Abstract
Wetlands are characterized by changing water tables, which have an influence on the activity of microorganisms. Particularly, the effect of oxygen on anaerobic methanogenic archaea is of importance for understanding greenhouse gas fluxes in wetlands. In this study the influence of oxygen on CH4 production in marshland soils was investigated in relation to varying soil water potentials. Water saturated samples as well as samples with drained macropores, and mesopores were used. Under anoxic conditions the CH4 production showed a dependence on the water content. The CH4 production rates varied between about 213 and 51 nmol g−1 soil h−1. In the presence of oxygen a correlation between CH4 production activity and water potential of the samples could not be demonstrated. Under oxic conditions with defined water potentials the CH4 production rates varied between about 141 and 58 nmol g−1 soil h−1. Cell counts of methanogenic archaea showed similar numbers in oxic and anoxic soil layers, and further illustrated living methanogens in the aerobic horizons of the marshland soil. The presented results are of great importance for modelling of the CH4 release from wetlands, because up to 25% of the CH4 is produced in the oxic horizon of the investigated marshland soil.
Similar content being viewed by others
Introduction
Methane (CH4) is one of the most potent greenhouse gases in the atmosphere and thus influencing global climate change. It contributes to the enhanced greenhouse effect with a portion of approximately 20% of all greenhouse gases1. The reason is the higher potential of methane for absorbing infrared radiation, which was currently new calculated with 28 to 34 times of that of CO2 over an integrated period of 100 years2. The concentration of atmospheric methane has drastically increased from 0.7 to 1.8 ppmv since pre-industrial times3. Seventy to eighty percent of this methane originates from microbial processes4. Thus, for the understanding of the recent and future CH4 dynamics in wetlands, it is important to deepen our knowledge of CH4 cycling microbial processes and the response of the microbial communities to changing environmental conditions.
Organic matter in soils and sediments are decomposed under anoxic conditions by a sequence of different groups of microorganisms within the anaerobic food chain. Methane formation (methanogenesis) is the last step of decomposition in hydromorphic environments. Besides enteric fermentation, natural wetlands (marshlands, fens, tundra, swamps) and rice paddies are the most important sources of atmospheric methane5,6,7. Although methane fluxes has been quantified for many terrestrial ecosystems8,9,10,11,12 little is known about the mechanisms controlling methane production in wetland soils13,14,15,16,17. Wetlands often show great spatial or temporal variations in environmental conditions which influences the release of CH4 into the atmosphere. Soil water content, temperature, type and amount of organic matter, vegetation, and the potential for methane oxidation are important factors18,19,20,21. For example groundwater table fluctuations are typical for wetlands such as marshes, and thus these soils are characterized by changing oxygen concentrations. However, methanogenic archaea are regarded as strictly anaerobic organisms. Growth and methane production in pure methanogenic cultures were observed only under anaerobic conditions22. Therefore, the influence of oxygen on the activity of methanogenic archaea is a key parameter for the understanding of methane fluxes in wetlands.
Little is known about the existence of methanogenic archaea in oxic environments such as forest floors, arable soils and aerobic layers of groundwater influenced wetlands. Despite being strictly anaerobic and non-sporeforming organisms, methanogenic archaea in pure cultures can survive from several hours up to 3 days following exposure to air23,24. Strains with high oxygen tolerance were isolated from ecosystems (e.g., permafrost soils) with changing oxygen conditions. However, few hours of contact with oxygen led to a rapid decrease in viability.
In contrast to pure cultures, the methanogenic community in natural soils survives well in microniches in otherwise oxygen influenced environments. Mayer and Conrad25 found a small methanogenic population in unsaturated paddy soils. Even in oxic desert soils, which are not commonly considered as habitats for anaerobic microorganisms, low cell numbers of methanogenic archaea were detected26. A rapid initiation of methane production in air-dried soil samples was reported, when they were incubated under submerged and anaerobic conditions25.
A previous study conducted with marshland soil from the same study site demonstrated a low methane production rate (0.35 to 2.43 nmol g−1 soil h−1) in the presence of oxygen15. Experiments with aerated slurries of these soils and different textured model soils, consisting of clay, sand and gravel, respectively, showed that the aerobic and facultative anaerobic microflora in association with soil particles such as clay formed anoxic microniches, and enable methane production in the presents of oxygen. The results suggested that a spatial coupling of aerobic respiration and anaerobic methane formation represent a well-established part of the methane production process in natural ecosystems influenced by changing oxygen concentrations.
In contrast to soil slurries, which were made of homogenized soil material, natural soils are characterized by a definite pore system. The microbial activity in soil environments is influenced by the soil water potential27. Together with the pore size distribution it decisively determines the part of gas-filled pore volume and consequently the concentration of oxygen affecting the activity of anaerobic methanogenic archaea. The aim of this study was to verify the effect of different water potentials on the methane production in marshland soils. For this purpose undisturbed soil samples from a marshland soil in Northern Germany were used analysing the methane production in dependence of the soil water potentials under oxic and anoxic conditions.
Results
Cell numbers of methanogenic archaea
Cell numbers of methanogenic archaea were determined at in situ temperature (10 °C). The vertical profile showed the distribution of methanogens within the investigated marshland soil for the main methanogenic substrates acetate and H2/CO2 (Fig. 1).
Vertical profile of the abundance of methanogenic archaea. MPN counts were determined in fresh marshland soil. The incubation was done at in situ temperature (10 °C) with acetate (20 mM) or H2/CO2 (80:20 v/v, pressurized 150 kPa) as substrate. The 95% confidence limits amounted to log 0.670 in a three-tube MPN analysis. The horizontal broken line indicates the groundwater table level.
Methanogenic archaea were found in oxic (Go horizon) as well as in anoxic (Gr horizon) soil layers. Cell counts varied between 9.5 × 102 to 9.7 × 104 cells g−1 soil with acetate as substrate and between 3.7 × 102 and 8.9 × 103 cells g−1 soil with H2/CO2 as energy and carbon source. The average number of methanogens grown with H2/CO2 amounted to 2.7 × 103 cells g−1 soil, whereas the cell counts with acetate were about 10 times higher (2.5 × 104 cells g−1 soil).
Remarkable was that the cell numbers in oxic and anoxic soil layers were not significantly different, although methanogenic archaea are anaerobic organisms. The average number of methanogens in the upper 30 cm of soil amounted to 3.7 × 103 cell g−1 soil and the counts of acetotrophic and hydrogenotrophic methanogenic archaea were similar. The average cell number from a soil depth of 30–100 cm was about 7 times higher (1.8 × 104 cell g−1 soil) and acetotrophic methanogens occurred much more frequent.
In situ CH4 production
Figure 2 shows the vertical profile of the CH4 production rates, which were analysed at in situ temperature (10 °C) without any additional substrate. CH4 production was determined for all soil layers between 40 and 100 cm depth. The rates in this zone fluctuate considerably between 0.02 and 0.75 nmol CH4 h−1 g−1 soil. The highest CH4 production rate was observed in the soil depth 60–70 cm (samples from this soil layer were therefore used for all other experiments). The results indicate that in situ methane production not only occurred in the reduced soil horizons (Gr, fAhGr) but also in the transition zone between oxic and anoxic conditions (Gor).
Inhibition of CH4 oxidation by acetylene
For the study of methane production in the presence of oxygen soil material with the indigenous microflora was used. Besides methanogenic archaea this microflora included among other bacteria also methane-oxidizers, which convert methane to carbon dioxide. Therefore, it was necessary to inhibit the methane oxidation because otherwise it would lead to a falsification of the methane production activity in the experiment.
The results showed that a methane concentration of 2.5% in synthetic air remained constant in the presence of acetylene. Without acetylene the methane in the headspace was completely oxidized within 95 h (Fig. 3). Additionally, the influence of acetylene on methane production under anoxic conditions with actetate as substrate was investigated. Without acetylene the methane production rate amounted to 46.5 nmol g−1 soil h−1 whereas the rate in the presence of acetylene was 30.3 nmol g−1 soil h−1 (results are not shown). This difference corresponded to the variation of methane production in natural soil samples.
These results indicated that the used acetylene concentration completely inhibited the methane oxidation without an influence on the methane formation.
Methane production activity in relation to the soil water potential
The soil matrix volume of the marsh amounted to an average of 26.6% and the total pore volume was 73.4%. The pore volume was differentiated in pore size ranges due to the following pore size distribution (Fig. 4): the coarse macropores (>50 µm) varied between 9.1 and 16.4% with an average of 11.7%, whereas the part of fine macropores (50–10 µm) was similar for each layer of the vertical profile (average of 5.4%). The total portion of macropores was 17.1%. The portion of mesopores (10–0.2 µm) amounted to an average of 40.2% and increased with the profile depth. The portion of micropores (<0.2 µm) varied in a large range between 6.5 and 22.9% (average 16.1%). Whereas the volume of the soil matrix and the distribution of maropores were homogeneous for the whole profile, the portion of micropores decreased in relation to the increase of the portion of mesopores.
First of all, methane production of undisturbed soil samples was investigated under anoxic conditions to estimate the influence of the availability of water for methanogenic archaea. Therefore, different soil water potentials between 0 and −100 kPa were adjusted. On account of the required pressure it was possible to assign a definite part of the pore system to all drainage levels. The soil water characteristic (water retention curve) of undisturbed samples illustrated the connection between the soil water potential, the pore size, and the soil water content (Fig. 5). Marshland soil samples were water saturated at a water content of 54%. At a water potential of −6 kPa the samples had a water content of 48%, at −30 kPa the water content was 41% and at −100 kPa the water content decreased to 39%.
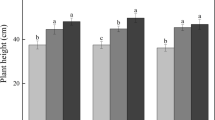
The progress of methane production of differently drained, undisturbed soil samples was almost linear under oxic and anoxic conditions for the whole incubation time (Fig. 6). The samples incubated with oxygen and acetylene showed a constant oxygen concentration of 15% O2 in the headspace.
Progress of methane production of undisturbed soil samples related to different soil water potentials; (a) under anoxic and (b) under oxic conditions. As a substrate H2/CO2 (80:20 v/v) was used (n = 3 for anoxic conditions, n = 4 for oxic condition, for a better overview standard errors are not shown).
Despite of the high variation of methane production, the rates under anoxic conditions showed for each sample a dependence on the soil water potential (Fig. 7b, r = 0.957, P = 0.043). The highest methane production rate amounted to 213.37 ± 46.4 nmol g−1 soil h−1 in the samples with 100% water saturation and the lowest rate (51.27 ± 25.52 nmol g−1 soil h−1) was analysed for the high drained samples with a water potential of −100 kPa.
In the presence of oxygen the dependence of methane production activity in relation to the soil water potential, which was observed under anoxic conditions, was overlaid by the influence of oxygen on the anaerobic methanogenic archaea (Fig. 7a, r = 0.848, P = 0.152). The water saturated samples had a methane production rate of 141.28 ± 16.99 nmol g−1 soil h−1, whereas all other drainage levels showed only small differences in methane production between 58.43 ± 9.32 and 80.71 ± 10.18 nmol g−1 soil h−1.
A comparison of the methane production rates in the presence and in the absence of oxygen showed that the rates under oxic conditions for the water saturated soil samples reached about 66% of the rates under anoxic conditions (Fig. 7a,b). The methane production under oxic conditions of the other water potentials were about 33% compared to rates under anoxic conditions related to the water saturated soil samples. At a water potential of −6 and −30 kPa the methane production rates were only half in the presence of oxygen compared to the anoxic incubated samples. At a water potential of −100 kPa the methane production rate remained the same irrespective of oxygen levels. In the presence of oxygen no significant difference was found between methane production rates at water potentials between −6 and −100 kPa.
Discussion
Some methanogenic archaea in soils can tolerate oxygen for a short period of time23,24,28, although these microorganisms are described as strictly anaerobic organisms. The presented results of methanogenic cell counts show, that these microorganisms were found within the whole profile of the marshland. Even in the upper oxic layer (Go horizon) about 103 cells g−1 soil were determined with the MPN approach, which is also a validation for living and potential active methanogens. The detected cell numbers were only 7 times less than in the anoxic soil layers (Gr horizon). Methane production under in situ conditions at the time of sampling revealed activity in the oxic/anoxic transition zone and in the deeper soil layers with reduced redox conditions. However, a previous study demonstrated that even in aerated marshland and different textured model soils methane production in the presence of oxygen was possible15. A combination of biotic and abiotic soil parameters is responsible for the development of microniches and for protecting methanogenic archaea against oxygen.
Natural soils are characterized by a structure, which is assembled by the mineral and organic compounds and mostly by swelling and shrinking processes. The soil structure and the soil pore system are important for the water regime and the gas fluxes. The water content and the pore size distribution determines the water potential and the part of gas-filled pore volume and thus the influence of oxygen on the activity of anaerobic methanogenic archaea. Therefore, it was necessary to carry out the experiments with varying water potentials under aerobic and anaerobic conditions to investigate the influence of water potential independent from the influence of oxygen.
It is well known, that the activity of soil microorganisms is decisively controlled by the water potential19. The methane production activity under anoxic conditions of the undisturbed soil samples showed, according to the Kruskal-Wallis analysis, a significant dependence on the increasing water potential. In contrast, the methane production rates in the presence of oxygen for water potentials of −6, −30 and −100 kPa were not significant different. This indicates that the activity of methanogens in relation to the soil water potential under aerobic conditions was overlapped by the influence of oxygen. A step by step increase of the soil water potential should lead to a gradual decrease of the methane production activity.
The exchange of gases between soils and the atmosphere as well as the gas transport within soils differs due to the soil water potential because of different diffusion coefficients of water-filled and air-filled pores29. Oxygen for example is transported in the gas phase 104 times faster (2.1 × 10−1 cm−2 s−1) than in the water phase (2.6 × 10−5 cm−2 s−1). The insignificant differences of methane production under oxic conditions indicated that the methanogenic archaea as well as the facultative anaerobic microorganisms had probably their greatest extent in the pores of 1 to 3 µm size, which were not drained in these experiments. The effect of oxygen was the same at all drainage levels and did not increase with increasing water potential. This interpretation is supported by the fact that the methane production rates of the high drained samples with a water potential of −100 kPa in the presence and in the absence of oxygen were similar.
This observation is in accordance with the results of Schricker30, who showed that the optimal water potential for microorganisms certainly was higher than −30 kPa, but a significant decline of the microbial activity was only observed when the pores ≤ 1 µm were drained. An influence of reduced substrate availability could be also excluded, because this effect can only be observed at a water potential of −600 kPa or higher31.
The results presented in a previous study by Wagner et al.15 and the data presented here, show, that methane production in aerobic horizons in wetland soils is possible by a spatial coupling with aerobic respiration. Only in combination with the absorbing capacity of soil particles such as clay, silt, and organic matter the indigenous microflora is able to develop a protective effect on the methanogenic archaea against oxygen. This potential of the investigated soil and the indigenous microbial community is of ecological significance because soils influenced by groundwater as well as other anaerobic habitats are periodically in contact with oxygen32,33,34,35. This particularly holds also for the investigated marshland soil, which is characterized by changing ground water tables.
Currently existing models for the calculation of methane release from wetlands often focusing on specific small-scale applications36,37 or oversimplifying the complexity of microbial processes, particularly the conditions under which methane production and oxidation may take place38,39. Best to our knowledge, there is only one process-based methane model published. It integrates for the first time the oxygen content into the modelling approach, in order to take into account methane oxidation potentials in periglacial landscapes40. However, of particular interest, is the potential activity of methanogenic archaea at the oxic-anoxic interface (Gor horizons) of marshlands shown in this study and eventually in other aerobic soils contributing to the methane production and therefore also to the methane emission. Figure 8 shows a descriptive model which integrates the soil water regime into the methane production potential in the presence of oxygen.
Descriptive model imaging the conditions for soil water and soil air in the investigated marshland soil at different water contents; (a) in the case when all pores (meso- and macropores) are drained (up to −100 kPa water potential), (b) in the case when only the coarse macropores are drained (−6 kPa water potential). The differences in the water content refer to the degree of filling of the pores (highlighted in blue).
Below the water table (below 50 cm soil depth) all pores are filled with water and the diffusion of oxygen is mostly restricted (Eh~ -200 mV), which causes best conditions for methanogenic archaea and methane production. Above the water table the clay fraction, which had a portion of 20% in the investigated soil, stored enough water for biological processes within the Gor horizons41. At first, the macropores are drained under these conditions (Fig. 8b). At a pore oxygen concentration of 15–21% a sufficient supply of respiration processes are still guaranteed at a thickness of the water film of 1 cm42. The portion of coarse macropores of the investigated profile amounted to 10%. Therefore relatively high oxygen diffusion under these conditions is assumed. Despite this fact a more or less high methane production was determined, because the meso- and micropores, which are the preferred habitat of microorganisms, were water saturated. In these pores of the Gor horizon the diffusion of oxygen remains permanently restricted.
All coarse and fine macropores and the mesopores were drained in the Go horizons, especially during dry periods when the water table became very low. In this case only a small water film43 existed around sand and silt particles (Fig. 8a). An inhibition of the methanogenic archaea as a result of a higher oxygen transport rate and therefore a significant increase of methane oxidation were conceivable. Our results show, that under these circumstances (soil water/soil air conditions) 25% of the methane production rate in comparison of completely anoxic conditions will be obtained (Fig. 7). The presence of clay minerals enhances this potential. Under these drained conditions clay minerals are still swollen and reduce the oxygen diffusion to methanogens which are in close association with soil particles41,44,45.
Conclusions
The investigations indicate, that methanogenic archaea survive not only in the aerobic layers of the marshland soil, but on account of their activity in an oxygen-influenced environment contribute also to methane production. Therefore, it is expected that other aerobic habitats such as forest floors and arable soils have a possible methane production potential and could be temporary a source of methane. The presented results are of great importance for modelling of methane release from natural wetlands, because this study shows that up to 25% of CH4 is produced in the oxic and transition layers (Go, Gor) of the investigated marshland soil, which can be a significant contribution to the methane production. This conclusion is supported by the studies of Walter38 for instance, who showed a difference of the calculated methane emission and the real determined methane release from North American wetlands, if the soils were not completely water saturated. Based on the presented results, it seems to be necessary to integrate the methane production activity in anaerobic niches in oxic soil horizons into future process-based methane modelling approaches.
Methods
Investigation site
The investigation area, which is called ‘Asseler Sand’ is located at the Lower Elbe near the city of Hamburg, Northern Germany (53°42′N, 9°27′E). The study site represents a typical freshwater marshland soil of perimarine silty loamy sediments. Further details on the properties of the investigated Elbe river marshland was given by Wagner et al.15.
MPN counts
The number of methanogenic archaea was determined in a three-tube most probable number (MPN) analysis using a ten-fold serial dilution of soil in growth medium. The composition of the minimal medium was described by Wagner and Pfeiffer46.
The MPN-method was applied to count the cell numbers for a vertical profile of the investigated marshland. The vertical profile was determined to a depth of 100 cm. As energy and carbon source acetate (20 mM) or H2/CO2 (80:20 v/v, pressurized 150 kPa) were used. The bottles were incubated at 10 °C (in situ temperature at the time of sampling) for 12 weeks in darkness.
Methane production activity
Fresh soil material (15 g) from 5 cm soil layers each was weighed into 25-ml glass jars and closed with black rubber stoppers. The samples were evacuated and flushed with ultra-pure N2. The prepared soil samples were incubated at 10°C without any additional substrate. Gas samples were taken every 24 h out of the jars headspace with a gastight syringe. CH4 production rates were calculated from the linear increase in CH4 concentration analysed by gas chromatography.
Acetylene as a specific inhibitor for CH4 oxidation
To suppress CH4 oxidation in the presence of oxygen acetylene was used as a specific inhibitor47. A first test should show whether the CH4 oxidation activity was completely stopped with the used acetylene concentration and whether the CH4 production activity was influenced or not.
Therefore, fresh soil samples, taken from the anoxic layer (60–70 cm depth) of the profile, were passed through a stainless steel sieve of 2 mm mesh size. Then 100 g of the homogenized soil were weighed into 250 ml Erlenmeyer flasks and mixed with 20 ml sterile and anoxic tap water. These samples were used to study CH4 oxidation as well as CH4 production in the presence and in the absence of acetylene. In the case of CH4 oxidation the flasks were flushed with synthetic air containing 2.5% CH4, whereas the effect on CH4 production was investigated under anoxic conditions. In each case 3 samples were incubated with and without acetylene (60 nl acetylene ml−1 headspace). All slurries were shaken continuously at 28 °C. CH4 consumption and production was analysed during 170 h by gas chromatography.
Pore size distribution and CH4 production at defined water potentials
The study of methane production activity in dependence on different soil water potentials was carried out using undisturbed soil samples taken with high-grade steel cylinder (100 cm3). The soils were sampled at a soil depth of 60–70 cm at the investigation site Asseler Sand. This soil horizon represented the layer with significant in situ methane production in the zone of changing groundwater table (Fig. 2).
To adjust water contents to specific pores (drained coarse macropores with < 50 µm, fine macropores of 50–10 µm and part of the mesopores with 10–3 µm) the undisturbed soil samples were drained using pressures of 0.3, 6, 30 and 100 kPa according to the method of Richards and Fireman48. To determine remaining pore size distribution disturbed soil material of above samples was also drained with a pressure of 300 and 1500 kPa. The total pore volume of the samples was analysed with a vacuum-air-pycnometer.
The 100 cm3 steel cylinder with the drained soil samples were placed into larger cylinders (Ø 10 cm, 35 cm hight, high grade steel) with a special fixture for 3 undisturbed soil samples46. The cylinders were closed with gastight seals at both ends. The top had two ports with ball taps. One port was used to exchange the headspace atmosphere and the other one had a screw cap with septum used as a syringe port.
The samples were used to study methane production of undisturbed marshland soils under oxic and anoxic conditions. In the case of anoxic methane production the cylinder was flushed with N2/CO2 (80:20 v/v). For the determination of methane production under oxic conditions the cylinder was flushed with synthetic air containing 20% oxygen. To inhibit methane oxidation, all samples (oxic and anoxic) were supplied with 60 nl acetylene ml−1 headspace. Additional H2/CO2 (20% H2, 100 kPa pressurized) was given as a substrate. Three replicates were used for the anoxic experiments and four replicates for the experiments under oxic conditions. All cylinders were incubated at 25 °C. Gas samples were taken from the headspace of the cylinder with a gastight syringe and analysed for the concentration of CH4 and O2 by gas chromatography. Methane production rates were calculated from the linear increase in methane concentration.
Gas analysis
Methane and oxygen concentrations were determined with a Carlo Erba (GC 6000 vega series 2) gas chromatograph. The instrument was equipped with a Heyesep D (100/120 mesh, 20 ft) and a Molesieve 5 A (60/80 mesh, 7 ft) stainless steel column connected with a switching valve. Methane was analysed by a flame ionization detector (FID) and oxygen by a hot wire detector (HWD). All gas sample analyses were done after calibration with standards of known concentrations of the respective gases. The injector temperature was set at 100 °C, the columns at 70 °C and the FID at 200 °C. The detector temperature of the HWD was 100 °C and the filament cell temperature was 180 °C. Helium was used as carrier gas.
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Change history
09 May 2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
Wuebbles, J. & Hayhoe, K. Atmospheric methane and global change. Earth-Sci. Rev. 57, 177–210 (2002).
Myhre, G. et al. Anthropogenic and Natural Radiative Forcing in Climate Change 2013: The Physical Science Basis, Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds. Stocker, T. F. et al.) 659–740 (Cambridge University Press, 2013).
Ito, A. & Inatomi, M. Use of a process-based model for assessing the methane budgets of global terrestrial ecosystems and evaluation of uncertainty. Biogeosciences 9, 759–773 (2012).
Wahlen, M. et al. Carbon-14 in methane sources and in atmospheric methane: the contribution from fossil carbon. Science 245, 286–290 (1989).
Wille, C., Kutzbach, L., Sachs, T., Wagner, D. & Pfeiffer, E.-M. Methane emission from Siberian Arctic polygonal tundra: eddy covariance measurements and modeling. Glob. Change Biol. 14, 1395–1408 (2008).
Bridgham, S. D., Cadillo-Quiroz, H., Keller, J. K. & Zhuang, Q. Methane emissions from wetlands: biogeochemical, microbial, and modeling perspectives from local to global scales. Glob. Change Biol. 19, 1325–1346 (2013).
Kirschke, S. et al. Three decades of global methane sources and sinks. Nat. Geosci. 6, 813–823 (2013).
Priemé, A. Production and emission of methane in a brackish and a freshwater wetland. Soil Biol. Biochem. 26, 7–18 (1994).
Shannon, R. D. & White, J. R. A three-year staudy of controls on methane emissions from two Michigan peatlands. Biogeochemistry 27, 35–60 (1994).
Chen, H. et al. Methane emissions from rice paddies natural wetlands, lakes in China: synthesis new estimate. Glob. Change Biol. 19, 19–32 (2013).
Liebner, S. et al. Shifts in methanogenic community composition and methane fluxes along the degradation of discontinuous permafrost. Front. Microbiol. 6, 356 (2015).
Pandey, S. et al. Enhanced methane emissions from tropical wetlands during the 2011 La Niña. Sci. Rep. 7, 45759 (2017).
Schimel, J. P., Holland, E. A. & Valentine, D. Controls on methane flux from terrestrial ecosystems in Agricultural ecosystem effects on trace gases and climate change. (eds. Harper, L. A., Mosier, A. R, Duxbury, J. M. & Rolston, D. E.) 167–182 (American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, Medison, 1993).
Segers, R. & Kengen, S. W. M. Methane production as a function of anaerobic carbon mineralization: a process model. Soil Biol. Biochem. 30, 1107–1117 (1998).
Wagner, D., Pfeiffer, E.-M. & Bock, E. Methane production in aerated marshland and model soils: effects of microflora and soil texture. Soil Biol. Biochem. 31, 999–1006 (1999).
Wagner, D., Kobabe, S., Pfeiffer, E.-M. & Hubberten, H.-W. Microbial controls on methane fluxes from a polygonal tundra of the Lena Delta, Siberia. Permafrost Periglac. Process. 14, 173–185 (2003).
Jerman, V., Danevčič, T. & Mandic-Mulec, I. Methane cycling in a drained wetland soil profile. J Soils Sediments, https://doi.org/10.1007/s11368-016-1648-2 (2017).
Wagner, D., Kobabe, S. & Liebner, S. Bacterial community structure and carbon turnover in permafrost-affected soils of the Lena Delta. Canadian J. Microbiol. 55, 73–83 (2009).
Christiansen, J. R., Levy-Booth, D., Prescott, C. E. & Grayston, S. J. Microbial and Environmental Controls of Methane Fluxes Along a Soil Moisture Gradient in a Pacific Coastal Temperate Rainforest. Ecosystems 19, 1255–1270 (2016).
Meijide, A., Gruening, C., Goded, I., Seufert, G. & Cescatti, A. Water management reduces greenhouse gas emissions in a Mediterranean rice paddy field. Agric. Ecosyst. Environ. 238, 168–178 (2017).
Vizza, C., West, W. E., Jones, S. E., Hart, J. A. & Lamberti, G. A. Regulators of coastal wetland methane production and responses to simulated global change. Biogeosciences 14, 431 (2017).
Whitman, W. B., Bowen, T. C. & Boone, D. R. The methanogenic bacteria in The prokaryotes (eds Balows, A., Trüper, H. G., Dworkin, M., Harder, W. & Schleifer, K. H.) 719–767 (Springer, 1992).
Kiener, A. & Leisinger, T. Oxygen sensitivity of methanogenic bacteria. Syst. Appl. Microbiol. 4, 305–312 (1983).
Morozova, D. & Wagner, D. Stress response of methanogenic archaea from Siberian permafrost compared to methanogens from non-permafrost habitats. FEMS Microbiol. Ecol. 61, 16–25 (2007).
Mayer, H.-P. & Conrad, R. Factors influencing the population of methanogenic bacteria and the initiation of methane production upon flooding of paddy soils. FEMS Microbiol. Ecol. 73, 103–112 (1990).
Peters, V. & Conrad, R. Methanogenic and other strictly anaerobic bacteria in desert soil and other oxic soils. Appl.Environ. Microbiol. 61, 1673–1676 (1995).
Griffin, D. M. Water potential as a selective factor in the microbial ecology of soils in Water potential relations in soil microbiology. (eds Parr, J. F., Gardner, W. R. & Elliott, L. F.) 141–151 (Soil Science Society of America, 1981).
Kato, M. T., Field, J. A. & Lettinga, G. High tolerance of methanogens in granular sludge to oxygen. Biotech. Bioeng. 42, 1360–1366 (1993).
Castro, M. S., Steudler, P. A. & Bowden, R. D. Factors controlling atmospheric methane consumption by temperate forest soils. Glob. Biogeochem. Cyc. 9, 1–10 (1995).
Schricker, K. Mikrobielle Aktivitäten und Populationen in Abhängigkeit vom Wasserpotential und anderen Bodenparametern (English summary). Bayreuther Forum Ökol. 34, 1–191 (1996).
Stark, J. M. & Firestone, M. K. Mechanisms for soil moisture effects on activity of nitrifying bacteria. Appl. Environ. Microbiol. 61, 218–221 (1995).
Benstead, J. & Lloyd, D. Spatial and temporal variations of dissolved gases (CH4, CO2 and O2) in peat cores. Microb. Ecol. 31, 57–66 (1996).
Brocke, H. J. et al. High dissolved organic carbon release by benthic cyanobacterial mats in a Caribbean reef ecosystem. Sci. Rep. 5, 8852 (2015).
D’Hondt, S. et al. Presence of oxygen and aerobic communities from sea floor to basement in deep-sea sediments. Nat. Geosci. 84, 299–304 (2015).
Waddington, J. M. et al. Hydrological feedbacks in northern peatlands. Ecohydrology 8, 113–127 (2015).
Walter, B. P. & Heimann, M. A process-based, climate-sensitive model to derive methane emissions from natural wetlands: Application to five wetland sites, sensitivity to model parameters, and climate. Glob. Biogeochem. Cycles 14, 745–765 (2000).
Mi, Y. et al. Improving a plot-scale methane emission model and its performance at a northeastern Siberian tundra site. Biogeosciences 11, 3985–3999 (2014).
Walter, B. Development of a process-based model to derive methane emissions from natural wetlands for climate studies. Dissertation, University of Hamburg (1998).
Zhu, Q. et al. Modelling methane emissions from natural wetlands by development and application of the TRIPLEX-GHG model. Geosci. Model Dev. 7, 981–999 (2014).
Kaiser, S. et al. Process-based modelling of the methane balance in periglacial landscapes (JSBACH-methane). Geosci. Model Dev. 10, 333–358 (2017).
Strotzky, G. Influence of soil mineral colloids on metabolic processes, growth, adhesion, and ecology of microbes and viruses in Interactions of soil minerals with natural organics and microbes (eds Huang, P. M. & Schnitzer, M.) 305–428 (Soil Science Society of America, 1986).
Rowell, D. L. Bodenkunde - Untersuchungsmethoden und ihre Anwendung. Springer-Verlag (1994).
Harris, R. F. Effect of water potential on microbial growth and activity in Water potential relations in soil microbiology (eds Parr, J. F., Gardner, W. R. & Elliott, L. F.) 23–96 (Soil Science Society of America, 1981)
Strotzky, G. & Rem, L. T. Influence of caly minerals on microorganisms: I. Montmorillonite and kaolinite on bacteria. Canadian J. Microbiol. 12, 547–563 (1966).
Cygan, R. T. & Tazaki, K. Interactions of kaolin minerals in the environment. Elements 10, 195–200 (2014).
Wagner, D. & Pfeiffer, E.-M. Two temperature optima of methane production in a typical soil of the Elbe river marshland. FEMS Microbiol. Ecol. 22, 145–153 (1997).
Watanabe, A., Satho, Y. & Kimura, M. Estimation of the increase in CH4 emission from paddy soils by rice straw application. Plant Soil 173, 225–231 (1995).
Richards, L. A. & Fireman, M. Pressure plate apparatus for measuring moisture and transmission by soils. Soil Sci. 56, 395–404 (1943).
Acknowledgements
I am thankful to Eberhard. Bock and Reiner Mansch (Bio Centre Klein Flottbek, University of Hamburg) for stimulating discussion as well as Eva-Maria Pfeiffer (Institute of Soil Science, University of Hamburg) for supporting the soil experiments. Special thanks go to Birgit Grabellus and Holger Becker (Institute of Soil Science, University of Hamburg) for soil sampling under ‘critical’ conditions, to Peter Kühn (Eberhard Karls University Tübingen) for critical reading of the manuscript and Patryk Krauze (GFZ German Research Centre for Geosciences) for his IT support.
Author information
Authors and Affiliations
Contributions
D.W. designed the study, performed all analysis, interpreted the results and wrote the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wagner, D. Effect of varying soil water potentials on methanogenesis in aerated marshland soils. Sci Rep 7, 14706 (2017). https://doi.org/10.1038/s41598-017-14980-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-14980-y
- Springer Nature Limited