Abstract
Cervical cancer (CC) screening in women comprises human papillomavirus (HPV) testing followed by cytology triage of positive cases. Drawbacks, including cytology’s low reproducibility and requirement for short screening intervals, raise the need for alternative triage methods. Here we used an innovative triage technique, the WID-qCIN test, to assess the DNA methylation of human genes DPP6, RALYL and GSX1 in a real-life cohort of 28,017 women aged ≥30 years who attended CC screening in Stockholm between January and March 2017. In the analysis of all 2,377 HPV-positive samples, a combination of WID-qCIN (with a predefined threshold) and HPV16 and/or HPV18 (HPV16/18) detected 93.4% of cervical intraepithelial neoplasia grade 3 and 100% of invasive CCs. The WID-qCIN/HPV16/18 combination predicted 69.4% of incident cervical intraepithelial neoplasia grade 2 or worse compared with 18.2% predicted by cytology. Cytology or WID-qCIN/HPV16/18 triage would require 4.1 and 2.4 colposcopy referrals to detect one cervical intraepithelial neoplasia grade 2 or worse, respectively, during the 6 year period. These findings support the use of WID-qCIN/HPV16/18 as an improved triage strategy for HPV-positive women.
Similar content being viewed by others
Main
Cervical cancer (CC) screening is among the most successful strategies for cancer prevention. In combination with high human papillomavirus (HPV) vaccination coverage, cervical screening with substantial uptake is an essential part of the global strategy to eventually eliminate CC1. Cytology-based cervical screening requires a complex infrastructure, well-trained workforce and short screening intervals. The proven superiority of testing for oncogenic HPVs (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 or 68) as an objective and examiner-independent technique with high sensitivity and prolonged protection against cervical intraepithelial neoplasia (CIN; grades 1, 2 or 3) grade 2 or worse (CIN2+)2,3,4,5,6 has resulted in international guidelines recommending a transition from primary cytology-based to primary HPV-based screening7,8,9. Due to increased prevalence of HPV in women <30 years of age10 leading to low specificity of HPV testing, primary HPV screening is only recommended in women ≥30 years of age. An HPV screen-positive result requires cytology-based triaging so that only HPV- and cytology-positive women are referred for colposcopy and biopsy7. In high-income countries, HPV-positive women typically undergo triaging with cytology. In contrast, patients positive for the main oncogenic HPV types (that is, HPV16 and HPV18) are referred directly for colposcopy in the United States11,12. At present, most HPV screening tests provide at least partial information on HPV genotypes. Emphasis is increasing to utilize the information on HPV16/18, indicating higher risk for disease progression, in the screening algorithms11.
Cytology shows limited and highly variable sensitivity13 that has been observed to decrease over time14. It requires equipment and expertise that differs from HPV testing, without being applicable for self-samples. The patients who test positive for HPV through self-sampling need to be reinvited for a separate cytology sample, potentially impacting attendance rates adversely. Therefore, improved strategies for triaging HPV-positive women are essential.
The proof of principle strategy to utilize DNA methylation (DNAme) tests on a noncytological sample collected from the cervicovaginal region for detection of cervical (pre)cancer was demonstrated 20 years ago15. Since then, several DNAme-based markers have been developed and applied in different settings16,17,18,19,20,21,22,23,24,25, predominantly representing case–control studies or small cohort sets with fewer than a thousand volunteers. Recently, we developed the WID-qCIN test, assessing DNAme across three regions of the human genes: DPP6, RALYL and GSX1. The latter were selected from an epigenome-wide screen of 850,000 CpGs in 170 CIN3+ cases and 202 controls. A preliminary assay validation was conducted in both a diagnostic (case–control) and a predictive setting (nested case–control) and a total of 761 samples26. In this article, we optimized the WID-qCIN test and applied it to HPV-positive women from a real-life population-based cohort of the 28,017 women ≥30 years of age having attended screening in the capital region of Sweden between 1 January and 31 March 2017. We assessed the predictive performance of the WID-qCIN test in combination with HPV16/18 genotyping compared with cytology to triage HPV-positive women.
Results
Study population
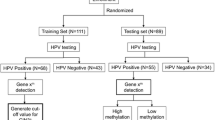
Between 1 January and 31 March 2017, 28,017 women ≥30 years of age participated in cervical screening in the capital region of Stockholm (the KI-q1-2017 cohort). A total of 2,377 women tested positive for HPV (that is, positive for one or more of the HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68) and cytology was assessed. Of these, 711 were cytology positive (atypical squamous cells of undetermined significance or worse (ASC-US+)) and were then referred for colposcopy and histological assessment. The biopsies obtained within the first 12 months as a consequence of the baseline screen were defined as prevalent cases (306 CIN2+, of which 11 were CCs) (Fig. 1). Biopsies obtained after 12 months were defined as incident cases (271 CIN2+ of which 11 were CCs; prevalent CIN2+ cases for whom data from follow-up screens or colposcopy were also reported between months 13 and 72 were excluded from the incidence analyses). The average follow-up time of women without CIN2+ was 40.3 months (range from 0.3 to 71.4 months).
The mean age of HPV-positive women was 40.8 years (range 30–64 years); 654 (27.5%) were HPV16 and/or HPV18 (HPV16/18) positive, and 686 (28.9%) were WID-qCIN positive. Among WID-qCIN-positive women, 49.4% and 41.4% were cytology positive and HPV16/18 positive, respectively (Table 1). A total of five women had inadequate cytology results, three were missing HPV subtype information and 90 had inconclusive WID-qCIN results (Supplementary Note 1).
Detection of prevalent disease
The sensitivity of cytology to detect prevalent CIN2+ cases defined by histopathology was, by definition, very high at 98.4% since only cytology-positive women in the cohort had been referred for colposcopy and thus histopathological biopsy. Whereas HPV16/18 genotyping detected approximately half of the CIN2+ cases (53.3%, 95% confidence interval (CI) 47.5–58.9), the sensitivity of the stand-alone WID-qCIN testing (77.0%, 95% CI 71.6–81.6) or WID-qCIN testing in combination with HPV16/18 genotyping (further on referred to as WID-qCIN/HPV16/18 (85.9%, 95% CI 81.3–89.6)) was significantly higher (P < 0.01 and P < 0.01, respectively). Importantly, HPV16/18 alone would have missed >40% of CIN3 cases (sensitivity 58.9%, 95% CI 48.4–68.8), and the sensitivity of the WID-qCIN test alone (85.7%, 95% CI 76.4–91.9) or the WID-qCIN/HPV16/18 (93.4%, 95% CI 85.7–97.3) was significantly higher for CIN3 detection (P < 0.01 and P < 0.01, respectively). All CCs were detected by HPV16/18 or the WID-qCIN/HPV16/18. The WID-qCIN test detected 10 out of 11 CCs (Table 2).
The specificity (≤CIN1) was comparable for cytology (80.1%, 95% CI 78.3–81.8), HPV16/18 (76.3%, 95% CI 74.4–78.1) and WID-qCIN (76.9%, 95% CI 74.9–78.7). The specificity for the WID-qCIN/HPV16/18 was significantly lower (60.7%, 95% CI 58.5–62.9) when compared with either HPV16/18 alone (P < 0.01) or the WID-qCIN test alone (P < 0.01) (Table 2). All incident cases (diagnosed 13–72 months) were regarded as disease free for the purposes of calculating sensitivity and specificity in the prevalent setting (0–12 months).
The association between the numerical values of the WID-qCIN test (defined as the sum percentage methylation across the three genes) and the histological outcomes further substantiates the ability of the WID-qCIN test to indicate disease progression (Supplementary Note 2, Supplementary Fig. 1 and Supplementary Fig. 2).
Prediction of incident disease
Based on baseline test results, triage with cytology, HPV16/18, the WID-qCIN and the WID-qCIN/HPV16/18 predicted 18.2% (hazard ratio 0.96, 95% CI 0.70–1.31), 45.6% (hazard ratio 2.72, 95% CI 2.14–3.45), 46.3% (hazard ratio 3.01, 95% CI 2.36–3.85) and 69.4% (hazard ratio 3.55, 95% CI 2.73–4.63) of incident CIN2+ cases, respectively (Table 3 and Fig. 2). Whereas cytology only predicted 20.0% of incident CCs (that is, cancers detected more than 12 months after sample collection), HPV16/18 or the WID-qCIN predicted 54.5% and 40.0% respectively. The WID-qCIN/HPV16/18 identified 80.0% of all invasive CCs that developed 13–72 months after the cervical sample collection. The hazard ratios and Kaplan–Meier curves are shown in Table 3 and Fig. 2, respectively. The nonproportional hazards were observed for incident CIN2+ cases stratified according to cytology. This is probably an artifact due to disease detection primarily triggered by a cytology-positive test result. The majority of CIN2+ cases identified because of a (baseline) cytology positive test were detected within 0–12 months (Extended Data Fig. 1), rather than within 13–72 months. Furthermore, the women who were cytology negative at baseline may have tested positive at the second screening round (at approximately 3 years) but are still classified as cytology negative for the purposes of our analysis, which potentially explains why the two curves cross shortly after 3 years.
a–d, Results are stratified according to baseline cytology (a), HPV16/18 (b), WID-qCIN (c) and WID-qCIN/HPV16/18 (d). The insets are cumulative incidence curves corresponding to CCs only. The 95% CIs are shown as gray shaded areas. The P values assessed using log-rank tests are displayed in light gray within a–d.
We also implemented a previously described prevalence–incidence statistical model that considers two features of our cohort27. First, it allows for the possibility of undiagnosed prevalent cases (since some incident CIN2+ cases may be prevalent cases that were previously undiagnosed) and, second, the fact that incident cases are interval censored (since disease onset is known only to occur between irregular visits). According to the model, the WID-qCIN test had a hazard ratio of 2.31 (95% CI 1.31–4.08), HPV16/18 had a hazard ratio of 2.47 (95% CI 1.40–4.37) and the WID-qCIN/HPV16/18 had a hazard ratio of 2.83 (95% CI 1.55–5.16) (Supplementary Table 1 and Extended Data Fig. 1). The model failed to converge when the cohort was stratified by cytology, potentially due to poor model fit to the observed nonproportional hazards in cytology-negative and cytology-positive women.
Cumulative risk by baseline triage test status
Out of 28,017 screened women, 2,377 were HPV positive in the primary screening test. In this HPV-positive subset of women, cytology-based triaging identified 60.8% of CIN2+ cases and 63.6% of CCs over the 72-month study period (Table 4). This required a total of 1,432 colposcopy referrals (split over two screening rounds), resulting in an average of 4.1 colposcopy referrals required to detect one CIN2+ case.
HPV16/18 triage (based on a single baseline screen) would have detected 49.7% of CIN2+ cases and 75.0% of CCs. Assuming that a positive HPV16/18 result would trigger a colposcopy referral, a total of 654 referrals would be required for this strategy, resulting in an average of 2.3 referrals to detect one CIN2+ case.
WID-qCIN triaging would have detected 62.5% of CIN2+ cases and 69.6% of CCs. A total of 686 referrals would be required, resulting in an average of 2.0 referrals to detect one CIN2+ case.
Finally, WID-qCIN/HPV16/18 triaging would have detected 78.1% of CIN2+ cases and 91.3% of CCs. Importantly, this includes seven out of eight CCs in cytology negative women detected from 13 to 72 months after the baseline screen. A total of 1,033 colposcopy referrals would be required, resulting in an average of 2.4 referrals to detect one CIN2+ case.
Discussion
We report a large-scale, population-based longitudinal evaluation of triaging HPV-positive women ≥30 years of age participating in cervical screening using an automated molecular test (HPV16/18 in combination with WID-qCIN). The combined approach detects 85.9% (100%) of prevalent and 69.4% (80.0%) of incident CIN2+ (CC) cases. Importantly, the WID-qCIN/HPV16/18 identified seven out of eight cytology-negative women who were subsequently diagnosed with invasive CC, an important finding considering patient outcome. The WID-qCIN/HPV16/18 combination identified almost three times more women with CIN2+ but, nevertheless, resulted in a much lower number of women requiring colposcopy to identify one woman with CIN2+.
The strength of our study lies in the population-based real-life setting, which minimizes biases such as healthy volunteer or greater compliance effects that may be observed with clinical trials. Although only residual samples (that is, after testing for HPV and cytology) were available, 96.2% of samples from all HPV-positive women were suitable for WID-qCIN testing. This is higher compared with data from a study17 that assessed the performance of DNAme markers for prevalent disease detection and only included 66.8% of eligible patients that are HPV positive. Despite our unbiased real-life setting, the sensitivity of the WID-qCIN alone to detect prevalent CIN2+ was significantly higher (77.0%, 95% CI 71.6–81.6) than the sensitivity reported in a pooled analysis that assessed the best DNAme markers and excluded highly biased studies (CIN2+ pooled sensitivity was 66.0%, 95% CI 61.0–70.0). The specificity of the WID-qCIN test was also superior (76.9%, 95% CI 74.9–78.7) when compared with other DNAme markers (74.0%, 95% CI 69.0–78.0). Notably, the pooled prevalence of CIN2+ in the published studies was two to three times higher, potentially resulting in an artificial reduction of false-positive cases in these settings16.
Whereas no study has assessed the performance of HPV16/18 genotyping in combination with DNAme markers to predict incident and detect prevalent disease, Kremer et al. described a complementary effect of HPV16 and DNAme in predicting the likelihood of CIN2/CIN3 lesions spontaneously regressing, albeit this study was limited by a high proportion (74%) of CIN2 lesions of which only 36% were methylation positive and a low proportion of CIN3 (26%) cases, of which 79% showed methylation28. The fact that the WID-qCIN/HPV16/18 detects 93.4% of all prevalent CIN3 cases and predicts 87.5% of all triage cytology-negative invasive CCs indicates that replacing cytology-based triaging by a WID-qCIN/HPV16/18 triage strategy could almost eliminate invasive cancers in an HPV-screened population.
It is important to acknowledge a few limitations of this study. The gradual transition of Swedish pathology laboratories from reporting CIN2–3 lesions both as high-grade squamous intraepithelial lesion (HSIL) limits our analyses of the more severe incident CIN3 cases, equivalent to cervical carcinoma in situ. Furthermore, the ascertainment bias in identifying prevalent cases, where only women with positive cytology results underwent colposcopy with or without biopsy, poses challenges in accurately evaluating the impact of the proposed molecular triage, covering HPV16/18 with WID-qCIN analyses, on prevention of invasive CCs. Ideally, this testing aims to prevent invasive CCs by identifying preinvasive lesions (that is, CIN2, CIN3, adenocarcinoma in situ (AIS) and HSIL), which may be treated before they progress to invasion. Precise assessment of this necessitates the conduction of a prospective randomized clinical trial. Yet, the fact that combined WID-qCIN/HPV16/18 testing predicted all but two cancers developing within 72 months following sample collection indicates that this DNA-based triaging strategy of HPV-positive women has the potential to greatly diminish the number of invasive cancers in the screened population. This further suggests that screening intervals for HPV-positive WID-qCIN/HPV16/18 negative women could be extended to 5 years29.
Overall, our data indicate that the DNAme-based WID-qCIN test may complement HPV16/18 genotyping in triaging HPV-positive women with improved performance compared with widely used cytology. The fact that this triage test does not rely on assessment of cellular morphology and can be performed purely on DNA, renders it suitable for screening strategies based on self-sampling. The implementation of WID-qCIN in combination with HPV16/18 screening could help to overcome the issue of resampling patients for triaging after positive HPV results on self-samples.
Cervical screening is an essential pillar of the global strategy to eliminate CC. Furthermore, the World Health Organization advocates for HPV-based screening using self-sampling as a simple strategy that could work also in resource-limited settings. High-performance molecular triaging strategies, such as the WID-qCIN test, which could be readily automated and do not require complex infrastructures, should further facilitate these efforts.
Methods
Ethics statement
Ethical approval for the use of samples and linked disease status information in the current study was granted by the Swedish Ethical Review Authority (document number 2014/1242-31/4 and 2022-04693-02) and the Medical University Innsbruck Ethical Committee (reference number 1411/2020). This study was conducted in strict adherence to ethical guidelines and principles.
Study population
In 2017, the Swedish CC screening program invited all women 23–70 years of age to provide a cervical smear sample every 3–5 years depending on age. The liquid-based cytology specimens were collected by midwives and subjected to HPV testing and cytological assessment30. As recommended in the European guidelines for triaging, women aged 30–70 years were primary HPV screened and triaged with cytology upon a positive HPV result. The HPV testing was conducted using the Cobas 4800 platform, which provides genotyping information on HPV16/18 and other oncogenic HPV31 strains. All cervical liquid-based cytology samples obtained in Greater Stockholm were biobanked at −25 °C at the Karolinska University Hospital26,32. With participation in the nationwide CC screening program, women consented in writing to sample collection and diagnosis, as well as potential sample reuse for future research, as approved by the Swedish Ethical Review Authority. No additional written informed consent was collected from the study participants. Patient consent followed an opt-out principle (that is, samples were biobanked by default unless participant opted out). Due to the screening-based character of the described study, no form of patient compensation was provided.
Women with HPV-positive and cytology-negative results at the baseline visit were invited for follow-up screens within 36 months. Women with HPV-positive and cytology-positive (ASC-US+) results were referred for colposcopy. Upon clinical indication, cervical biopsies were taken and histopathologically assessed. The histopathological findings were reported as CIN2 or CIN3, HSIL, AIS or CC. In 2017, Swedish pathology laboratories gradually replaced reporting of CIN2 and CIN3 separately with HSIL (which includes CIN2 or CIN3 without differentiating between the two). Positive histopathological findings (that is, CIN2+) triggered immediate treatment of the patient according to national guidelines.
All data on screening invitations, HPV, cytologies and histopathological assessments from the cervix are uploaded to the Swedish National Cervical Screening Registry (NKCx) once per year30. NKCx was complete up until 31 December 2022. To ensure accurate ascertainment of invasive CC cases, information regarding this disease was obtained from two independent sources. Apart from the NKCx data on cervical histopathologies, we additionally obtained data on invasive CC from the Swedish National Quality Register for Gynecological Cancers (GCR)33. For nine women, the NKCx and the GCR disagreed on the diagnosis of invasive cancer. For eight women, the original diagnostic slides and medical charts were reviewed by a pathologist who was unaware of HPV and cytology status. For one woman, the original slides could not be located. In this case, the diagnosis provided by the NKCx was carried forward. The data on race and ethnicity were not collected for this study cohort.
Study conduct
We conducted a population-based cohort study including all women ≥30 years of age who attended the CC screening program in the capital region of Stockholm between 1 January and 31 March 2017 (the KI-q1-2017 cohort) (Fig. 1). Given the focus on CC screening, only samples from women or those with a cervix were evaluated for this study.
We assessed cervical samples from all HPV-positive women of the KI-q1-2017 cohort with the optimized WID-qCIN test (Supplementary Note 3) and a prespecified threshold (Supplementary Note 4) for what was considered DNAme or WID-qCIN positive. We retrieved information on age, HPV status (negative or positive), HPV16/18 status (for HPV-positive cases only) and cytology outcomes on women having attended the CC screening program between 1 January and 31 March 2017 from NKCx to identify all HPV-positive specimens. Histopathological diagnoses made between 0 and 12 months and between 13 and 72 months were extracted from NKCx, and invasive CC cases were independently verified using data retrieved from the GCR. Furthermore, dates of histopathological diagnosis, last HPV-positive test, last HPV-negative test, last cytology-positive test and last cytology-negative test results between 1 January 2017 and 31 December 2022 were extracted from NKCx.
Optimized WID-qCIN test
The WID-qCIN, a quantitative real-time PCR test, assesses DNAme in bisulfite-modified DNA in three human gene target regions26. The assay has been optimized as described in Supplementary Information (Supplementary Note 3 and Supplementary Fig. 3). Samples of the KI-q1-2017 cohort were analyzed with the optimized and calibrated duplex setup of the WID-qCIN test using a predefined threshold (Supplementary Note 4, Supplementary Table 2 and Supplementary Table 3). The percentage of fully methylated reference (PMR) values were calculated as previously described (Supplementary Note 5)26. The samples with SUM−PMR > 0 were defined as WID-qCIN positive and the samples with SUM−PMR = 0 as WID-qCIN negative (Supplementary Fig. 4).
Statistical analyses
Statistical significance was set to 5%, and 95% CIs were computed for all estimates. The analyses were performed using R (version 4.3.1). The 95% CIs for proportions were computed using the Wilson method in the prop.test function in the stats R package (version 4.3.1). Where applicable, sensitivity or specificity estimates were compared using a two-sided chi-squared test without Yates’ continuity correction using the prop.test function.
Time from sample collection to incident (from 13 to 72 months) CIN2+ (or CC) diagnosis was represented using Kaplan–Meier estimators of cumulative incidence curves using the survfit function in the survival R package (version 3.5–7). The hazard ratios and 95% CIs were calculated using the Cox proportional hazards model using the coxph function in the survival R package (version 3.5–7). The log-rank tests were performed using the survdiff function in the survival R package. The censoring time was defined as the time to the most recent negative test (Supplementary Note 6).
A logistic Weibull mixture model, which considers undiagnosed prevalent disease and interval-censored incident disease, was implemented using the PIMixture R package (version 0.4.4). The odds ratios, hazard ratios and 6-year cumulative incidence estimates, along with 95% CIs, were computed34.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
In consideration of the General Data Protection Regulation by the European Union and the potential risk of patient identification, supplementary analyzed data will not be made publicly available. Specific inquiries requesting additional supplementary data should be directed to M. Widschwendter, MD (Martin.Widschwendter@uibk.ac.at) or J. Dillner, MD, PhD (Joakim.Dillner@ki.se) and will be collaboratively reviewed to ascertain any confidentiality constraints. The evaluation criteria for requests will include overall scientific merit, required anonymization and adherence to data transfer agreements. The response timelines are anticipated to range between 2 and 4 weeks.
References
Brisson, M. et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 395, 575–590 (2020).
Ronco, G. et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet 383, 524–532 (2014).
Gage, J. C. et al. Age-stratified 5-year risks of cervical precancer among women with enrollment and newly detected HPV infection. Int J. Cancer 136, 1665–1671 (2015).
Sankaranarayanan, R. et al. HPV screening for cervical cancer in rural India. N. Engl. J. Med. 360, 1385–1394 (2009).
Mayrand, M. H. et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N. Engl. J. Med. 357, 1579–1588 (2007).
Naucler, P. et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N. Engl. J. Med. 357, 1589–1597 (2007).
Bouvard, V. et al. The IARC perspective on cervical cancer screening. N. Engl. J. Med. 385, 1908–1918 (2021).
Bruni, L. et al. Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: a review and synthetic analysis. Lancet Glob. Health 10, e1115–e1127 (2022).
Simms, K. T. et al. Benefits, harms and cost-effectiveness of cervical screening, triage and treatment strategies for women in the general population. Nat. Med. 29, 3050–3058 (2023).
Dunne, E. F. et al. Prevalence of HPV infection among females in the United States. JAMA 297, 813–819 (2007).
Perkins, R. B. et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J. Low. Genit. Trac. Dis. 24, 102–131 (2020).
Perkins, R. B., Wentzensen, N., Guido, R. S. & Schiffman, M. Cervical cancer screening: a review. JAMA 330, 547–558 (2023).
Ramirez, A. T. et al. Performance of cervical cytology and HPV testing for primary cervical cancer screening in Latin America: an analysis within the ESTAMPA study. Lancet Reg. Health Am. 26, 100593 (2023).
Wang, J. et al. Long-term follow-up of cervical cancer incidence after normal cytological findings. Int. J. Cancer 154, 448–453 (2023).
Widschwendter, A. et al. Analysis of aberrant DNA methylation and human papillomavirus DNA in cervicovaginal specimens to detect invasive cervical cancer and its precursors. Clin. Cancer Res. 10, 3396–3400 (2004).
Salta, S., Lobo, J., Magalhaes, B., Henrique, R. & Jeronimo, C. DNA methylation as a triage marker for colposcopy referral in HPV-based cervical cancer screening: a systematic review and meta-analysis. Clin. Epigenetics 15, 125 (2023).
Bonde, J. et al. Methylation markers FAM19A4 and miR124-2 as triage strategy for primary HPV screen positive women: a large European multi-center study. Int. J. Cancer 148, 396–405 (2020).
Adcock, R. et al. DNA methylation testing with S5 for triage of high-risk HPV positive women. Int. J. Cancer 151, 993–1004 (2022).
Dippmann, C. et al. Triage of hrHPV-positive women: comparison of two commercial methylation-specific PCR assays. Clin. Epigenetics 12, 171 (2020).
Verhoef, L. et al. Performance of DNA methylation analysis of ASCL1, LHX8, ST6GALNAC5, GHSR, ZIC1 and SST for the triage of HPV-positive women: results from a Dutch primary HPV-based screening cohort. Int. J. Cancer 150, 440–449 (2022).
Schmitz, M. et al. Performance of a DNA methylation marker panel using liquid-based cervical scrapes to detect cervical cancer and its precancerous stages. BMC Cancer 18, 1197 (2018).
Zhu, P. et al. ZNF671 methylation test in cervical scrapings for cervical intraepithelial neoplasia grade 3 and cervical cancer detection. Cell Rep. Med. 4, 101143 (2023).
Apostolidou, S. et al. DNA methylation analysis in liquid-based cytology for cervical cancer screening. Int. J. Cancer 125, 2995–3002 (2009).
Teschendorff, A. E. et al. Epigenetic variability in cells of normal cytology is associated with the risk of future morphological transformation. Genome Med. 4, 24 (2012).
Zhuang, J. et al. The dynamics and prognostic potential of DNA methylation changes at stem cell gene loci in women’s cancer. PLoS Genet. 8, e1002517 (2012).
Herzog, C. et al. DNA methylation-based detection and prediction of cervical intraepithelial neoplasia grade 3 and invasive cervical cancer with the WID-qCIN test. Clin. Epigenetics 14, 150 (2022).
Clarke, M. A. et al. Five-year risk of cervical precancer following p16/Ki-67 dual-stain triage of HPV-positive women. JAMA Oncol. 5, 181–186 (2019).
Kremer, W. W. et al. Clinical regression of high-grade cervical intraepithelial neoplasia is associated with absence of FAM19A4/miR124-2 DNA methylation (CONCERVE Study). J. Clin. Oncol. 40, 3037–3046 (2022).
Kim, J. J., Burger, E. A., Regan, C. & Sy, S. Screening for cervical cancer in primary care: a decision analysis for the US Preventive Services Task Force. JAMA 320, 706–714 (2018).
Elfström, K. M. et al. Registry-based assessment of the status of cervical screening in Sweden. J. Med. Screen. 23, 217–226 (2016).
Hortlund, M., Sundstrom, K., Lamin, H., Hjerpe, A. & Dillner, J. Laboratory audit as part of the quality assessment of a primary HPV-screening program. J. Clin. Virol. 75, 33–36 (2016).
Perskvist, N., Norman, I., Eklund, C., Litton, J. E. & Dillner, J. The Swedish cervical cytology biobank: sample handling and storage process. Biopreserv. Biobank. 11, 19–24 (2013).
Ludvigsson, J. F., Otterblad-Olausson, P., Pettersson, B. U. & Ekbom, A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur. J. Epidemiol. 24, 659–667 (2009).
Cheung, L. C. et al. Mixture models for undiagnosed prevalent disease and interval-censored incident disease: applications to a cohort assembled from electronic health records. Stat. Med 36, 3583–3595 (2017).
Acknowledgements
We thank all volunteers who participated in this study, and N. Pashayan and S. Sleigh for critical assessment of the manuscript. We thank I. Ishaq-Parveen, J. Rothärmel and A. Oberhuber for their assistance with the wet laboratory work. This study received financial support from The Land Tirol, which was allocated to M.W., and from the European Union’s Horizon 2020 Research and Innovation program (grant agreement no. 874662; HEAP), which was allocated to M.W. and J.D.
Author information
Authors and Affiliations
Contributions
M.W. and J.D. conceived the study, supervised the study, received funding and contributed equally to this study. J.W., with support from K.S., identified samples in the biobank and collated all outcome data. L.S. and E.R. carried out the wet laboratory work and optimized the WID-qCIN test. J.E.B., supported by C.H. and C.D.V., carried out the statistical analysis and, together with L.S., produced the display items. M.W. wrote the original draft of the manuscript and, together with L.S., conducted the initial literature search. L.S., J.E.B. and J.W. contributed equally to this study. All authors had access to the study data, contributed to data interpretation, critically reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
J.E.B. and M.W. are named as inventors on a patent (submitted by University College London Business Ltd (UCLB); no. WO2021255460), which covers developmental aspects of the WID-qCIN test. M.W., L.S. and E.R. are listed as inventors on a priority patent application (submitted by Sola Diagnostics GmbH; no. GB2401477.1) covering the optimization of the WID-qCIN test. J.E.B., C.H. and M.W. are shareholders in Sola Diagnostics GmbH, which holds exclusive licenses to the respective intellectual property rights that protect the commercialization of the WID-qCIN test. K.S. has received consulting fees and research grants from Merck Sharp and Dohme Corp. to her affiliating institution for studies on HPV vaccination in Sweden. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Partha Basu, Silvia DeSanjose and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling editor: Sonia Muliyil, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Logistic Weibull mixture model analysis of the KI-q1-2017 cohort.
Graphs depict cumulative incidence rates of incident CIN2+ cases stratified according to a) WID-qCIN, b) HPV16/18, and c) WID-qCIN/HPV16/18 analyses. 95% confidence intervals are shown as gray shaded areas. CIN2+ cervical intraepithelial neoplasia or worse, HPV human papillomavirus.
Supplementary information
Supplementary Information
Supplementary Notes 1–6, Figs. 1–4, Tables 1–3 and References.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schreiberhuber, L., Barrett, J.E., Wang, J. et al. Cervical cancer screening using DNA methylation triage in a real-world population. Nat Med (2024). https://doi.org/10.1038/s41591-024-03014-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41591-024-03014-6
- Springer Nature America, Inc.