Abstract
Brain metastases (BMs) and high-grade gliomas (HGGs) are the most common and aggressive types of malignant brain tumors in adults, with often poor prognosis and short survival. As their clinical symptoms and image appearances on conventional magnetic resonance imaging (MRI) can be astonishingly similar, their accurate differentiation based solely on clinical and radiological information can be very challenging, particularly for “cancer of unknown primary”, where no systemic malignancy is known or found. Non-invasive multiparametric MRI and radiomics offer the potential to identify these distinct biological properties, aiding in the characterization and differentiation of HGGs and BMs. However, there is a scarcity of publicly available multi-origin brain tumor imaging data for tumor characterization. In this paper, we introduce a multi-center, multi-origin brain tumor MRI (MOTUM) imaging dataset obtained from 67 patients: 29 with high-grade gliomas, 20 with lung metastases, 10 with breast metastases, 2 with gastric metastasis, 4 with ovarian metastasis, and 2 with melanoma metastasis. This dataset includes anonymized DICOM files alongside processed FLAIR, T1-weighted, contrast-enhanced T1-weighted, T2-weighted sequences images, segmentation masks of two tumor regions, and clinical data. Our data-sharing initiative is to support the benchmarking of automated tumor segmentation, multi-modal machine learning, and disease differentiation of multi-origin brain tumors in a multi-center setting.
Similar content being viewed by others
Background & Summary
High-grade gliomas (HGGs) and intracranial brain metastases (BMs) are the most prevalent malignant brain tumors in adults. They have incidence rates of 4.26 and 7–14 per 100,000 population per year, respectively1,2. HGG is characterized by its high malignancy due to rapid progression and spread. Recent research findings estimate that these tumors account for over 50% of malignant primary brain and central nervous system (CNS) cancers1. The prognosis for HGG is typically bleak, especially for the most aggressive subtype glioblastoma (GBM). Fewer than 5% of diagnosed individuals survive beyond five years. The median survival rate for newly diagnosed GBM patients is between 15 and 18 months3. On the other hand, BMs, which are more prevalent than primary brain tumors, also present a poor prognosis and unique diagnostic and therapeutic challenges. Median survival often only spans a few months, even with therapy4. The rising incidence rates can be attributed to advancements in imaging tools for detection and increased survival rates from primary malignancies5.
Differentiating between BMs and HGGs is clinically significant due to the contrasting surgical and therapeutic strategies6,7,8,9. For HGGs, a comprehensive systemic evaluation is not recommended due to the rare incidence of extracranial spread. However, for suspected brain metastatic lesions without a known systemic cancer history, it is imperative to first identify the primary malignancy and perform comprehensive systemic staging before initiating surgical or pharmacological interventions7. However, there are situations when no primary tumor can be found in a patient with metastases (dubbed “cancer of unknown primary”), which presents a unique challenge. While histopathological examination is currently the gold standard for definitive diagnosis10,11, biopsy procedures to obtain tumor tissue for this analysis can differentiate between tumor types. Nonetheless, non-invasive and rapid methods would be preferable. This is especially pertinent for patients who are not suitable for biopsy, such as those with tumors located in or near eloquent areas, or patients too debilitated to undergo biopsy or surgery12. In addition, it is essential to consider that the accuracy of diagnoses based on histopathology can be influenced by several factors, including the quality of the tissue sample obtained, the presence of biological heterogeneity and variety (particularly in HGGs), and the specific procedures used for processing the specimens13,14,15. Effective diagnosis hinges on the accurate integration of clinical, radiological, and histological data. Deviations or inaccuracies in the two previously mentioned factors might ultimately result in interpretative errors during the analysis of surgical specimens.
Magnetic Resonance Imaging (MRI) is the favored technique for assessing individuals with brain malignancies. Differentiating between HGGs and BMs requires considering morphological features, variety, location, and the patient’s clinical history. Challenges arise, particularly with solitary-enhancing brain lesions7,16,17. Intriguingly, a significant portion of BM cases present as solitary metastases, while HGGs occasionally manifest multifocal lesions18,19. Distinguishing between HGGs and single BMs is a significant challenge when there is a lack of documented clinical history, as these two conditions exhibit comparable radiological characteristics on MRI. Both HGGs and BMs have comparable features, including the presence of necrotic cores, uneven enhancing borders, and peritumoral edema. As a result, they commonly display identical morphological appearances on MRI scans7. According to existing research, it has been found that HGGs are characterized by their ability to infiltrate neighboring regions, but BMs do not possess this invasive property20. Subtle distinctions may arise between the two tumor types with respect to peritumoral enhancement zones, especially pertaining to the nature of edema and angiogenesis21. Nevertheless, conventional MRI methods continue to have challenges in accurately capturing these subtle distinctions and distinguishing between HGGs and BMs. The conventional MRI imaging sequences - FLAIR, T1-w, T1-ce, and T2-w - provide valuable information regarding tumor size, morphology, and adjacent brain structures, but they often fall short in predicting treatment outcomes or differentiating tumor subtypes22. As the standard of care has evolved to include more personalized treatment regimens, there is an increasing need for precise tumor differentiation and characterization.
Artificial intelligence (AI) presents a promising avenue for enhancing diagnostic accuracy23. Current endeavors focus on algorithms for automated lesion detection, segmentation, and differential diagnosis between HGGs and BMs24,25. AI can reduce human errors due to heavy workloads, thereby increasing the consistency of results. Radiomics represents an advanced technique that harnesses a multitude of features from radiographic images26. By quantifying a wide range of image attributes, including both conventional morphological features and intricate texture analyses, radiomics can uncover subtle imaging details that may elude human detection26. Studies highlight the efficacy of radiomics in assessing fundamental tumor pathophysiology and differentiating between various tumor types26,27.
Currently, the most comprehensive and widely used image repository for cancer imaging research is the Cancer Imaging Archive (TCIA). This archive houses imaging data for approximately 140 different types of human cancers28. Several databases are dedicated to gliomas, yet the TCIA only has one database specifically for BMs, comprising 156 whole-brain MRI studies29. Notably, there has been a recent addition of a BM database containing MRI data for 75 BM patients30, but it lacks data on HGG patients. This absence presents obstacles in the development of methods to differentiate between HGGs and BMs using imaging and clinical data.
Our work primarily offers multi-parametric, multi-center MRI scans and associated clinical data for patients diagnosed with both HGGs and BMs from various origins. This enriched dataset aims to facilitate the development of novel techniques for determining the origins of brain tumors. It encompasses pre-processed MRI data from 67 patients, each with unique MRI studies featuring FLAIR, T1, contrast-enhanced T1, and T2 sequences. Additionally, it includes semi-automated segmentation for all 67 lesions, leading to 67 segmentations based on both FLAIR and post-contrast T1-w sequences. Furthermore, the dataset is augmented with an extensive clinical database detailing patient demographics and treatment histories, positioning it as a valuable resource for automated tumor segmentation, disease differentiation, and the assessment of disease status for multi-origin brain tumors. Specifically, the dataset can significantly contribute to developing advanced machine-learning algorithms aimed at automated tumor segmentation. The diversity of tumor origins, despite the small sample sizes, provides a unique challenge set for developing robust algorithms that can generalize across different tumor types and origins. Furthermore, our dataset is poised to support radiogenomics research, which aims to correlate radiographic imaging features with genomic data. Although the sample sizes for some tumor origins are limited, these cases can still yield preliminary insights and hypotheses that can be further explored in larger follow-up studies. Another potential application lies in the development of personalized treatment strategies. Including clinical data alongside imaging data opens avenues for exploratory analyses that could identify imaging biomarkers predictive of treatment response or prognosis, even within the subsets of less common tumor origins. Lastly, our dataset can facilitate comparative studies between the various types of brain metastases and high-grade gliomas, contributing to a deeper understanding of their radiological distinctions and similarities. This, in turn, could aid in the differential diagnosis and treatment planning for patients presenting with these conditions.
Methods
Ethical approval
The dataset was retrospectively collected in accordance with the relevant ethical regulations established by the corresponding hospital’s Institutional Review Board, the Second Affiliated Hospital of Anhui Medical University (No.2023105), The First Affiliated Hospital of USTC (No. 2022-KY-242), and Changzheng Hospital (CZEC2021-068). For patients still alive, informed consent was obtained during their follow-up at the hospital, including a specific case where consent was provided by the parents of a minor. For deceased patients, consent was waived by the hospital’s ethics committee. All data utilized in this study was managed securely and de-identified to ensure the rights and privacy of the participants.
Data description
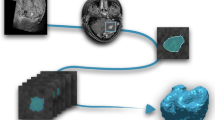
The multi-parametric MRI dataset for multi-origin brain tumors (MOTUM) contains 67 patients with brain tumors and provides five different sources of data, as shown in Figure 1:
-
1.
Structural MRI scans (including DICOM files and processed images) and tumor segmentations of contrast-enhancing tumor and non-enhancing FLAIR signal abnormalities.
-
2.
Pathological confirmation and labels specifying the origin of brain metastasis.
-
3.
Clinical data and records.
-
4.
Automated tumor segmentation tool.
-
5.
Radiomics features.
Overview of MOTUM dataset. (A) Structural MRIs and their derivatives including FLAIR, T1, contrast-enhanced T1, T2 and the segmentation of two tumor sub-structures. (B) Pathological and clinical confirmation of the tumor origin. (C) Clinical data and records. (D) Automated segmentation tool. (E) Radiomics feature extraction.
Subject characteristics
The collected data include imaging studies and clinical data of 67 HGGs and BM patients from the Second Affiliated Hospital of Anhui Medical University, Changzheng Hospital, and The First Affiliated Hospital of USTC. Inclusion criteria were defined as deceased adult patients with a pathologically confirmed diagnosis of HGGs or BMs between January 1, 2019, and January 1, 2022, availability of complete imaging studies, no noise or artifacts in the images, and availability of basic clinical data (age at diagnosis, sex, surgical result, molecular results, etc.). In addition to HGGs, which include GBM, WHO grade III and IV astrocytomas, and oligodendrogliomas (n = 29), the origins of brain metastasis were lung cancer (n = 20), breast cancer (n = 10), ovarian cancer (n = 4), gastric cancer (n = 2), and melanoma (n = 2).
Image acquisition
MRI scans were collected as part of the routine clinical care for each patient. Scans were acquired from two vendors - Siemens or Philips 3.0 T system. The slice thickness is 5 mm.
Pre-processing and quality control
Raw DICOM files were sorted by sequence and converted to NIfTI format. Four image sequences - FLAIR, T1, contrast-enhanced T1, and T2 - have been skull-stripped using HD-BET31 and rigidly co-registered with FSL32, using T1 as the reference. The results of skull-stripping and co-registration are visually checked. All sequences are acquired originally in a 2D manner. We opted to retain their 2D resolution instead of homogenizing them to isotropic resolutions, even though there are public tools available for this purpose33. Low-quality images caused by severe motion are excluded in this study. Afterward, each image is rated with two scores: mild motion (1) and no motion (2).
Semi-automated image segmentation
Contrast-enhanced tumors (CEs) and non-enhancing abnormalities (NCEs) presented in the T1-ce and FLAIR respectively, are segmented. Initially, 30 subjects were stratified, considering their origins. The tumor signals are manually segmented with ITK-SNAP (v3.6.0)34. Subsequently, they are corrected by two physicians (Z.G. and T.X.) after a visual inspection slice-by-slice, using a brush tool. The rest 37 subjects are automatically segmented after training a 2D nn-UNet model35 on initial manual segmentations. The automated segmentations are checked and corrected by the same physicians (Z.G. and T.X.).
Clinical data and anonymization
A comprehensive collection of clinical data was accomplished for the cohort. This dataset encompassed critical parameters such as age at diagnosis, gender, primary tumor classification, and histological subtypes, along with results from immunohistochemical staining assays. Furthermore, the dataset detailed the surgical interventions undertaken, including the scope of resection achieved. Initial anonymization of this dataset was performed at the source institutions, entailing the redaction of personally identifiable information. This was followed by the meticulous removal of private DICOM tags and any elements bearing sensitive personal data. The final step in data sanitization involved facial de-identification during the skull-stripping step, effectively precluding facial reconstruction. This rigorously anonymized dataset was then subject to a dual-review process, conducted independently by two physicians (Z.G. and F.H.), to ensure integrity and compliance with privacy standards.
Automated image segmentation tool
After the segmentation masks for 67 subjects were obtained, automated 2D segmentation models were trained with the nnUNet framework35, contained to be usable in popular operating systems (MacOS, Windows, and Linux), and released. To simply the following radiomics feature extraction process based on binary segmentation masks, we train two different models for NCE and CE taking FLAIR, T1, T2, and T1-ce as the inputs. We used a 2D model instead of a 3D model considering the large slice thickness (5 mm) and relatively small sample size. We initially trained the model with 30 subjects using extensive data augmentation, applied it for 37 subjects and then manually correct the segmentation. We observed that training on 2D slides from 30 subjects can reduce significant annotation effort.
Radiomic features
Using the PyRadiomics open-source Python library (version 2.2.0), we extracted a suite of 110 imaging features. This collection includes 16 shape-related descriptors, a range of intensity distribution metrics, and textural features linked to segmentation labels. The intensity-based features consist of basic first-order statistics along with those calculated from various matrices: 24 from the gray-level co-occurrence matrix (GLCM), 16 from the gray-level run-length matrix (GLRLM), 16 from the gray-level size-zone matrix (GLSZM), 5 from the neighboring gray-tone difference matrix (NGTDM), and 14 from the gray-level dependence matrix. Feature extraction from the pre-processed image sequences was conducted post-z-score normalization and intensity amplification by a factor of 100. Further modifications included an upward shift by 300 to maintain predominantly positive values for the first-order statistics and the application of a geometric tolerance threshold of 0.04.
Data Records
The dataset36 is available on a G-Node repository and can be accessed at https://doi.gin.g-node.org/10.12751/g-node.tvzqc5. All resources can be found in a GitHub repository: https://github.com/hongweilibran/MOTUM. All files are organized with BIDS format35. Tumor segmentation and the corresponding have been stored in the Neuroimaging Informatics Technology Initiative (NIfTI) format, maintaining raw medical image coordinates. For each subject (e.g., /sub-0001), the directory includes several NIfTI files containing native space FLAIR, T1-w, T1-ce, and T2 images. Their segmentation masks, radiomics features, and acquisition parameters are stored in a folder named ‘derivatives’. A general CSV file containing all the clinical data including gender, age, the origin of the tumor, the final pathological diagnosis, image quality rating, molecular information and surgery results, is created.
Technical Validation
Data collection
The collaborating expert board-certified neuroradiologists identified and collected the 67 HGGs and BMs patients included in the study. The tumors for each patient were pathologically confirmed and verified prior to inclusion in the study. Data curation and testing of the inclusion criteria were performed by three physicians (Z.G. T.X., and N.P.) with more than seven years’ experience in the management of medical images and then cross-checked.
Pre-processing and segmentation method
All images after skull-stripping and co-registration were carefully checked to avoid including corrupted cases. All semi-automated segmentations performed in this study were carefully validated and corrected by experienced physicians.
Evaluation of automated segmentation tool
The segmentation performance of the tool was rigorously evaluated by splitting 67 patients as training and test sets based on their origin ID. Specifically, 80% patients from each category were used for training, the rest 20% were for testing. Considering its long-tail nature, at least one patient from each category was involved for testing, resulting 16 patients for the test set. Dice score which calculates the overlap between the predicted segmentation and reference segmentation, is used to quantify to segmentation. The evaluation result is shown in Fig. 2, achieving Dices scores of 0.902 and 0.587 for NCE and CE, respectively.
Code availability
All processing pipeline scripts are openly available. Code to generate pre-processed outputs can be accessed via https://github.com/hongweilibran/MOTUM. The automated segmentation tool can be used by following the instruction in the DockerHub page: https://hub.docker.com/repository/docker/branhongweili/motum_seg/. Pre-processing scripts for skull-stripping and co-registration are available.
References
Ostrom, Q. T. et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro Oncol 24, V1–V95 (2022).
Achrol, A. S. et al. Brain metastases. Nat Rev Dis Primers 5, (2019).
Ostrom, Q. T., Gittleman, H., Stetson, L., Virk, S. M. & Barnholtz-Sloan, J. S. Epidemiology of Gliomas. Cancer Treat Res 163, 1–14 (2015).
Ostrom, Q. T., Wright, C. H. & Barnholtz-Sloan, J. S. Brain metastases: epidemiology. Handb Clin Neurol 149, 27–42 (2018).
Nayak, L., Lee, E. Q. & Wen, P. Y. Epidemiology of brain metastases. Curr Oncol Rep 14, 48–54 (2012).
Giese, A. & Westphal, M. Treatment of malignant glioma: a problem beyond the margins of resection. J Cancer Res Clin Oncol 127, 217–225 (2001).
Cha, S. et al. Differentiation of glioblastoma multiforme and single brain metastasis by peak height and percentage of signal intensity recovery derived from dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR. Am J Neuroradiol 28, 1078–1084 (2007).
O’Neill, B. P., Buckner, J. C., Coffey, R. J., Dinapoli, R. P. & Shaw, E. G. Brain metastatic lesions. Mayo Clin Proc 69, 1062–1068 (1994).
Le Rhun, E. et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol 32, 1332–1347 (2021).
Weller, M. et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 18, 170–186 (2021).
Nabors, L. B. et al. Central Nervous System Cancers, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 18, 1537–1570 (2020).
Blanchet, L. et al. Discrimination between metastasis and glioblastoma multiforme based on morphometric analysis of MR images. AJNR Am J Neuroradiol 32, 67–73 (2011).
Chand, P., Amit, S., Gupta, R. & Agarwal, A. Errors, limitations, and pitfalls in the diagnosis of central and peripheral nervous system lesions in intraoperative cytology and frozen sections. J Cytol 33, 93–97 (2016).
Wesseling, P., Kros, J. M. & Jeuken, J. W. M. The pathological diagnosis of diffuse gliomas: towards a smart synthesis of microscopic and molecular information in a multidisciplinary context. Diagn Histopathol 17, 486–494 (2011).
Pollo, B. Pathological classification of brain tumors. The quarterly journal of nuclear medicine and molecular imaging: official publication of the Italian Association of Nuclear Medicine (AIMN) [and] the International Association of Radiopharmacology (IAR), [and] Section of the Society of… 56, 103–111 (2012).
Schiff, D. Single Brain Metastasis. Curr Treat Options Neurol 3, 89–99 (2001).
Server, A. et al. Diagnostic examination performance by using microvascular leakage, cerebral blood volume, and blood flow derived from 3-T dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging in the differentiation of glioblastoma multiforme and brain metastasis. Neuroradiology 53, 319–330 (2011).
Hassaneen, W. et al. Multiple craniotomies in the management of multifocal and multicentric glioblastoma. Clinical article. J Neurosurg 114, 576–584 (2011).
Loh, D. et al. Two-year experience of multi-disciplinary team (MDT) outcomes for brain metastases in a tertiary neuro-oncology centre. Br J Neurosurg 32, 53–60 (2018).
Artzi, M. et al. Differentiation between vasogenic edema and infiltrative tumor in patients with high-grade gliomas using texture patch-based analysis. Journal of Magnetic Resonance Imaging 48, 729–736 (2018).
Halshtok Neiman, O. et al. Perfusion-weighted imaging of peritumoral edema can aid in the differential diagnosis of glioblastoma mulltiforme versus brain metastasis. Isr Med Assoc J 15, 103–105 (2013).
Nilsson, M., Englund, E., Szczepankiewicz, F., van Westen, D. & Sundgren, P. C. Imaging brain tumour microstructure. Neuroimage 182, 232–250 (2018).
Najjar, R. Redefining Radiology: A Review of Artificial Intelligence Integration in Medical Imaging. Diagnostics 13, 2760 (2023).
Bakas, S., Reyes, M., Jakab, A. & Bauer Helbling, S. Identifying the Best Machine Learning Algorithms for Brain Tumor Segmentation, Progression Assessment, and Overall Survival Prediction in the BRATS Challenge. https://www.researchgate.net/publication/331576745.
Menze, B. H. et al. The Multimodal Brain Tumor Image Segmentation Benchmark (BRATS). IEEE Trans Med Imaging 34, 1993 (2015).
Gillies, R. J., Kinahan, P. E. & Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 278, 563–577 (2016).
Prasanna, P., Patel, J., Partovi, S., Madabhushi, A. & Tiwari, P. Radiomic features from the peritumoral brain parenchyma on treatment-naïve multi-parametric MR imaging predict long versus short-term survival in glioblastoma multiforme: Preliminary findings. Eur Radiol 27, 4188–4197 (2017).
Clark, K. et al. The cancer imaging archive (TCIA): Maintaining and operating a public information repository. J Digit Imaging 26, 1045–1057 (2013).
Grøvik, E. et al. Deep learning enables automatic detection and segmentation of brain metastases on multisequence MRI. Journal of Magnetic Resonance Imaging 51, 175–182 (2020).
Ocaña-Tienda, B. et al. A comprehensive dataset of annotated brain metastasis MR images with clinical and radiomic data. Scientific Data 10(1), 1–6 (2023).
Isensee, F. et al. Automated brain extraction of multisequence MRI using artificial neural networks. Hum Brain Mapp 40, 4952–4964 (2019).
Jenkinson, M., Bannister, P., Brady, M. & Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841 (2002).
Kofler, F. et al. BraTS Toolkit: Translating BraTS Brain Tumor Segmentation Algorithms Into Clinical and Scientific Practice. Front Neurosci 14, 501835 (2020).
Yushkevich, P. A. et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31, 1116–1128 (2006).
Isensee, F., Jaeger, P. F., Kohl, S. A. A., Petersen, J. & Maier-Hein, K. H. nnU-Net: a self-configuring method for deep learning-based biomedical image segmentation. Nature Methods 18(2), 203–211 (2020).
G-Node Open Data:A multi-center, multi-parametric MRI dataset of primary and secondary brain tumors.G-Node https://doi.org/10.12751/g-node.tvzqc5 (2024).
Acknowledgements
H.B.L. is supported by a Swiss Postdoc Mobility Fellowship.
Author information
Authors and Affiliations
Contributions
Z.G., F.H., and H.B.L. conceived of the presented idea; Z.G. and T.X. performed and confirmed the segmentations; Z.G. and F.H. performed full data anonymization; N. P., F. H., and T.X. collected data; Z.G., T.X., and H.B.L. wrote the initial draft. X.C., C.N., and B.W. completed the critical review and revision of the manuscript and datasets, as well as proofreading.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gong, Z., Xu, T., Peng, N. et al. A Multi-Center, Multi-Parametric MRI Dataset of Primary and Secondary Brain Tumors. Sci Data 11, 789 (2024). https://doi.org/10.1038/s41597-024-03634-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-024-03634-0
- Springer Nature Limited