOD, CAT and GSH-Px, indicating that these enzymes play a critical effect in ROS overproduction induced by oxidative stress. Pretreatment of PC12 cells with shikonin attenuated these Aβ1–42-treated alterations in a concentration-dependent manner, indicating that the protective activity of shikonin may be mediated by its antioxidant properties.
It is generally accepted that the mitochondrial membrane plays an important role in cell survival and death, especially under the influence of oxidative stress27,37. Mitochondrial dysfunction, leading to irreversible mitochondrial depolarization, has been found in Aβ-induced neurotoxicity in AD patients. ROS are produced in the mitochondria and then released into the cytoplasm, triggering oxidative stress and initiating cellular apoptosis. Our results illustrated that the mitochondrial membrane potential of PC12 cells treated with Aβ1–42 decreased, but this change was attenuated by shikonin. Therefore, according to our findings, we found that shikonin holds neuroprotective activity by inhibiting oxidative stress and apoptosis.
The Bcl-2 family plays an important factor in the mitochondrial apoptosis pathway induced by oxidative stress in PC12 cells exposed to Aβ1–42, and Bcl-2 family members consist of several pro-apoptotic including Bax and antiapoptotic including Bcl-238. It has been reported that Bcl-2 has the ability to inhibit mitochondrial depolarization and ROS production, while Bax induces both of these processes27. Therefore, the ratio of Bcl-2 to Bax plays a pivotal role for cell survival and death. In addition, excessive ROS can induce the intermembrane protein to be released into the cytoplasm and eventually triggered caspase-3 activation, leading to cell apoptosis. Our present study indicated that Aβ1–42 ameliorated the Bcl-2/Bax expression ratio and improved the activation of caspase-3, consistent with previous researches in PC12 cells suggesting that these proteins play an important effect in mitochondria-mediated apoptosis induced by oxidative stress39,40,41. Our results suggested that pretreatment of shikonin in PC12 cells partially attenuated these Aβ1–42-treated alterations, indicating that the neuroprotective activities of shikonin may associate with its modulation on the expression of the Bcl-2 family.
In conclusion, our findings demonstrate that shikonin has a significant protective effect on Aβ1–42-induced neuronal injury. According to these in vitro studies, the protective effect of shikonin may be mediated by preventing oxidative stress and neuronal apoptosis. Additionally, further research is needed to verify and explore potential mechanisms underlying the findings.
Materials and Methods
Shikonin, neuron growth factor (NGF), Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), horse serum (HS), and Aβ1–42 were obtained from Sigma–Aldrich Inc. (St Louis, MO, USA). The kits for malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px) and lactate dehydrogenase (LDH) were provided from Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). Reactive oxygen species (ROS) assay kit and mitochondrial membrance potential assay kit with JC-1 were obtained from Beyotime Institute of Biotechnology (Haimen, Jiangsu, China). Polyclonal antibodies against cleaved caspase-3, Bax and Bcl-2 were purchased from Cell Signaling Technology (Danvers, MA, USA).
Cell culture
Undifferentiated rat pheochromocytoma PC12 cells were obtained from the American Type Culture Collection (Rockville, MD, USA) which were from passage 3 to 20. PC12 cells were cultured in high glucose DMEM containing 10% horse serum, 5% fetal bovine serum of penicillin-streptomycin and were maintained in a humidified atmosphere containing 5% CO2. PC12 cells were placed in poly D-lysine-coated plates and were treated with 5 ng/ml NGF to induce differentiation. The medium was changed every two days38.
Cell Viability and LDH Assay
Cell viability was assessed by measuring the metabolism of 3-(4, 5-dimethyldiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Briefly, PC12 cells were seeded into 96-well plates with 8 × 103 cells per well and incubated overnight for viability detection. After pretreatment with serial concentrations of shikonin (3.47, 10.42, 34.72 µM) for 12 h, the culture medium was replaced with medium containing 100 µM Aβ1–42 for 12 h. Then the cells were treated with 5 mg/ml MTT solution for 4 h at room temperature. The supernatants were then discarded, 150 µl of DMSO was added to solubilize the formazan crystals with shaking for 5 min. The absorbance at 570 nm of solution was detected using a in a microplate reader (Thermo, Varioskan Flash).
The activity of released LDH is an in vitro marker for cell injury. The extracellular and intracellular LDH content were assessed with a commercial assay kit. Briefly, PC12 cells were plated in 6-multiwell plates at 4 × 105 cells/well for LDH activity determination. The cells were pretreated with serial concentrations of shikonin (3.47, 10.42, 34.72 µM) for 12 h, and then, the PC12 cells were insulted with 100 µM Aβ1–42 for 12 h. The culture medium was collected for the extracellular LDH activity assay. After washing three times in cold PBS, the adherent cells were collected by scraping and homogenized for the intracellular LDH activity assay. The absorbance of each sample was measured at 450 nm via microplate reader. LDH release was calculated as follows:
TUNEL staining
The degree of DNA fragmentation was determined by a terminal deoxynucleotidyl transferase nickend labeling (TUNEL) assay. In brief, PC12 cells were cultured on coverslips. After incubation with shikonin (34.72 µM) for 12 h and exposure with 100 µM Aβ1–42 for 12 h, media was aspirated and plates were rinsed with cold PBS buffer. The cells were fixed with 4% paraformaldehyde at 4 °C for 10 min. The cells were further incubated in blocking and treated with 0.1% TritonX-100 in 0.1% sodium acetate for 5 min. Thereafter, the cells were labeled by incubation with the TUNEL reagent and fluorescent dUTP mixture for 1 h at 37 °C. Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI). Subsequently, the cells were observed with a fluorescence microscope (Carl Zeiss Shanghai Co., Ltd).
Lipid Peroxidation Assay
The malondialdehyde (MDA) content, a marker of lipid peroxidation, was tested by biochemical methods according to the instructions of reagent kits. Briefly, 4 × 105 cells per well were cultured into 6-well plates. After pretreatment with serial concentrations of shikonin (3.47, 10.42, 34.72 µM) for 12 h, the cells were insulted with 100 µM Aβ1–42 for 12 h. At the end of the treatment, PC12 cells were rinsed with cold phosphate-buffered saline and homogenized in 0.5 ml of buffer solution (0.1 M, containing 0.05 mM EDTA). The homogenate was centrifuged at 4000 g for 10 min. The supernatant was collected for the MDA content assay.
Dichlorofluorescein assay for ROS
The ROS level was monitored using a fluorescent probe 2’, 7’-dichlorodihydrofluorescin diacetate (DCFH-DA). Intracellular ROS oxidize nonfluorescent compound DCFH-DA to highly fluorescent compound dichlorofluorescein (DCF), which can be detected with a fluorescence microscope and flow cytometry. In brief, 4 × 105 cells per well were cultured into 6-well plates. After pretreatment with serial concentrations of shikonin (3.47, 10.42, 34.72 µM) for 12 h, then the cells were insulted with 100 µM Aβ1–42 for 12 h. At the end of treatment, PC12 cells were rinsed with cold phosphate-buffered saline and treated with 10 µM DCFH-DA for 30 min at room temperature in a humid and dark environment. At the end of the incubation, cold phosphate-buffered saline was used to wash away the extracellular DCFH-DA molecules. Finally, the fluorescence of DCF was detected with a fluorescence microscope (Carl Zeiss Shanghai Co., Ltd.) and flow cytometry (Beckman Coulter).
Antioxidant Systems Assay
The cultured cells were seeded into 6-well plates at 4 × 105 cells per well. After pretreatment with serial concentrations of shikonin (3.47, 10.42, 34.72 µM) for 12 h, the cells were insulted with 100 µM Aβ1–42 for 12 h. At the end of the treatment, PC12 cells were rinsed with cold phosphate-buffered saline and homogenized in 0.5 ml of buffer solution. The homogenates were centrifuged, and the supernatants were used for the assay.
SOD, CAT and GSH-Px activities in PC12 cells were measured by means of kit assays according to the instructions of the manufacturers. The SOD activity was determined using the xanthine oxidase method at 550 nm wavelength with a spectrophotometer. The CAT activity was detected by biochemical method according to the instruction of reagent kit, and the absorbance of the test solution was measured at 405 nm wavelength with a spectrophotometer. The GSH-Px activity was assayed by quantifying the rate of oxidation of reduced glutathione to oxidized glutathione by H2O2 at 412 nm wavelength.
Measurement of Mitochondrial Membrane Potential
The membrane-permeant JC-1 dye was used to monitor mitochondrial membrane potential of Aβ1–42-induced PC12 cells. 4 × 105 cells per well were cultured into 6-well plates. After pretreatment with serial concentrations of shikonin (3.47, 10.42, 34.72 µM) for 12 h, the cells were insulted with 100 µM Aβ1–42 for 12 h. At the end of the treatment, PC12 cells were treated with JC-1 dye for 30 min. After incubation, the cells were washed three times with phosphate-buffered saline. The fluorescence was measured by flow cytometry (Beckman Coulter), depolarization in mitochondrial membrane potential of PC12 cells was quantified as the ratio of the red to green signal.
Immunofluorescence Assay
Immunofluorescence assay was used to evaluate the levels of Bax, Bcl-2, and activated caspase-3 protein. Briefly, PC12 cells were cultured onto coverslips after incubation with shikonin (34.72 µM) for 12 h and exposured to 100 µM Aβ1–42 for 12 h. The medium was aspirated from the plates, and the cells were rinsed with phosphate-buffered saline. At the end of the treatment, PC12 cells were fixed for 10 min with 4% paraformaldehyde at 37 °C and rinsed with phosphate-buffered saline. Fixed cells were incubated with primary antibodies diluted in PBS (anti-cleaved caspase-3, 1:1000; anti-Bax, 1:1000; anti-Bcl-2, 1:500) overnight. The coverslips were washed and treated with Cy3-conjugated secondary antibody diluted 1:500 in PBS for 4 h. Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI). Subsequently, the cells were observed with a fluorescence microscope (Carl Zeiss Shanghai Co., Ltd.).
Data analysis
Results are shown as the mean ± SD. Differences between two experimental conditions were evaluated with one-way ANOVA followed by Dunnett’s post-hoc test (version 17.0 software, SPSS Inc.). Differences were accepted statistically significant for P < 0.05.
References
Zheng, W. H., Bastianetto, S., Mennicken, F., Ma, W. & Kar, S. Amyloid β peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience 115, 201–211 (2002).
Forman, M. S., Trojanowski, J. Q. & Lee, V. M. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nature Medicine 10, 1055 (2004).
Nordberg, A. PET imaging of amyloid in Alzheimer’s disease. Lancet Neurology 3, 519–527 (2004).
Hardy, J. & Selkoe, D. J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 297, 353–356 (2002).
Khodadadi, S. et al. Effect of N -homocysteinylation on physicochemical and cytotoxic properties of amyloid β-peptide. Febs Letters 586, 127–131 (2012).
Pearson, H. A. & Peers, C. Physiological roles for amyloid β peptides. Journal of Physiology 575, 5–10 (2006).
Selkoe, D. J. Soluble Oligomers of the Amyloid β-Protein: Impair Synaptic Plasticity and Behavior. Behavioural Brain Research 192, 106–113 (2008).
Knopman, D. S. Current treatment of mild cognitive impairment and alzheimer’s disease. Current Neurology and Neuroscience Reports 6, 365–371 (2006).
Yamada, K. & Nabeshima, T. Animal models of Alzheimer’s disease and evaluation of anti-dementia drugs. Pharmacology & therapeutics 88, 93 (2000).
Thomas, T., Nadackal, T. G. & Thomas, K. Aspirin and non-steroidal anti-inflammatory drugs inhibit amyloid-beta aggregation. Neuroreport 12, 3263–3267 (2001).
Schenk, D. Hopes remain for an Alzheimer’s vaccine. Nature 431, 398 (2004).
Frisardi, V. et al. Towards disease-modifying treatment of Alzheimer’s disease: drugs targeting beta-amyloid. Current Alzheimer Research 7, 40–55 (2010).
Wang, F. et al. A Peptide That Binds Specifically to the β-Amyloid of Alzheimer’s Disease: Selection and Assessment of Anti-β-Amyloid Neurotoxic Effects. Plos One 6, e27649 (2011).
Papageorgiou, V. P., Assimopoulou, A. N. & Ballis, A. C. Alkannins and shikonins: a new class of wound healing agents. Current Medicinal Chemistry 15, 3248 (2008).
Tanaka, S., Tajima, M., Tsukada, M. & Tabata, M. A Comparative Study on Anti-Inflammatory Activities of the Enantiomers, Shikonin and Alkannin. Journal of Natural Products 49, 466 (1986).
Yoshimi, N. et al. Modifying effects of fungal and herb metabolites on azoxymethane-induced intestinal carcinogenesis in rats. Cancer Science 83, 1273–1278 (2005).
Hisa, T., Kimura, Y., Takada, K., Suzuki, F. & Takigawa, M. Shikonin, an ingredient of Lithospermum erythrorhizon, inhibits angiogenesis in vivo and in vitro. Anticancer Research 18, 783–790 (1998).
Sekine, T., Masumizu, T., Maitani, Y. & Nagai, T. Evaluation of superoxide anion radical scavenging activity of shikonin by electron spin resonance. International Journal of Pharmaceutics 174, 133–139 (1998).
Assimopoulou, A. N., Boskou, D. & Papageorgiou, V. P. Antioxidant activities of alkannin, shikonin and Alkanna tinctoria root extracts in oil substrates. Food Chemistry 87, 433–438 (2004).
An, S., Park, Y. D., Paik, Y. K., Jeong, T. S. & Lee, W. S. Human ACAT inhibitory effects of shikonin derivatives from Lithospermum erythrorhizon. Bioorganic & Medicinal Chemistry Letters 17, 1112–1116 (2007).
Kourounakis, A. P., Assimopoulou, A. N., Papageorgiou, V. P., Gavalas, A. & Kourounakis, P. N. Alkannin and Shikonin: Effect on Free Radical Processes and on Inflammation - A Preliminary Pharmacochemical Investigation. Archiv Der Pharmazie 335, 262–266 (2002).
Esmaeilzadeh, E., Gardaneh, M., Gharib, E. & Sabouni, F. Shikonin protects dopaminergic cell line PC12 against 6-hydroxydopamine-mediated neurotoxicity via both glutathione-dependent and independent pathways and by inhibiting apoptosis. Neurochemical Research 38, 1590–1604 (2013).
Gao, D., Kakuma, M., Oka, S., Sugino, K. & Sakurai, H. Reaction of beta-alkannin (shikonin) with reactive oxygen species: detection of beta-alkannin free radicals. Bioorganic & Medicinal Chemistry 8, 2561–2569 (2000).
Wang, Z. et al. Shikonin protects mouse brain against cerebral ischemia/reperfusion injury through its antioxidant activity. European Journal of Pharmacology 643, 211–217 (2010).
Liu, T. et al. The protective effects of shikonin on hepatic ischemia/reperfusion injury are mediated by the activation of the PI3K/Akt pathway. Scientific Reports 7, 44785 (2017).
Li, G. et al. Protective effect of erythropoietin on beta-amyloid-induced PC12 cell death through antioxidant mechanisms. Neuroscience Letters 442, 143 (2008).
Y. F., X. et al. Protective effect of isorhynchophylline against β-amyloid-induced neurotoxicity in PC12 cells. Cellular and Molecular Neurobiology 32, 353–360 (2012).
Tan, J. et al. Bcl-X(L) inhibits apoptosis and necrosis produced by Alzheimer’s beta-amyloid1-40 peptide in PC12 cells. Neuroscience Letters 272, 5 (1999).
Hensley, K. et al. A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America 91, 3270–3274 (1994).
Chauhan, V. & Chauhan, A. Oxidative stress in Alzheimer’s disease. Biochimica Et Biophysica Acta 13, 139–144 (2006).
Zhao, Z., Wang, W., Guo, H. & Zhou, D. Antidepressant-like effect of liquiritin from Glycyrrhiza uralensis in chronic variable stress induced depression model rats. Behavioural Brain Research 194, 108–113 (2008).
Mao, Q. Q., Xian, Y. F., Ip, S. P., Tsai, S. H. & Che, C. T. Protective effects of peony glycosides against corticosterone-induced cell death in PC12 cells through antioxidant action. Journal of Ethnopharmacology 133, 1121–1125 (2011).
Hu, J. F. et al. Protective effect of (−)clausenamide against Abeta-induced neurotoxicity in differentiated PC12 cells. Neuroscience Letters 483, 78–82 (2010).
Zheng, W. X. et al. Baicalin protects PC-12 cells from oxidative stress induced by hydrogen peroxide via anti-apoptotic effects. Brain Injury 28, 227–234 (2014).
Lee, I. K. et al. Mitochondria protection of baicalein against oxidative damage via induction of manganese superoxide dismutase. Environmental Toxicology & Pharmacology 31, 233–241 (2011).
Olsvik, P. A. et al. mRNA expression of antioxidant enzymes (SOD, CAT and GSH-Px) and lipid peroxidative stress in liver of Atlantic salmon (Salmo salar) exposed to hyperoxic water during smoltification. Comparative Biochemistry & Physiology Part C Toxicology & Pharmacology 141, 314–323 (2005).
Chen, J. X. & Yan, S. D. Pathogenic role of mitochondrial amyloid-β peptide. Expert Review of Neurotherapeutics 7, 1517–1525 (2007).
Zhu, Y., Sun, X., Gong, T., He, Q. & Zhang, Z. Antioxidant and Antiapoptotic Effects of 1,1′-(Biphenyl-4,4′-diyl)-bis(3-(dimethylamino)-propan-1-one) on Protecting PC12 Cells from Aβ-Induced Injury. Molecular Pharmaceutics 11, 428–435 (2013).
Pias, E. K. & Aw, T. Y. Early redox imbalance mediates hydroperoxide-induced apoptosis in mitotic competent undifferentiated PC-12 cells. Cell Death & Differentiation 9, 1007 (2002).
Saito, Y. et al. Molecular mechanisms of 6-hydroxydopamine-induced cytotoxicity in PC12 cells: involvement of hydrogen peroxide-dependent and -independent action. Free Radical Biology & Medicine 42, 675 (2007).
Yu, Y., Du, J. R., Wang, C. Y. & Qian, Z. M. Protection against hydrogen peroxide-induced injury by Z-ligustilide in PC12 cells. Experimental Brain Research 184, 307–312 (2008).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81603050), the Foundation of Healthy and Family Planning Commission Program of Sichuan Provincial (No. 16PJ046, 16PJ478), the Central Universities Foundation of University of Electronic Science and Technology of China (No. ZYGX2015J130) and the Foundation of Sichuan Provincial People’s Hospital (No. 2016LY01). We are thankful to Dr. Wescoe Zak of Department of Bioengineering of University of Washington for English correction in our manuscript.
Author information
Authors and Affiliations
Contributions
Y.T. and Y.Z. conceived the study and participated in its design. Y.T., L.B. and J.C. contributed samples and carried out the experiments. R.G. and X.D. analyzed the data. Y.T. and L.B. drafted the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tong, Y., Bai, L., Gong, R. et al. Shikonin Protects PC12 Cells Against β-amyloid Peptide-Induced Cell Injury Through Antioxidant and Antiapoptotic Activities. Sci Rep 8, 26 (2018). https://doi.org/10.1038/s41598-017-18058-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-18058-7
- Springer Nature Limited
This article is cited by
-
Shikonin inhibits neuronal apoptosis via regulating endoplasmic reticulum stress in the rat model of double-level chronic cervical cord compression
Cell Biology and Toxicology (2023)
-
Shikonin induces programmed death of fibroblast synovial cells in rheumatoid arthritis by inhibiting energy pathways
Scientific Reports (2021)
-
Effect of gold nanoparticles on the structure and neuroprotective function of protein L-isoaspartyl methyltransferase (PIMT)
Scientific Reports (2021)