Abstract
The data described in this paper were collected from the Circum-Bohai Bay and Loess Plateau regions of northern China. Soil, leaf and fruit nutrients from 225 typical pear orchards in these regions were measured. Soil data included pH, organic matter, total N, alkaline hydrolysable N, available P and available K concentrations of 3 different soil layers, 0–20 cm, 20–40 cm and 40–60 cm, from different orchards. Leaf and fruit data included N, P, K, Ca, Fe, Mn, Cu, Zn and B concentrations of pear trees from different orchards. These data can be used to assess the soil nutrient supply and leaf and fruit nutrient status of pear orchards in two major producing areas, Circum-Bohai Bay and Loess Plateau. Additionally, this dataset provides data to support the development of regionalized and standardized soil nutrient management programs for pear orchards, as well as regionalized layouts of the main varieties in the two producing areas.
Similar content being viewed by others
Background & Summary
China is the largest pear producer in the world. The main areas in which pear trees are distributed in China are the Circum-Bohai Bay, Loess Plateau and Yangtze River Basin regions, among which Circum-Bohai Bay and Loess Plateau are located in northern China. The cultivated area and yield of pears planted in these two regions are 338 kha and 7.96 Gkg, accounting for 24.53% and 33.29% of the world’s total cultivated area and total yield, respectively1.
The Circum-Bohai Bay pear producing area includes the Hebei, Shandong, and Liaoning provinces and Beijing. This area contains various landforms, such as plains, hills and piedmont slopes, with various soil types, such as brown soil, cinnamon soil and fluvo-aquic soil. The pear varieties in these regions are diverse, including Yali (Pyrus bretschneideri Rehd.) and Xuehuali (Pyrus bretschneideri Rehd.) in Hebei, Laiyangchili (Pyrus bretschneideri Rehd.) and Pyrus communis L. in Beijing and Nanguoli (Pyrus ussuriensis Maxim.) in Liaoning. The Loess Plateau pear producing area is located on Loess Plateau. It includes all of Shanxi and the Shaanxi Provinces and parts of Gansu Province and is approximately 1000 m above sea level, with sufficient light, a large temperature difference between day and night, a deep loess layer, large terrain variations, frequent droughts and a lack of irrigation. Dangshansuli (Pyrus bretschneideri Rehd.) and Yuluxiangli (Pyrus bretschneideri Rehd.) are the main cultivated varieties in this region.
Fruit mineral nutrient concentrations are not only closely related to fruit quality, nutritional value and physiological diseases during storage2,3, but also play a key role in promoting human health4. Pear tree growth and fruit yield and quality are greatly affected by soil conditions and tree nutrient status, which can be reflected in the leaf mineral nutrient status5 and fruit nutrient status6. Researchers have investigated and studied the soil nutrient status7, the leaf nutrient status8 and the relationship between soil nutrients and leaf nutrients9 of pear orchards in parts of Circum-Bohai Bay and Loess Plateau, which play an important role in guiding pear production. However, the relevant datasets on fruit nutrition of major cultivars planted in Circum-Bohai Bay and Loess Plateau regions are missing and the datasets on leaf and soil nutrients are incomplete. Thus, we present datasets of soil available nutrient concentrations and leaf and fruit mineral nutrient concentrations of 225 typical pear orchards in Circum-Bohai Bay and Loess Plateau to provide guidance for the accurate management of pear tree nutrients in these regions.
This dataset can be used to obtain a more in-depth and systematic understanding of the soil nutrient supply and leaf and fruit nutrient status of pear trees in the two major producing areas. This dataset can be analysed in a variety of ways, such as by correlation analysis of leaf mineral nutrient concentration and soil mineral nutrient concentration, and by multivariate statistical analysis and partial least squares regression analysis on fruit mineral nutrient concentration and soil mineral nutrient concentration. These analyses will be helpful in clarifying the influence of the main mineral soil nutrient factors on leaf and fruit mineral concentrations in Circum-Bohai Bay and Loess Plateau regions and then determine the optimum soil mineral nutrient concentration that ensures abundant mineral concentrations in leaves and fruits for high quality and high yield. In addition, prominent problems, such as large differences in nutrient management and slow improvement in fruit yield and quality caused by natural factors such as topography, soil types, irrigation conditions and main varieties, can be eliminated by comparing the differences in soil mineral nutrient management between the Circum-Bohai Bay and Loess Plateau regions. This study provides theoretical support for the development of regionalized and standardized soil nutrient management programs in pear orchards, as well as for a regionalized layout of the main varieties in the two producing areas.
Methods
Orchard site selection
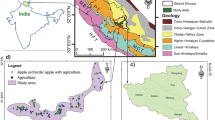
The survey was conducted from 2018 to 2019 in the Circum-Bohai Bay region, which included Shandong, Hebei, and Liaoning provinces and Beijing, and the Loess Plateau region, which included Shanxi and Shaanxi provinces. Five typical production counties were selected in each province or city. Representative orchards were selected according to the production of the main varieties in each county (orchard area was greater than 1.0 ha; the pear trees were 15 to 25 years old; and the yield of orchards ranged from 40 to 60 t ha−1). A total of 225 orchards were investigated (Fig. 1), including 150 in the Circum-Bohai Bay region and 75 in the Loess Plateau region (Table 1).
Sample collection and pretreatment
Soil and leaf samples were collected at the stage in which the growth of new shoots ceased, from July 1 to July 1510. Eleven sampling sites were determined in each orchard according to an “S” shape sampling method (Fig. 2), and soil samples from the 0–20 cm, 20–40 cm and 40–60 cm layers were collected. The soil samples of the same soil layer at each sampling site were mixed into one sample. Then, the soil samples were air-dried, ground and sifted with a nylon sieve for determination of nutrient concentrations.
Ten to fifteen pear trees in each orchard of the same size and vigour and 5 to 10 mature leaves from the middle of a long shoot from the periphery of each tree were selected for leaf sampling11. Then, all the leaves from the same orchard were mixed into one leaf sample. The leaves were washed with tap water containing a detergent, with deionized water, with 0.01 M hydrochloric acid and then with deionized water again and then dried at 100 °C for 30 min and at 70 °C to a constant weight. Then, the leaf samples were crushed into a powder and sifted with a nylon sieve for nutrient determination.
Fruit samples were collected at the ripening stage. Pear trees from which leaf samples were collected from each orchard were selected for fruit sample collection. Three to five peripheral fruits of the same size were collected from each tree, and fruit samples from the same orchard were mixed into one sample. The fruits were washed with tap water containing a detergent, with deionized water, with 0.01 M hydrochloric acid and then with deionized water again, cut into slices and then dried at 100 °C for 30 min and at 70 °C to a constant weight. Then, the fruit samples were crushed into a powder and sifted with a nylon sieve for nutrient determination.
Sample determination
Various indicators of soil and plant samples were determined according to the method of Cui et al.12 and Bao13.
Soil pH determination
A potentiometric method was used to measure soil pH. Carbon dioxide-free water was added to soil that had been passed through a 2 mm sieve at a water-soil ratio of 2.5:1. The soil solution was stirred for 1 min and left undisturbed for 30 min. Each soil sample was measured more than three times with a pH meter (FE20K PLUS PH, Mettler-Toledo, Switzerland), and the difference in the parallel determination results was less than 0.2 pH units. The electrode was washed with deionized water and dried with filter paper after each sample measurement. A calibration solution was used to calibrate the electrode between measurements after every 10 soil samples.
Soil organic matter determination
Soil organic matter was measured according to the Schollenberger method using chromic acid redox titration. Five millilitres of a 0.8 M 1/6 K2Cr2O7 solution was added to a test tube with approximately 0.5000 g of soil that had been passed through a 0.25 mm sieve. The mixture was then added to 5 mL concentrated sulfuric acid and shaken gently to disperse the soil. The tube was placed in a phosphoric acid bath, heated to 170 °C and boiled for 5 min. To condense the water vapour that escaped during the heating process, a small funnel was placed on the top of the test tube. The substances in the test tube and funnel were transferred to a conical flask after cooling. Then, the solution was added to 1,10-phenanthroline hydrate and titrated with 0.2 M FeSO4 until it turned maroon. A blank experiment was performed when each batch of samples was measured. The soil organic matter content was calculated according to the following formula:
ω(OM): soil organic matter content; c: standard FeSO4 solution concentration; V: volume of the standard FeSO4 used in titration; V0: volume of standard FeSO4 used in titrating control sample; 3: molar mass of a quarter of carbon; 1.724: the conversion factor from organic carbon to organic matter; f: oxidation correction coefficient (the value was 1.1); m: mass of oven-dried soil sample.
Soil total N determination
Total N was determined by the semitrace Kjeldahl method. Approximately 1.0000 g of air-dried soil that had been passed through a 0.25 mm sieve was added to a digestion tube. Meanwhile, the soil moisture content was measured to calculate the mass of the oven-dried soil. Two grams of accelerator and 5 mL of concentrated sulfuric acid were added to the tube. The tube was then covered with a small funnel, and the sample was digested at 360 °C for 15–20 min. The mixture was digested for 1 h until the colour changed from brown to greyish green or greyish white. Two digested soilless samples were used as controls. After the digestion tube cooled, it was placed in a distiller, and a small amount of deionized water was added. Five millilitres of a 2% boric acid indicator was added to a 150 mL conical flask, and the flask was placed at the end of the condenser tube. Then, the digestion solution was distilled until the distillate volume was approximately 75 mL. The distillate was titrated with 0.01 M standard hydrochloric acid to a purplish red colour endpoint. The soil total N concentration was calculated according to the following formula:
ω(N): soil total N concentration; c: standard acid concentration; V: volume of the standard acid used in titration; V0: volume of standard acid used in titrating control sample; 14: molar mass of N; m: mass of oven-dried soil sample.
Soil alkaline hydrolysable N determination
Approximately 2.00 g of air-dried soil that have been passed through a 2 mm sieve was placed in the outer chamber of a diffuser. The diffuser was gently rotated to evenly distribute the soil in the outer chamber. Two millilitres of H3BO3 indicator was placed in the inner chamber of the diffusion dish. The edge of the frosted glass surface of the diffuser was coated with alkaline glycerin and covered with frosted glass. The diffuser was covered tightly and secured with rubber bands after 10.00 mL of 1 M NaOH was injected into the diffuser through a hole in the frosted glass. The diffuser was placed in a 40 °C incubator for alkaline hydrolysis diffusion for 24 h. Then, the mixture was titrated with 0.01 M standard hydrochloric acid until it turned purplish red. A blank test was performed at the same time as the samples. The soil alkaline hydrolysable N concentration was calculated according to the following formula:
ω(N): soil alkaline hydrolysable N concentration; c: standard acid solution concentration; V: volume of the standard acid used in titration; V0: volume of standard acid used in titrating control sample; 14: molar mass of N; m: mass of air-dried soil sample.
Soil available P determination
Approximately 2.50 g of air-dried soil that had been passed through a 2 mm sieve was placed in a plastic bottle and 50 mL of 0.5 M NaHCO3 was added. After the bottle was shaken for 30 min, the mixture was immediately filtered with phosphorus-free filter paper. Ten millilitres of the filtrate was accurately measured into a conical flask, and 5.00 mL of Mo-Sb-Vc colour developer and 10 mL of deionized water were added. The absorbance of the mixture was measured at approximately 700 nm after 30 min using a UV-Vis spectrophotometer (UV1900PC, AuCy Instrument, Shanghai, China). Finally, the P concentration was calculated according to a standard curve prepared with solutions of different P concentrations. A blank test was performed at the same time that the samples were determined.
Soil available K determination
Approximately 5.00 g of air-dried soil that had been passed through a 2 mm sieve was placed in a plastic bottle, and 50 mL of 1.0 M NH4OAc was added. After the sample was shaken for 30 min, the mixture was immediately filtered with dry filter paper. The concentration of K in the filtrate was determined directly by a flame photometer (LM12-FP6430, Haifuda, China) according to a standard curve prepared with solutions of different K concentrations. A blank test was performed at the same time that the samples were determined.
Leaf and fruit N determination
Approximately 0.3000 g of plant powder that had been passed through a 0.5 mm sieve was placed into a digestion tube and 5 mL concentrated sulfuric acid was added. Then, the digestion tube was placed onto a digestion stove at 360 °C after two doses of 2 mL H2O2, and the sample was digested until the mixture turned brown. After the tube cooled, 2 mL H2O2 was added, and the digestion was continued for 5 min. This process was repeated until the mixture turned clear. The mixture was diluted to 100 mL in a volumetric flask for testing after it cooled. Then, 5 to 10 mL of the liquid to be tested was accurately measured into a distiller for distillation. The distillation and titration processes were the same as those used for ammonium in the Soil total N determination section. A blank test was performed at the same time as sample measurement. The leaf or fruit N concentration was calculated according to the following formula:
ω(N): total N concentration; c: standard acid concentration; V: volume of the standard acid used in titration; V0: volume of standard acid used in titrating control sample; 14: molar mass of N; m: mass of oven-dried sample; V1: volume of the digestion solution after constant volume; V2: measured volume of digestion solution after constant volume.
Leaf and fruit P, K, Ca, Fe, Mn, Cu, Zn, B determination
Approximately 0.5000 g of plant powder that had been passed through a 0.5 mm sieve was placed in a digestion tube and a 10 mL mixture of concentrated nitric acid and hypochlorous acid (4:1) was added. After the sample was left undisturbed for more than 4 h, it was placed onto a digestion stove and heated to 150 °C so that NO2 could volatilize slowly. Then, the temperature was appropriately increased to a temperature not higher than 250 °C until the digestive solution was transparent and approximately 2 mL remained. The solution was transferred into a volumetric flask after cooling and adjusted to a constant volume of 50 mL. The solution was then filtered, and the concentration of each element in the solution was determined by a plasma emission spectrometer (ICP-OES, OPTIMA 3300 DV, 75 Perkin-Elmer, USA). A blank test was performed at the same time as sample measurement. The leaf or fruit P, K, Ca, Fe, Mn, Cu, Zn, and B concentrations were calculated according to the following formula:
ω(P, K, Ca, Fe, Mn, Cu, Zn, B): P, K, Ca, Fe, Mn, Cu, Zn, B concentration in leaf or fruit; ρ(P, K, Ca, Fe, Mn, Cu, Zn, B): the concentration of P, K, Ca, Fe, Mn, Cu, Zn or B in the liquid to be measured; V: volume of the liquid to be measured after constant volume; f: dilution ratio of the liquid to be measured; m: mass of oven-dried sample.
Data Records
The dataset is accessible from figshare14. The dataset for the soil, leaf and fruit nutrient concentrations of pears in different orchards are displayed in the database. Soil data included pH, organic matter, total N, alkaline hydrolysable N, available P and available K concentrations of 3 different soil layers, 0–20 cm, 20–40 cm and 40–60 cm (named Soil 0–20 cm, Soil 20–40 cm and Soil 40–60 cm, respectively). Leaf and fruit data (named Leaf and Fruit, respectively) included N, P, K, Ca, Fe, Mn, Cu, Zn and B concentrations. In the dataset, CBB represents Circum-Bohai Bay, LP represents Loess Plateau, BJ represents Beijing, HB represents Hebei, SD represents Shandong, LN represents Liaoning, SX represents Shanxi, SaX represents Shaanxi, and different numbers in the same province indicate orchards at different locations.
Technical Validation
The investigated area
The Circum-Bohai Bay and Loess Plateau regions are two of the three recognized major pear-producing areas in China. The typical survey counties were collaboratively determined with the local fruit tree scientific research institute or technology extension departments based on comprehensive consideration of the main cultivars and distributions in the provinces, as well as the cultivation area and yield of each county.
The investigated pear orchards
To ensure the representativeness of the selected orchards, the research team conducted a field investigation and comprehensively considered the areas and yields of the orchards and the growth status and ages of the pear trees in the orchards.
Investigation method
This survey was carried out by researchers with backgrounds in fruit tree, plant nutrition and soil science. Before sample collection, the investigation team developed a detailed manual for soil, leaf and fruit sample collection based on the biological habits of pear trees. For sample collection, pear trees with essentially the same size and growth states were selected.
Determination of the samples
The soil samples were pretreated and analysed at the Key Laboratory of Plant-Soil Interaction, Ministry of Education, College of Resources and Environmental Sciences, China Agricultural University. Leaf and fruit samples were pretreated and tested at the Test Center of Vegetable Seed Quality, Ministry of Agriculture. These institutions are authoritative testing agencies. The sample pretreatment and determination methods were in line with testing standards to ensure the accuracy of the sample analysis results.
Code availability
No custom code was used in this study.
References
Fallahi, E., Conway, W. S., Hickey, K. D. & Sams, C. E. The role of calcium and nitrogen in postharvest quality and disease resistance of apples. HortScience 30, 748–914, https://doi.org/10.21273/HORTSCI.30.4.751C (1995).
Bennewitz, E., Fredes, C., Losak, T., Martínez, C. & Hlusek, J. Effects on fruit production and quality of different dormant pruning intensities in ‘Bing’/‘Gisela®6’ sweet cherries (Prunus avium) in Central Chile. Cien. Inv. Agr. 38, 339–344, https://doi.org/10.4067/s0718-16202011000300003 (2011).
Steffan, J. J., Brevik, E. C., Burgess, L. C. & Cerdà, A. The effect of soil on human health: An overview. Eur. J. Soil Sci. 69, 159–171, https://doi.org/10.1111/ejss.12451 (2018).
Han, Z. & Wang, Q. Current situation and prospects of research on fruit mineral nutrition in China: A literature review. Acta Horticulturae Sinica 22, 138–146 (1995).
Fallahi, E., Neilsen, D., Neilsen, G. H., Fallahi, B. & Shafii, B. Efficient irrigation for optimum fruit quality and yield in apples. HortScience 45, 1616–1619, https://www.researchgate.net/publication/236735562 (2010).
An, H. et al. Soil nutrient state of main pear gardens in Fuquan city. Northern Horticulture 8, 23–26 (2008).
Yu, N. et al. Investigation and analysis on nutrient status of soil and leaves in ‘Nanguoli’ pear orchards in Liaoning province. Journal of Fruit Science 30, 254–259 (2013).
Bai, M., Hao, G., Zhang, X., Yang, S. & Guo, H. Nutrients of soil and leaf in pear orchards of Shanxi province. Journal of Agriculture 9, 23–27 (2019).
Li, G., Su, R., Shen, T. & Wu, J. Studies on the preparation of standard reference material of orchard leaves. Acta Horticulturae Sinica 12, 217–222 (1985).
Li, G., Su, R. & Shen, T. The prospect of improving the yield and quality of some major fruits in China as seen from the results of leaf analysis. Acta Horticulturae Sinica 21, 56–63 (1988).
Cui, J. & Jiang, R. Experimental Course For Soil Testing, Plant Diagnosis And Environmental Monitoring (China Agricultural University Press, 2019).
Bao, S. Soil And Agricultural Chemistry Analysis (China Agriculture Press, 2000).
Sun, M. et al. Soil, leaf and fruit nutrient data for pear orchards located in the Circum-Bohai Bay and Loess Plateau regions, figshare, https://doi.org/10.6084/m9.figshare.c.6165858 (2023).
Acknowledgements
This study was funded by the National Key R&D Program of China (2020YFD1000200), the Science and Technology Innovation Capacity Building Program of Beijing Academy of Agriculture and Forestry (KJCX20210437 and KJCX20180701), and the National Science and Technology Support Program of China (2014BAD16B03).
Author information
Authors and Affiliations
Contributions
M.S. and Y.Z. led the field survey, collected the data and wrote the manuscript. Z.L., Y.W. and R.D. participated in the field surveys and assisted in sample pretreatment. J.L. participated in the preparation of sample collection. F.Y. participated in manuscript revision and provided technical guidance for sample testing. S.L. designed the survey, prepared the sample collection methods and provided funding support.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, M., Zhao, Y., Liang, Z. et al. Soil, leaf and fruit nutrient data for pear orchards located in the Circum-Bohai Bay and Loess Plateau regions. Sci Data 10, 88 (2023). https://doi.org/10.1038/s41597-023-01999-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41597-023-01999-2
- Springer Nature Limited