Abstract
Neuropsychiatric symptoms can be interrelated to delirium. We aimed to investigate an association between pre-stroke neuropsychiatric symptoms and the risk of delirium in stroke patients. We included 606 patients (median age: 73, 53% female) with stroke or transient ischemic attack admitted within 48 hours from symptoms onset. We assessed delirium on a daily basis during the first 7 days of hospitalization. To make diagnosis of delirium we used DSM-5 criteria. We used Neuropsychiatric Inventory to assess neuropsychiatric symptoms occurring within 4 weeks prior to stroke. We diagnosed delirium in 28.2% of patients. On univariate analysis, higher score of pre-stroke depression (OR: 1.58, 95% CI: 1.04–2.40, P = 0.03), apathy (OR: 2.23, 95% CI: 1.44–3.45, P < 0.01), delusions (OR: 2.00, 95% CI: 1.09–3.68, P = 0.03), hallucinations (OR: 2.39, 95% CI: 1.19–4.81, P = 0.01) and disinhibition (OR: 2.10, 95% CI: 1.04–4.25, P = 0.04) was associated with the increased risk of delirium. On multivariate analysis adjusted for age, atrial fibrillation, diabetes mellitus, stroke severity, right hemisphere lesion, pre-stroke cognitive decline, pre-stroke disability and infections, higher apathy score (OR: 2.03, 95% CI: 1.17–3.50, P = 0.01), but no other neuropsychiatric symptoms, remained independent predictor of delirium. We conclude that pre-stroke apathy symptoms are associated with increased risk of delirium in stroke patients.
Similar content being viewed by others
Introduction
Delirium is a transient neurocognitive disorder characterized by cognitive, psychomotor and behavioral symptoms. The core features include attention and awareness disturbances, acute onset and fluctuations in symptoms severity1. Between 10% and 48% of stroke patients develop delirium2. Patients with post-stroke delirium have higher mortality, longer hospital stay and worse functional outcome3, 4.
Although delirium is widely recognized and has important clinical implications, its etiology is not fully explained. Delirium can be considered as a sign of the vulnerable brain with reduced resilience to insults5. According to this hypothesis, predisposing factors might be understood as preexisting conditions that diminish cognitive reserve and disturb brain compensatory mechanisms6. Therefore, identification of predisposing factors is important for understanding delirium’s pathophysiology.
Neuropsychiatric symptoms are not rare in elderly persons. In population-based study 27% of cognitively normal participants had at least one neuropsychiatric symptom7. These symptoms are even more frequent in patients with mild cognitive impairment7 or dementia8. Neuropsychiatric disturbances can be potentially inter-related to delirium. Both conditions can involve disruption of common neuronal networks responsible for mood and cognition, share risk factors and have similar pathophysiological mechanisms9. Numerous studies showed that depression is a risk factor for delirium9, 10. The relationship between other neuropsychiatric symptoms and delirium is poorly understood.
The aim of our study was to determine an association between preexisting neuropsychiatric symptoms and risk of delirium in stroke patients.
Methods
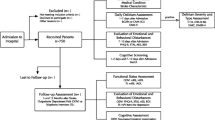
PRospective Observational POLIsh Study on post-stroke delirium (PROPOLIS) is a prospective, single center study conducted in Department of Neurology, University Hospital, Krakow, Poland. The main goal of PROPOLIS is to determine frequency, predisposing factors and consequences of delirium in stroke patients11. We recruited participants to this study between May 2014 and March 2016. The Bioethics Committee of Jagiellonian University approved the study’s protocol. All methods were performed in accordance with approved guidelines and regulations. Each patient or his/her legal guardian gave an informed consent.
Inclusion criteria to this study were: (1) acute stroke (brain infarction, transient ischemic attack, intracerebral hemorrhage); (2) age ≥18 years; (3) admission within 48 h from symptoms onset; (4) Polish as a native language; (5) informed consent of a patient or a legal guardian. The exclusion criteria were: coma, brain tumor, alcohol withdrawal syndrome, cerebral venous thrombosis, subarachnoid hemorrhage, head trauma, vasculitis and diseases with life expectancy ≤1 year.
We screened patients for delirium on a daily basis during the first 7 days after admission to hospital. To assess core features of delirium we used Brief Confusion Assessment Method (bCAM) for verbal12 and Intensive Care Units version (CAM-ICU) for non-verbal patients13. When delirium was suspected, we also used Delirium Rating Scale-Revised-9814. In addition, nurses completed daily questionnaire about patients’ behavior and cognitive fluctuations. For the final diagnosis of delirium, we analyzed collected data in clinical context and assessed them according to DSM-5 criteria15. Patients with severe aphasia were assessed with Delirium Rating Scale-Revised–98, nurses daily questionnaire and DSM-5 criteria.
We gathered information about pre-stroke functional, cognitive and neuropsychiatric status using a structured interview with the close relative or caregiver who knew the patient well. This interview was completed during 48 hours from admission. We review patients’ medications for anticholinergic properties according to Anticholinergic Risk Scale (ARS)16. The scale ranks medication from 0 (no or low potential) to 3 (high potential). The total score is the sum of points for all patient’s medications.
We used Neuropsychiatric Inventory (NPI) to assess neuropsychiatric disturbances occurring within the four weeks prior to admission17. The NPI-Q10 subscale includes 10 behavioral items: delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritability and aberrant motor behavior. We calculated score for each item (from 0 to 12) as a product of severity scale (from 0 to 3) and frequency scale (from 0 to 4).
To assess pre-stroke functional status, we used modified Rankin Scale (mRS)18. We defined functional dependency as mRS 3–5.
To diagnose pre-stroke cognitive decline, we used a validated Polish version of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE)19. The questionnaire consists of 26 items that rate the change in patients’ intellectual abilities over the past ten years. Each item is rated from 1 - much improved to 5 - much worse. Several cut-off scores for cognitive decline are used in practice20. For the purpose of our study, we chose the threshold of 3.3, because it has yielded the highest sensitivity20.
We assessed neurological deficit on admission using National Institute of Health Stroke Scale (NIHSS)21. Patients who received 2 points in speech item were classified as non-verbal. Patients who received 2 or more points in language item were classified as severely aphasic. For diagnosis of pneumonia and urinary tract infections we used Centers for Disease Control and Prevention criteria for clinically defined pneumonia and symptomatic urinary tract infection22.
We used χ2 test to compare proportions and Mann-Whitney test to compare continuous variables. We used uni- and multivariate logistic regression analyses to determine an association between independent variables and delirium. To multivariate analysis we included all variables with P value below 0.05 on univariate analysis. In analyses, we compared upper quartile of NPI scores to other quartiles. We performed 1:2 propensity score matching without replacement using nearest neighbor algorithm. For calculations, we used Statistica for Windows (version 10; Statsoft, Poland).
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Results
PROPOLIS study included 750 stroke patients (median age: 73, interquartiles: 63–82; 53.1% women; median NIHSS score on admission: 6, interquartiles: 3–15).
Information about pre-stroke neuropsychiatric symptoms was available for 606 patients (80.8%) and we included them into further analyses. Median age in this group was 73 (interquartiles: 63–82) and median NIHSS score was 6 (interquartiles: 3–14). Fifty-three per cent of patients were women. Baseline characteristic (age, sex and NIHSS score on admission) of patients included into analyses did not differ from the total cohort of patients participated in PROPOLIS.
We diagnosed delirium in 171 patients (28.2%). Table 1 shows baseline characteristics and Table 2 shows neuropsychiatric symptom scores in patients with delirium and patients without delirium.
Compared to patients without delirium, delirious patients were older, more often suffered from atrial fibrillation, diabetes mellitus, pre-stroke cognitive decline and pre-stroke disability. They had more severe neurological deficit on admission and more often suffered from in-hospital pneumonia and urinary tract infections. Right hemisphere lesions were more frequent in delirious patients. Total NPI-Q10 score was higher in patients with delirium compared to those without delirium. Among neuropsychiatric symptoms, only apathy had significantly higher score in patients with delirium.
In logistic regression analysis, we compared the upper quartile of NPI scores to other quartiles Table 3.
On univariate analysis, higher score of pre-stroke depression, apathy, delusions, hallucinations and disinhibition were associated with the increased risk of delirium.
In the next step, we separately analyzed an association between delirium and each neuropsychiatric symptom after adjusting for potential confounders (age, atrial fibrillation, diabetes mellitus, NIHSS score on admission, right hemisphere lesion, pre-stroke cognitive decline, pre-stroke disability, pneumonia and urinary tract infections). Higher apathy score was associated with the increased risk of delirium (OR: 2.03, 95% CI: 1.17–3.50, P = 0.01). The association between other neuropsychiatric symptoms and delirium was non-significant on multivariate logistic regression analysis.
Finally, we put into the statistical model all-above mentioned confounders and all neuropsychiatric symptoms that had P value < 0.05 on univariate analysis (depression, apathy, delusions, hallucinations, disinhibition). Higher apathy score remained independent predictor of delirium (OR: 2.22, 95% CI: 1.22–4.02, P = 0.01).
Other significant predictors of delirium in our study were: older age (OR: 1.02, 95% CI: 1.00–1.04, P = 0.04), diabetes mellitus (OR: 1.91, 95% CI: 1.21–3.02, P < 0.01), right hemisphere lesion (OR: 1.78, 95% CI: 1.15–2.75, P = 0.01), pneumonia (OR: 2.29, 95% CI: 1.34–3.94, P < 0.01), and higher NIHSS score (OR: 1.11, 95% CI: 1.07–1.14, P < 0.01).
Data about pre-stroke medications were available for 536 patients (Table S1 in supplementary file).
Exclusion of patients who took anti-depressants (N = 18), neuroleptics (N = 7) or benzodiazepines (N = 12) before stroke, did not substantially change the results of multivariate analysis.
In our study, ARS score above 0 (OR: 2.02, 95% CI: 0.79–5.14, P = 0.14) or ARS score above 1 (OR: 1.82, 95% CI: 0.51–6.58, P = 0.36) was not associated with delirium on univariate analysis.
Next, we checked if pre-stroke medications could confound our results. After adding diuretics, insulin and angiotensin converting enzyme inhibitors to the to the statistical model (containing age, NIHSS score, atrial fibrillation, pre-stroke cognitive decline, pre-stroke disability and infections), the odds ratio of delirium in patients with higher apathy score was 1.80 (95% CI: 1.00–3.24, P = 0.049). The variable “diabetes mellitus” was not put into the model due to its interaction with variable “insulin”.
To confirm the obtained results, we performed cohort study matching. Propensity scores of patients with delirium were matched with propensity scores of patients without delirium taking into account the most important confounders (age, NIHSS score, diabetes mellitus, atrial fibrillation, infections, pre-stroke cognitive decline). A logistic regression analysis performed on balanced groups showed that higher pre-stroke apathy score is associated with an increased risk of delirium (OR: 1.89, CI: 1.19–2.99, P < 0.01).
Discussion
Among 10 neuropsychiatric symptoms only apathy was independently associated with an increased risk of post-stroke delirium. Apathy is generally defined as a loss of motivation23. It can be considered as a separate syndrome or as a symptom of other disorders e.g. depression. Almost twenty per cent of community-dwelling elderly persons who are free of dementia and depression have symptoms of apathy24. Apathy is associated with a higher risk of functional impairment, lower cognitive performance and dementia25.
The association between apathy and delirium has not receive much attention so far. Hölttä and colleagues examined a cohort of 425 patients hospitalized in geriatric wards and nursing homes26. Apathy was assessed by trained nurses or geriatricians without use of any validated neuropsychiatric questionnaire. Twenty-three per cent of patients suffered from apathy. Patients with apathy more often had delirium (37.8% vs 21.1%, P < 0.01). The authors did not report results of multivariate analysis.
Apathy and delirium could be related to each other in several ways. First, structural disruption of common neuronal networks can be seen in apathy and delirium. In both entities diffusion tensor imaging studies showed microstructure abnormalities in frontal lobe, corpus callosum, thalamus and limbic structures27, 28. These structures are important for regulation of emotions and maintenance of consciousness and attention28, 29. Further studies are needed to identify common neuronal substrate for delirium and apathy. Second, the same risk factors could be relevant for delirium and apathy. Neurodegenerative and vascular pathologies can predispose to both of them. Apathy is common in neurocognitive disorders such as mild cognitive impairment or Alzheimer’s disease and its incidence increases with dementia progression25. In elderly, apathy is also associated with vascular risk factors (systolic blood pressure, body mass index, C-reactive protein level) and cardiovascular diseases including stroke24. Cognitive decline and vascular risk factors are predictors of delirium5, 30. In our multivariate analysis, apathy score predicted delirium independently from pre-stroke cognitive decline and vascular risk factors. Third, we previously mentioned that delirium can be a sign of brain vulnerability. In this context, apathy may be an early marker of diminished cognitive reserve, which appears before cognitive decline becomes detectable with screening tools. Given relatively low specificity of IQCODE in detecting patients who would develop dementia, this theory needs further investigation with the use of more accurate test31. Finally, apathy can be associated with depression, which is a risk factor for delirium in certain group of patients9, 22. Adding depressive symptoms score to our model did not change the association between apathy and delirium.
We did not find the association between delirium and depressive symptoms. Although depression was found to predispose to delirium in many studies9, some authors did not confirm this relation32, 33. We proposed several explanations for a lack of association between depression and delirium in our study. First, we used NPI-Q10 item to diagnose depressive symptoms. This instrument is based on interview with informants. Many previous studies assessed depression with tools based on self-report inventory such as Geriatric Depression Scale – 15 or Beck Depression Inventory9. Therefore, depression in our group might have been underestimated. Second, predictive role of depression may not refer to the whole syndrome, but only to some specific symptoms such as dysphoric mood and feeling of hopelessness34. Furthermore, the association between depression and delirium may be seen only in certain populations. In the literature review performed by Nelson and colleagues the connection between delirium and depression was found only in postoperative patients10. Finally, apathetic patients might meet criteria for depression35. Not all authors differentiated depression from apathy as we did in our study. Consequently, they might have misdiagnosed apathy as depression.
Apart from depression, anxiety has been considered as a predisposing factor for delirium. Two studies yielded negative results about relationship between delirium and anxiety33, 36. Schneider and colleagues found that psychopathological symptoms predicted higher delirium severity but they did not analyze separate symptoms37. We found on univariate analysis that delusions, hallucinations, and disinhibition were related to increased risk of delirium. However, this association did not withstand adjustment for potential confounders including pre-stroke cognitive decline.
Advantages of our study include: comprehensive assessment of delirium on a daily basis, complex evaluation of neuropsychiatric symptoms and relatively large group of unselected stroke patients.
We also need to address some limitations. NPI is commonly used tool for assessment of neuropsychiatric symptoms but it does not acknowledge self-reported symptoms. Given low agreement between self and informant report38, future studies should include these two complementary sources. We did not have information about pre-stroke neuropsychiatric symptoms for 19% of participants. It could be a source of potential bias, however, the total cohort of patients participated in PROPOLIS study and those included into this analysis did not differ in baseline characteristic. We cannot exclude the possibility that we missed delirium in patients who had symptoms only before admission to hospital. We also realized that stroke sequelae such as aphasia, inattention or neglect might interfere with delirium assessment. To avoid a misdiagnosis of delirium, we daily repeated assessment using validated tools, which cover multiple delirium manifestations and we paid special attention to symptoms fluctuations. Based on previous studies we did not exclude stroke patients due to dysphasia or attention disturbances on admission4, 39, 40. Exclusion of patients with potentially interfering stroke sequelae would lead to an underestimation of delirium in a whole cohort of stroke patients. In single cases, even after meticulous examination, it might be difficult to differentiate whether disturbances in attention, awareness and cognition are due to delirium or they are secondary to neurological and neuropsychiatric consequences of stroke. In clinical practice, including such patients under the umbrella of delirium will result in increased patient safety through delirium identification and prevention. Furthermore, due to a lack of normative data, NPI does not allow us to differentiate between normal and pathological intensity of neuropsychiatric symptoms. Lastly, predisposing factors for delirium vary among different cohorts of patients5, therefore, our results cannot be generalized and need to be confirmed in non-stroke patients.
Apathy may be considered as a predisposing factor for post-stroke delirium but can be also a marker of other conditions which contribute to delirium risk, such as undetected cognitive decline. This needs clarification in future studies. Nevertheless, our findings could have potential clinical implications. Identification of patients with premorbid apathy symptoms might be important for a selection of persons who are at risk of delirium. These patients may demand extensive delirium prevention and closer monitoring for early detection and escalated treatment of delirium.
In conclusion, we found apathy symptoms as a predisposing factor for post-stroke delirium.
References
Meagher, D. J., MacLullich, A. M. J. & Laurila, J. V. Defining delirium for the International Classification of Diseases, 11th Revision. J Psychosom Res 65, 207–214 (2008).
Klimiec, E., Dziedzic, T., Kowalska, K., Slowik, A. & Klimkowicz-Mrowiec, A. Knowns and Unknowns About Delirium in Stroke: A Review. Cogn Behav Neurol 29, 174–189 (2016).
Shi, Q., Presutti, R., Selchen, D. & Saposnik, G. Delirium in acute stroke: a systematic review and meta-analysis. Stroke 43, 645–649 (2012).
Oldenbeuving, A. W. et al. Delirium in the acute phase after stroke: incidence, risk factors, and outcome. Neurology 76, 993–999 (2011).
Inouye, S. K., Westendorp, R. G. J. & Saczynski, J. S. Delirium in elderly people. Lancet 383, 911–922 (2014).
Inouye, S. K. & Charpentier, P. A. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA 275, 852–857 (1996).
Geda, Y. E. et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry 65, 1193–1198 (2008).
Lyketsos, C. G. et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 288, 1475–1483 (2002).
O’Sullivan, R., Inouye, S. K. & Meagher, P. D. Review Delirium and depression: inter-relationship and clinical overlap in elderly people. Lancet Psychiatry 1, 303–311 (2014).
Nelson, S., Rustad, J. K., Catalano, G., Stern, T. A. & Kozel, F. A. Review Article Depressive Symptoms Before, During, and After Delirium: A Literature Review. Psychosomatics 57, 131–141 (2016).
Klimiec, E., Dziedzic, T., Kowalska, K., Slowik, A. & Klimkowicz-Mrowiec, A. PRospective Observational POLIsh Study on post-stroke delirium (PROPOLIS): methodology of hospital-based cohort study on delirium prevalence, predictors and diagnostic tools. BMC Neurology 15, 94 (2015).
Han, J. H. et al. Diagnosing delirium in older emergency department patients: validity and reliability of the delirium triage screen and the brief confusion assessment method. Ann Emerg Med 62, 457–465 (2013).
Ely, E. W. E. et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 286, 2703–2710 (2001).
Trzepacz, P. T. Validation of the Delirium Rating Scale-Revised-98: comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci 13, 229–242 (2001).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed., (APA, Arlington, VA, 2013).
Rudolph, J. L., Salow, M. J., Angelini, M. C. & McGlinchey, R. E. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med 168, 508–13 (2008).
Cummings, J. L. The Neuropsychiatric Inventory Assessing psychopathology in dementia patients. Neurology 48, 10S–16S (1997).
Bonita, R. & Beaglehole, R. Recovery of motor function after stroke. Stroke 19, 1497–1500 (1988).
Jorm, A. F. & Korten, A. E. Assessment of cognitive decline in the elderly by informant interview. Br J Psychiatry 152, 209–213 (1988).
Harrison, J. K. et al. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) for the diagnosis of dementia within a secondary care setting. Cochrane Database Syst Rev 3, CD010772 (2015).
Brott, T. et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20, 864–870 (1989).
Horan, T. C., Andrus, M. & Dudeck, M. A. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J of Infect Control 36, 309–332 (2008).
Marin, R. S. Differential diagnosis and classification of apathy. Am J Psych 147, 22–30 (1990).
Ligthart, S. A. et al. Association of vascular factors with apathy in community-dwelling elderly individuals. Arch Gen Psychiatry 69, 636–642 (2012).
Lanctôt, K. L. et al. Apathy associated with neurocognitive disorders: recent progress and future directions. Alzheimers Dement 16, 1–17 (2016).
Hölttä, E. H. et al. Apathy: prevalence, associated factors, and prognostic value among frail, older inpatients. J Am Med Dir Assoc. 13, 541–545 (2012).
Cavallari, M. et al. Neural substrates of vulnerability to postsurgical delirium as revealed by presurgical diffusion MRI. Brain 139, 1282–1294 (2016).
Hollocks, M. J. et al. Differential relationships between apathy and depression with white matter microstructural changes and functional outcomes. Brain 138, 3803–3815 (2015).
Steriade, M. Awakening the brain. Nature 383, 24–25 (1996).
Rudolph, J. L. et al. Independent vascular and cognitive risk factors for postoperative delirium. Am J Med 120, 807–813 (2007).
Harrison, J. K. et al. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) for the early diagnosis of dementia across a variety of healthcare settings. Cochrane Database Syst Rev 11, CD011333 (2016).
Jankowski, C. J. et al. Cognitive and functional predictors and sequelae of postoperative delirium in elderly patients undergoing elective joint arthroplasty. Anesth Analg 112, 1186–1193 (2011).
Detroyer, E. et al. Is preoperative anxiety and depression associated with onset of delirium after cardiac surgery in older patients? A prospective cohort study. J Am Geri Soc 56, 2278–2284 (2008).
McAvay, G. J. et al. Depressive Symptoms and the Risk of Incident Delirium in Older Hospitalized Adults. J Am Geri Soc 55, 684–691 (2007).
Mortby, M. E., Maercker, A. & Forstmeier, S. Apathy: a separate syndrome from depression in dementia? A critical review. Aging Clin Exp Res 24, 305–316 (2012).
Van Grootven, B. et al. Is preoperative state anxiety a risk factor for postoperative delirium among elderly hip fracture patients? Geriatr Gerontol Intl 16, 948–955 (2015).
Schneider, F. et al. Risk factors for postoperative delirium in vascular surgery. Gen Hosp Psychiatry 24, 28–34 (2002).
McKinlay, A. et al. Neuropsychiatric problems in Parkinson’s disease: Comparisons between self and caregiver report. Aging Ment Health 12, 647–653 (2008).
Oldenbeuving, A. W., de Kort, P. L., van Eck van der Sluijs, J. F., Kappelle, L. J. & Roks, G. An early prediction of delirium in the acute phase after stroke. J Neurol Neurosurg Psychiatry 85, 431–434 (2014).
Mitasova, A. et al. Poststroke delirium incidence and outcomes: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med 40, 484–490 (2012).
Author information
Authors and Affiliations
Contributions
E.K., K.K., A.K.M., J.P. and T.D. prepared study protocol. E.K., K.K., P.P. and A.Sz. collected the data. T.D. supervised the study. E.K. and T.D. wrote the manuscript. J.P. and A.S. revised the manuscript for intellectual content.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Klimiec, E., Kowalska, K., Pasinska, P. et al. Pre-stroke apathy symptoms are associated with an increased risk of delirium in stroke patients. Sci Rep 7, 7658 (2017). https://doi.org/10.1038/s41598-017-08087-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-08087-7
- Springer Nature Limited
This article is cited by
-
Clinical utility of brain computed tomography in prediction of post-stroke delirium
Journal of Neural Transmission (2021)