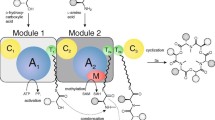
Abstract
Aromatic polyketides are natural polyphenolic compounds with a broad spectrum of pharmacological activities. Production of those metabolites in the model organisms Escherichia coli and Saccharomyces cerevisiae has been limited by the extensive cellular engineering needed for the coordinated biosynthesis of polyketides and their precursors. In contrast, the amoeba Dictyostelium discoideum is a native producer of secondary metabolites and harbors a wide, but largely unexplored, repertoire of genes for the biosynthesis of polyketides and terpenoids. Here we present D. discoideum as an advantageous chassis for the production of aromatic polyketides. By expressing its native and cognate plant polyketide synthase genes in D. discoideum, we demonstrate production of phlorocaprophenone, methyl-olivetol, resveratrol and olivetolic acid (OA), which is the central intermediate in the biosynthesis of cannabinoids. To facilitate OA synthesis, we further engineered an amoeba/plant inter-kingdom hybrid enzyme that produced OA from primary metabolites in two enzymatic steps, providing a shortcut in a synthetic cannabinoid pathway using the D. discoideum host system.





Similar content being viewed by others
Data availability
Nucleotide sequence data were obtained from the GenBank (https://www.ncbi.nlm.nih.gov/genbank/). The Codon Usage for organisms was obtained from the Kazusa Codon Usage Database (https://www.kazusa.or.jp/codon/). The minimal datasets generated during the study are available as Supplementary Information or as source data. Biological and genetic materials are available from V.V. and F.H. upon reasonable request and completion of a material transfer agreement. Source data are provided with this paper.
Change history
23 November 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41587-022-01607-5
References
Hertweck, C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. Engl. 48, 4688–4716 (2009).
Austin, M. B. & Noel, J. P. The chalcone synthase superfamily of type III polyketide synthases. Nat. Prod. Rep. 20, 79–110 (2003).
Lim, Y. P., Go, M. K. & Yew, W. S. Exploiting the biosynthetic potential of type III polyketide synthases. Molecules 21, 806 (2016).
Morita, H., Wong, C. P. & Abe, I. How structural subtleties lead to molecular diversity for the type III polyketide synthases. J. Biol. Chem. 294, 15121–15136 (2019).
Sunil, C. & Xu, B. An insight into the health-promoting effects of taxifolin (dihydroquercetin). Phytochemistry 166, 112066 (2019).
de la Lastra, C. A. & Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 35, 1156–1160 (2007).
Staunton, J. & Weissman, K. J. Polyketide biosynthesis: a millennium review. Nat. Prod. Rep. 18, 380–416 (2001).
Fellermeier, M. & Zenk, M. H. Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol. FEBS Lett. 427, 283–285 (1998).
Whiting, P. F. et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 313, 2456–2473 (2015).
Nivina, A., Yuet, K. P., Hsu, J. & Khosla, C. Evolution and diversity of assembly-line polyketide synthases. Chem. Rev. 119, 12524–12547 (2019).
Palmer, C. M. & Alper, H. S. Expanding the chemical palette of industrial microbes: metabolic engineering for type III PKS-derived polyketides. Biotechnol. J. 14, 1700463 (2019).
Luo, X. et al. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 567, 123–126 (2019).
Chen, X. et al. Terpene synthase genes in eukaryotes beyond plants and fungi: occurrence in social amoebae. Proc. Natl Acad. Sci. USA 113, 12132–12137 (2016).
Barnett, R. & Stallforth, P. Natural products from social amoebae. Chemistry 24, 4202–4214 (2018).
Eichinger, L. et al. The genome of the social amoeba Dictyostelium discoideum. Nature 435, 43–57 (2005).
Zucko, J. et al. Polyketide synthase genes and the natural products potential of Dictyostelium discoideum. Bioinformatics 23, 2543–2549 (2007).
Ghosh, R. et al. Dissecting the functional role of polyketide synthases in Dictyostelium discoideum: biosynthesis of the differentiation regulating factor 4-methyl-5-pentylbenzene-1,3-diol. J. Biol. Chem. 283, 11348–11354 (2008).
Heidel, A. J. et al. Phylogeny-wide analysis of social amoeba genomes highlights ancient origins for complex intercellular communication. Genome Res. 21, 1882–1891 (2011).
Austin, M. B. et al. Biosynthesis of Dictyostelium discoideum differentiation-inducing factor by a hybrid type I fatty acid-type III polyketide synthase. Nat. Chem. Biol. 2, 494–502 (2006).
Herbst, D. A., Townsend, C. A. & Maier, T. The architectures of iterative type I PKS and FAS. Nat. Prod. Rep. 35, 1046–1069 (2018).
Shimizu, Y., Ogata, H. & Goto, S. Type III polyketide synthases: functional classification and phylogenomics. ChemBioChem 18, 50–65 (2017).
Fey, P., Kowal, A. S., Gaudet, P., Pilcher, K. E. & Chisholm, R. L. Protocols for growth and development of Dictyostelium discoideum. Nat. Protoc. 2, 1307–1316 (2007).
Morris, H. R., Taylor, G. W., Masento, M. S., Jermyn, K. A. & Kay, R. R. Chemical structure of the morphogen differentiation inducing factor from Dictyostelium discoideum. Nature 328, 811–814 (1987).
Kay, R. R. & Jermyn, K. A. A possible morphogen controlling differentiation in Dictyostelium. Nature 303, 242–244 (1983).
Carvalho, A., Hansen, E. H., Kayser, O., Carlsen, S. & Stehle, F. Designing microorganisms for heterologous biosynthesis of cannabinoids. FEMS Yeast Res. 17, fox037 (2017).
Taura, F. et al. Characterization of olivetol synthase, a polyketide synthase putatively involved in cannabinoid biosynthetic pathway. FEBS Lett. 583, 2061–2066 (2009).
Gagne, S. J. et al. Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides. Proc. Natl Acad. Sci. USA 109, 12811–12816 (2012).
Morgan-Kiss, R. M. & Cronan, J. E. The Escherichia coli fadK (ydiD) gene encodes an anerobically regulated short chain acyl-CoA synthetase. J. Biol. Chem. 279, 37324–37333 (2004).
Kay, R. R. The biosynthesis of differentiation-inducing factor, a chlorinated signal molecule regulating Dictyostelium development. J. Biol. Chem. 273, 2669–2675 (1998).
Narita, T. B., Koide, K., Morita, N. & Saito, T. Dictyostelium hybrid polyketide synthase, SteelyA, produces 4-methyl-5-pentylbenzene-1,3-diol and induces spore maturation. FEMS Microbiol. Lett. 319, 82–87 (2011).
Saito, T. et al. Identification of new differentiation inducing factors from Dictyostelium discoideum. Biochim. Biophys. Acta 1760, 754–761 (2006).
Mookerjee, S., Campbell, A. J., Wiltshire, Z. D. & Chen, K. J. Method and cell line for production of phytocannabinoids and phytocannabinoid analogues in yeast. US patent no. 20200283807A1 (2018).
Mookerjee, S., Campbell, A. J., Wiltshire, Z. D. & Chen, K. J. Method and cell line for production of polyketides in yeast. US patent no. 10975395B2 (2020).
Salehi, B. et al. Resveratrol: a double-edged sword in health benefits. Biomedicines 6, 91 (2018).
Hoefgen, S. et al. Facile assembly and fluorescence-based screening method for heterologous expression of biosynthetic pathways in fungi. Metab. Eng. 48, 44–51 (2018).
Veltman, D. M., Keizer-Gunnink, I. & Haastert, P. J. An extrachromosomal, inducible expression system for Dictyostelium discoideum. Plasmid 61, 119–125 (2009).
Milke, L., Aschenbrenner, J., Marienhagen, J. & Kallscheuer, N. Production of plant-derived polyphenols in microorganisms: current state and perspectives. Appl. Microbiol. Biotechnol. 102, 1575–1585 (2018).
Wang, S. et al. Metabolic engineering of Escherichia coli for the biosynthesis of various phenylpropanoid derivatives. Metab. Eng. 29, 153–159 (2015).
Unkles, S. E., Valiante, V., Mattern, D. J. & Brakhage, A. A. Synthetic biology tools for bioprospecting of natural products in eukaryotes. Chem. Biol. 21, 502–508 (2014).
Katz, K. S. & Ratner, D. I. Homologous recombination and the repair of double-strand breaks during cotransformation of Dictyostelium discoideum. Mol. Cell. Biol. 8, 2779–2786 (1988).
Wiegand, S., Kruse, J., Gronemann, S. & Hammann, C. Efficient generation of gene knockout plasmids for Dictyostelium discoideum using one-step cloning. Genomics 97, 321–325 (2011).
Kuspa, A. & Loomis, W. F. Tagging developmental genes in Dictyostelium by restriction enzyme-mediated integration of plasmid DNA. Proc. Natl Acad. Sci. USA 89, 8803–8807 (1992).
Sekine, R., Kawata, T. & Muramoto, T. CRISPR/Cas9 mediated targeting of multiple genes in Dictyostelium. Sci. Rep. 8, 8471 (2018).
Tan, Z., Clomburg, J. M. & Gonzalez, R. Synthetic pathway for the production of olivetolic acid in Escherichia coli. ACS Synth. Biol. 7, 1886–1896 (2018).
Lu, Y. et al. Production of the soluble human Fas ligand by Dictyostelium discoideum cultivated on a synthetic medium. J. Biotechnol. 108, 243–251 (2004).
Vaknin, Y. et al. Identification and characterization of a novel Aspergillus fumigatus rhomboid family putative protease, RbdA, involved in hypoxia sensing and virulence. Infect. Immun. 84, 1866–1878 (2016).
Levi, S., Polyakov, M. & Egelhoff, T. T. Green fluorescent protein and epitope tag fusion vectors for Dictyostelium discoideum. Plasmid 44, 231–238 (2000).
Fey, P., Dodson, R. J., Basu, S. & Chisholm, R. L. In: Dictyostelium discoideum Protocols (eds Eichinger, L. & Rivero, F.) 59–92 (Humana Press, 2013).
Hirst, J., Kay, R. R. & Traynor, D. Dictyostelium cultivation, transfection, microscopy and fractionation. Bio. Protoc. 5, 1485 (2015).
Meier, K. et al. Correlation for the maximum oxygen transfer capacity in shake flasks for a wide range of operating conditions and for different culture media. Biochem. Eng. J. 109, 228–235 (2016).
Acknowledgements
We thank H. Heinecke for conducting NMR experiments and L. Reimer for technical support. This work was supported by two grants of the European Social Fund ESF ‘Europe for Thuringia’ projects MiQWi (2015FGR0097, to F.H.) and SphinX (2017FGR0073, to V.V.), the Leibniz Research Cluster in the frame of the BMBF Strategic Process Biotechnology 2020+ (031A360A, to V.V.), the BMBF funding program ‘GO-Bio initial’ (FKZ161B097, to F.H.) and the BMBF-funded InfectControl consortium (03ZZ0813A, to L.R.).
Author information
Authors and Affiliations
Contributions
C.R., J.E.K., V.V. and F.H. designed the research. C.R. and J.E.K. performed experiments and analyzed the data. L.R. supervised the respiration activity measurements. J.R. analyzed the NMR data. C.R., J.E.K., V.V. and F.H. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
C.R., J.E.K., V.V. and F.H. declare the following competing financial interest: part of this work was used to file patent application PCT/EP2021/068240.
Additional information
Peer review information Nature Biotechnology would like to thank Rasmus Frandsen, Rob Kay and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–10 and Supplementary Tables 1–4.
Source data
Source Data Fig. 1
Unprocessed Western blots.
Source Data Fig. 2
Unprocessed Northern blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Reimer, C., Kufs, J.E., Rautschek, J. et al. Engineering the amoeba Dictyostelium discoideum for biosynthesis of a cannabinoid precursor and other polyketides. Nat Biotechnol 40, 751–758 (2022). https://doi.org/10.1038/s41587-021-01143-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-021-01143-8
- Springer Nature America, Inc.
This article is cited by
-
Scale-up of an amoeba-based process for the production of the cannabinoid precursor olivetolic acid
Microbial Cell Factories (2022)
-
Slime moulds souped up with plant genes churn out drugs
Nature (2022)
-
CRISPR/Cas9-based genome-wide screening of Dictyostelium
Scientific Reports (2022)