Abstract
The present study is focused on the use of cubic metal-organic frameworks-5 (MOF-5) and its functionalized form in the removal of phenolic pollutants by molecular dynamics (MD) and Well-tempered metadynamics (WTMD) simulation methods. It was found that the adsorption mechanism of MOF-5s/phenolic compounds is mostly due to the van der Waals and π–π interactions. However, electrostatic and hydrogen bond (HB) interactions also play a significant role in removing phenolic pollutants by MOF-5 and its functionalized form. The results show that the fluorine functional group (F-MOF-5) increases the adsorption capacity of phenol compounds on the adsorbent surface. By functionalizing the MOF-5 with a methyl functional group (CH3-MOF-5), the adsorption strength decreases. The WTMD calculation confirmed that at the most stable state, the free energy (FE) value of system II (the most stable system in functionalized systems with –F functional group) is about −289.528 kJ mol−1. This value is ~5.781 and 35.514 kJ mol−1 more negative than the FE of the I and III systems (the most stable systems in the pristine and CH3-MOF-5/pollutant systems, respectively). Altogether, the results indicate that F-MOF-5 can be considered a more suitable adsorbent than MOF-5 and CH3-MOF-5 for phenolic pollutants removal from the environment for more assessment.
Similar content being viewed by others
Introduction
Although technological and industrial advances have facilitated life conditions in the last 50 years compared to the past, the increase in pollution has caused concerns for human health and the environment1,2,3. Living organisms and environments are continuously exposed to many organic and inorganic toxic chemicals due to domestic, agricultural, and industrial activities4,5.
The phenol and its derivatives which represent the majority of toxic chemicals, have entered almost all areas of life6. Phenol derivatives are widely utilized in industry, and their presence in surface and wastewater is a problem requiring urgent solution due to their carcinogenic effects, bioaccumulation, weak biodegradability, and high toxicity7,8,9. Phenolic molecules are a kind of toxic organic pollutants commonly discharged from industrial effluents. The phenol molecule and its derivatives are often released during industrial and agricultural activities, including pharmaceutical synthesis, metal coating, paper coal conversion, pulp, oil refining, and so on10. Their widespread use may exist in wastewater and natural waters that have serious impacts on humans, animals, and aqueous mediums. As a result, phenolic pollutants in aqueous solutions pose a high threat to land security and environmental pollution, thus prompting a high demand for their detection and removal. For example, Shaheen et al.11 used biochar derived from date palm frond pyrolyzed at 600 °C as a low-cost, effective, and renewable adsorbent to remove phenol from phenol-contaminated aqueous solutions. From their thermodynamic and experimental results, it was concluded that pate palm frond pyrolyzed at 600 °C can be used successfully to remove/adsorb phenol from wastewater.
As mentioned removal of phenolic pollutants by using cost-efficient and eco-friendly techniques is challenging at present. The highly water-soluble and stable nature of phenolic compounds makes their degradation from wastewater more challenging using common techniques12,13.
Amongst the materials utilized for the removal of pollutants from an aqueous medium, metal-organic frameworks have become a promising candidate for improved wastewater treatment14,15,16,17. This is mainly due to their intrinsic features such as; structural diversity, high adsorption capacity, and high specific surface area18,19. Highly porous metal-organic frameworks (MOFs) are emerging as potential materials for adsorbing different types of hazardous organic and inorganic pollutants20,21. For example, the use of MOF is investigated for the removal of heavy metals from wastewater by Haso et al.22. The MOF illustrated a maximum removal efficiency of over 90% for Pb, Cr, and Cd metals, from wastewater samples. As a result, they showed that the MOF reveals very good potential for the simultaneous removal of toxic metals from high-volume contaminated wastewater.
The metal-organic frameworks-5 (MOF-5) is a zinc-based MOF formed of benzene dicarboxylic acid as the crystal bifunctional bridging ligand and a [Zn4O]6+ inorganic moiety as the vertex. The MOF-5 is one of the most well-known compounds of MOFs due to its large pores and high surface area23. In addition, MOF-5 has been used for wastewater treatment, such as removing Cu ions from aqueous solution24.
For example, Xie et al.25 reported the use of MOF-5 to remove phenol from aqueous solution. They showed that the phenol removal rate was 97% or more, thus MOF-5 has good potential for phenol removal.
Also, another advantage of the MOF-5 is that it is utilized as a suitable adsorbent with multiple active sites and porous architectures26.
The functionalization of the organic part of MOFs with various functional groups and the presence of numerous active sites make it possible to obtain other configurations27. The bulk form of MOFs may be an obstacle to their adsorption efficiency; therefore, it is necessary to modify their structure toward a more effective compound for pollutant removal28.
Considering the necessity of removing phenolic compounds as a main class of pollutants, this work aims to evaluate the MOF-5 potential in adsorbing phenolic compounds by MD simulation. Also, the MOF-5 is modified by the fluorine (-F) and methyl (–CH3) functional groups and their efficiency is compared and investigated. Furthermore, well-tempered metadynamics (WTMD) simulations are performed for the most stable systems in pristine and functionalized systems to obtain free energy (FE) profiles. Although numerous literatures have reviewed the application of MOF-based materials for environmental applications25,29,30. However, only a few focus on the role of MOF-5 as a potential adsorbent for the removal of phenolic compounds through MD and WTMD simulations. This study presents worthwhile information about the adsorption mechanism of phenolic compounds on the MOF-5 and functionalized MOF-5 surfaces.
Results
MD simulation results
In the following sections, the pollutant adsorption process is examined by parameters such as the total energy (Etot), number of contacts, the center of mass distance (dCOM), radial distribution function (RDF), number of hydrogen bonds (HBs), solvent accessible surface area (SASA), density, and pollutant molecules mobility around the adsorbents.
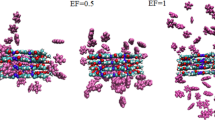
It is worth mentioning that pollutant molecules are located in parallel at a distance of ~2 nm from the adsorbent surface to minimize the effect of primitive orientations. Over time simulation, the distance between the adsorbents and the pollutant molecules is decreased (Supplementary Table 1), and finally, the pollutant adsorbed on the adsorbent surfaces. As can be observed in Fig. 1, a few pollutant molecules alter their orientation from parallel to perpendicular to the adsorbent surface during MD simulation. This behavior demonstrates that the parallel orientation is relatively more stable than the other orientations. This fact is consistent with our previous study31, which indicates that the distance between the adsorbent aromatic rings and the aromatic ring of the pollutant is shorter than the other interaction distances. Also, it can be seen that pollutant molecules lie on the MOF-5 surfaces with offset π–π interactions in which the vertical distance between pollutant molecules and MOF-5s is ~0.35 nm.
One of the most important indicators in the MD simulation is the analysis of energy values. The more negative energy shows a stronger interaction between adsorbent and adsorbed molecules. The average values of vdW, the electrostatic (elec), and Etot for pair adsorbents/pollutant molecules in all systems are listed in Table 1 and Supplementary Fig. 1. The negative values of energies during the process of system formation confirm that the adsorption process is favorable in terms of thermodynamics. The greater the values of vdW and elec interactions (more negative value of Etot), the more molecules are adsorbed onto the MOF-5 surfaces. Concerning the data in Table 1, the vdW energy is the dominant interaction between MOF-5s and pollutant molecules. This result can be related to the formation of the π–π interaction between the adsorbent and pollutant molecules in the studied systems. The higher elec energy values in the F-MOF-5/TCP and MOF-5/TCP systems show that the tendency of TCP molecule for adsorbing on the F-MOF-5 and MOF-5 surfaces is greater than the CH3-MOF-5 surface. This result confirms that the elec energy and HB formation have also a significant role in the adsorption of pollutants. Based on the obtained results, the Etots of chlorophenol compounds are higher than phenol molecules due to the higher polarity of the chlorophenol molecules. The presence of electronegative atoms like Cl (chlorine) in the chlorophenol structure causes asymmetric charge distribution in this molecule32. Consequently, the bipolar interactions between the chlorophenol and adsorbents are more significant than the adsorbent and phenol interaction, so, the adsorption strength of chlorophenol on the adsorbents is higher. This trend of increasing the interaction energy is also observed in the functionalized systems, and the most negative values of the Etots are observed for the F-MOF-5/TCP and CH3-MOF-5/TCP systems. These results show that energy values and complex formation are strongly related to the number of chlorine atoms in each system. Furthermore, by functionalizing the adsorbent with the –F functional group, the Etot becomes more negative, which shows the strength of the pollutant’s adsorption into the F-MOF-5 cavities/surface has increased. The increase in the Etot in these systems can be ascribed to the presence of the strong electron-withdrawing group (–F) and more pollutant adsorption. On the other hand, in the CH3-MOF-5/pollutant systems, the Etot is less negative (compared with pristine systems), which can facilitate the release of phenolic pollutants from the adsorbent surface for reuse. The reduction of Etot in these systems can be ascribed to the presence of the –CH3 electron-donating group, which can also create a steric hindrance and make it more difficult for pollutants to approach the adsorbents.
To better understand the interaction of pollutant molecules with MOF-5/functionalized MOF-5, dCOM between the adsorbent and adsorbed molecules as a function of time is calculated and represented in Fig. 2. This figure clearly shows that the dCOM of adsorbents-adsorbed molecules decreases drastically due to the adsorption of pollutant molecules into MOF-5/functionalized MOF-5. On the other hand, the number of contacts between the adsorbent and polluting molecules increases with their penetration into the MOF-5/functionalized MOF-5 (Fig. 3). In the F-MOF-5/pollutant systems, a less average dCOM is observed compared to the pristine and CH3-MOF-5/pollutant systems. This fact can explain why the Etot for the F-MOF-5/pollutant systems is more negative than the other systems.
The number of contacts between pollutant molecules and adsorbents is calculated for all systems and results are illustrated in Fig. 3. This figure displays that the TCP has a higher number of contacts than other pollutant molecules (in pristine systems), indicating more interaction of this molecule with MOF-5. The analysis of Fig. 3 shows that the pollutant molecules more tend to interact with the F-MOF-5, and the number of contacts reaches almost 3200 in the F-MOF-5/pollutant systems. Whereas, in the MOF-5/pollutant and CH3-MOF-5/pollutant systems, the number of contact values is almost 2200. The number of contacts is higher in the F-MOF-5/pollutant molecule systems, which makes this system more stable. In general, there is a significant correlation between the results obtained from the number of contact calculations and interaction energy values.
RDF calculations are analyzed from the MD results to evaluate the interaction distance between pollutant molecules and MOF-5/functionalized MOF-5. RDF is an important estimation of the probable separation distance of adsorbed molecules from the adsorbent surface. The RDF diagram related to the distribution of pollutant molecules around the MOF-5 and functionalized MOF-5 surfaces is shown in Fig. 4. As shown in this figure, the probability of finding TCP molecules around MOF-5 is higher than other pollutant molecules, which can be related to the stronger interaction between these molecules and MOF-5. In the MOF-5/PHE system, the RDF peak intensity of MOF-5-pollutant molecules is almost one-third of the peak intensity in the MOF-5/TCP system, which may be due to the lack of encapsulation of phenol inside MOF-5 cavities. Moreover, as it is clear from Fig. 4, the major interactions between MOF-5s and pollutant molecules are in the distance of ~0.3–1.5 nm, and the maximum interactions occur at 4.25–6.91 nm. The strongest RDF peaks of pollutant molecules and adsorbents can be observed in the MOF-5/TCP and F-MOF-5/TCP systems (among the pristine and functional systems, respectively) at distances of ~0.3–0.5 nm. These observations indicate that more TCP molecules are likely to be found around MOF-5 and F-MOF-5 surfaces/cavities than other phenolic compounds. These findings are consistent with the obtained results that show the vdW energy is higher in these systems than in other systems. According to RDF plots, in the CH3-MOF-5/pollutant systems, the probability of finding pollutants around the adsorbent surface is less than in the MOF-5/pollutant systems. As well as, pollutant molecules showed strong interactions with F-MOF-5, with a maximal g(r) of 6.91 and a peak distance of 0.42 nm. Further inspection of the RDF diagrams shows that in functionalized systems such as pristine systems, the intensity of the RDF peaks increases as the interaction energies become stronger. In other words, a similar trend is observed between these two parameters (energy values and RDF analysis).
Atomic RDF (aRDF) can help explore the molecular orientation of pollutant molecules with respect to the MOF-5 and functionalized MOF-5. For this purpose, the aRDF of the pollutant molecules in all systems is calculated and the obtained results are plotted in Supplementary Figs. 2–4. As depicted in this figure, in all systems, the strongest peak belongs to the H and C atoms (belonging to the aromatic group) of the pollutant molecules. These peaks are due to the formation of π–π interactions between the aromatic ring of pollutant molecules and the surface of adsorbents. In addition, it is found that the probability of the interaction between H and C atoms of phenolic compounds and MOF-5s is higher than the O and F atoms.
The number of HBs formed between the components of a simulated system over time provides valuable insight into the nature of the forces between them. In order to accurately evaluate the interaction mechanism between phenol compounds and MOF-5s, the number of HBs formed between them in a radius of 3.5 Å is calculated (Fig. 5). As shown in the figure, with the passage of simulation time and the completion of the adsorption process, the number of HBs between pollutant molecules and adsorbents increases. In systems with more Cl atoms (i.e., MOF-5/TCP, F-MOF-5/TCP, and CH3-MOF-5/TCP systems), the number of HBs also increases. This result shows that the increase in HB is directly related to the number of Cl atoms in these systems. A closer look at Fig. 5 shows that the number of HBs in the F-MOF-5/pollutant systems is more than in other systems. As a result, with MOF-5 surface functionalization with the -F functional group, the number of host-guest HBs has increased. This fact can be expressed in the way that in these systems, more pollutant molecules are adsorbed on the adsorbent surfaces. Generally, it can be concluded that HB can be a driving force for the adsorption of phenol molecules and their derivatives on the MOF-5/functionalized MOF-5 surfaces.
The SASA study can be used to determine the number of pollutant molecules dispersed in water. The average surface area of a molecule that is exposed to a solvent is described as the SASA33. This analysis compares the performance of MOF-5s in providing enough accessible areas to remove pollutant molecules. In general, more adsorption of pollutant molecules on MOF-5s leads to lower molecular dispersion in water and a lower average SASA value. According to Fig. 6, the lowest average SASA belonged to the TCP molecule in the MOF-5/TCP system with a value of 2.5736 nm2. Whereas, higher average SASA values in the MOF-5/PHE system confirm that phenol molecules are less adsorbed on the MOF-5 surface than the other pollutant molecules. The average values of SASA have been reduced for the functionalized systems (with –F group) compared to pristine systems. This result shows that the adsorption process is better performed by the F-MOF-5 than MOF-5. In addition, in these systems, the lowest level of contact of the pollutant molecules with the solvent is related to the F-MOF-5/TCP system with a SASA value of 2.5735 nm2. As a result, the adsorption of TCP on the F-MOF-5 surface is more favorable than other pollutant molecules. It should be noted that in the CH3-MOF-5/pollutant systems, the average SASA values are higher than those of the F-MOF-5/pollutant and MOF-5/pollutant systems. This result indicates that the pollutant molecules have less tendency for adsorption on the CH3-MOF-5 surface.
To better understand pollutant molecules’ movement from the water phase to the adsorbent surface, the density analysis for all systems is calculated and shown in Fig. 7. This figure shows that pollutant molecules tend to adsorb and penetrate the surface and cavities of MOF-5sIn the same way that the order of the Etot value in pristine systems is as MOF-5/TCP > MOF-5/DCP > MOF-5/CHP > MOF-5/PHE, the intensity of density peak for TCP molecule at closer distances is higher than other pollutant molecules. This result shows that TCP pollutant has a greater tendency for adsorption on the surface of MOF-5. As it is obvious from Fig. 7, in the functionalized substrates, systems where their Etot is more negative (CH3-MOF-5/TCP and F-MOF-5/TCP systems), the intensity of the peak density and the probability of pollutants present in the closer distances to the adsorbent increases. There is a similar trend between the density analysis and the Etots for pristine/functionalized systems, which confirms the previous results in this work.
The diffusion coefficient (D), which is obtained from the MSD analysis, provides information on the movement speed of pollutant molecules around the adsorbent cavities/surfaces34. In fact, MSD determines the mode of molecule (particle) displacement over time. It determines whether a molecule (particle) is bound, transported, or diffusing freely. The MSD diagrams and D values for the pollutant molecules in all systems are calculated and presented in Fig. 8 and Table 2. The obtained results from this table show that the D value in the pristine systems follows the following order: MOF-5/PHE > MOF-5/CHP > MOF-5/DCP > MOF-5/TCP systems. The D value and slope of the MSD diagram for the TCP molecule are less than the PHE, CHP, and DCP molecules. This trend of reducing D values is also observed in the F-MOF-5/pollutant and CH3-MOF-5/pollutant systems which can be attributed to greater weight and stronger adsorption of DCP molecule. In addition, in the F-MOF-5/pollutant systems, the D value of phenolic compounds has decreased compared to pristine systems, which can be due to the presence of -F functional groups on the adsorbent surface. While the presence of the –CH3 electron-donating group in the CH3-MOF-5/pollutant systems, increases the D value and slope of the MSD diagram. According to the obtained results, it can be concluded that the chemistry of the carrier surface also has a significant effect on the diffusion coefficient.
WTMD simulation results
Finally, FE surfaces are also investigated as a decisive clue for confirming all the interactions mentioned above and distribution patterns. Figure 9 reveals the FE surface of the adsorption process for the dCOM of TCP molecules relative to the dCOM of the adsorbents. The resulting patterns are provided in Fig. 9 and show that system II has the highest FE at its stable state, confirming the ٍEtots. For long distances of the pollutant molecules from the adsorbent surfaces, the FE is to be zero, while it becomes more and more negative as the TCP molecules move toward the adsorbent surface/cavities. The FE profile for the TCP adsorption on the MOF-5 shows a global minimum with an energy of ~−283.747 kJ mol−1 (at a distance of ~1.88 nm), which is higher than the system III (FE = −254.014 kJ mol−1). In system III, the TCP molecule, in the process of reaching its respective global minimums, experiences two energy barriers of ~+124.10 and +28.10 kJ mol−1. In system II, the value of energy corresponding to the most stable state is −289.528 kJ mol−1, which is ~5.78 kJ mol−1 more negative than the respective value in system I. This behavior can be explained by more HB formation between the TCP molecule and the F atoms at the edge of the F-MOF-5 surface. These findings show that the TCP molecule has a higher propensity for adsorption on the F-MOF-5 surface than other adsorbents.
Our results have good agreement with the recent experimental results obtained by Kumar et al.35. In their study, MOF-5 and MOF-5@GO are synthesized as follows and used as an adsorbent to removal of Rhodamine B (RhB) pollutants. 4.24 g of zinc acetate,1.26 g of benzene dicarboxylic acid, and N, N-dimethylformamide are continuously in a beaker for 2 h until a homogeneous mixture is obtained. Then, it is put in an oven at 125 °C for 24 h in a Teflon-lined sealed solvothermal vessel, as a result, a solid colorless is made. It is gradually cooled to room temperature and centrifuged at 7000 rpm for 15 min. After that, the obtained product is washed with N, N dimethylformamide, dried under vacuum at room temperature, and stored in a desiccator. The MOF-5@GO nanocomposite is synthesized using a grinding method at various ratios (i.e., 1:1, 1:2, and 2:1). They investigated the removal of RhB molecules by MOF-5 and MOF-5@GOX using techniques such as FTIR and XRD. They reported a removal efficiency of 95% for RhB removal and a surface area value of 29.95 m2 g−1 for the MOF-5. Their results showed that the synthesized adsorbent is appropriate for the removal of RhB, cost-effective, and reusable.
Discussion
The MOF-5/functionalized MOF-5 as adsorbents for the removal of phenolic pollutants are evaluated by the MD and WTMD simulation methods. The evaluation of the Etots in the absence and presence of the functional group shows that the vdW energy has a more important role in the adsorption process than the elec energy. By functionalizing MOF-5 with –CH3, the interaction between the adsorbent and pollutant molecules decreases, which can be related to the presence of the –CH3 electron-donating group. The results show that the functionalization of MOF-5 with –F increases the Etot, the radial distribution of phenol around F-MOF-5, as well as strengthens the HB between F-MOF-5 and phenol compounds. As a result, the –F functional group increases the adsorption capacity of phenol compounds on the MOF-5 surface. Hence, phenolic pollutants can be effectually removed using the F-MOF-5 compared to the MOF-5 and CH3-MOF-5. These results are well confirmed by the Etot, number of contacts, RDF, number of HBs, SASA, density, and MSD analyses. The WTMD calculation confirmed that the TCP molecule is facing small energy barriers to adsorption on the MOF-5 and functionalized MOF-5 surfaces. At the most stable state, the FE value of system II (the most stable system in functionalized systems with the –F group) is about −289.528 kJ mol−1. This value is about 5.781 and 35.514 kJ mol−1 more negative than the FE of the I and III systems (the most stable systems in the pristine and CH3-MOF-5/pollutant systems, respectively). In general, the present work emphasizes that the MOF-5/F-MOF-5 are suitable adsorbents for phenol and its derivatives and encourages researchers to use these adsorbents for removing pollutants.
Methods
Molecular models (initial structures) and simulation boxes
The 3D structure of the phenol and three of its derivatives namely, 4-chlorophenol (CHP), 2,4-dichlorophenol (DCP), and 2,4,6-trichlorophenol (TCP) molecules (Supplementary Fig. 5) are extracted from the PubChem database. The forcefield parameters of these compounds are obtained from the SwissParam web service. The 3D structure of MOF-5, the adsorbent model, is derived from the Chemtub3D website.
First, four systems are designed that are called MOF-5/PHE, MOF-5/CHP, MOF-5/DCP, and MOF-5/TCP systems, which include:
-
(1)
Ten phenol molecules + MOF-5 + water + ions,
-
(2)
Ten CHP molecules + MOF-5 + water + ions,
-
(3)
Ten DCP molecules + MOF-5 + water + ions, and
-
(4)
Ten TCP molecules, MOF-5, water + ions, respectively.
Then, to design functionalized systems, –F and –CH3 functional groups are added to the edge carbon atoms of MOF-5 (Fig. 10). More details for the functionalized systems are given in Supplementary Table 2. In all systems, the initial distance between pollutant molecules and adsorbent is considered to be about 2 nm. The dimensions of the intended simulation boxes are considered at 8 × 8 × 8 nm3, which is hydrated with water molecules. Snapshots of the initial and final configurations of the simulation boxes for the MOF-5s/PHE systems are shown in Supplementary Fig. 6.
MD simulation
The MD simulation is the computer simulation to measure the interaction between molecules and particles in a compound. This method can show many properties for specific molecules, such as interface interaction, temperature drop, heat flow through the molecule, particles’ movement, and others36. The MD simulation is based on calculating the kinematics between particles at the molecular level by using Newtonian or Langevin laws. The MD simulations are carried out via the GROMACS (ver 5.1.4) package37 to assess the pollutant molecules’ adsorption onto the surfaces/cavities of MOF-5 and F-MOF-5. The CHARMM36 force field is applied for all components of studied systems31,38. CHARMM has been developed over the last three decades, primarily focusing on complex structures, molecules of biological, and small molecule ligands. For the study of such systems, the program provides a large suite of computational tools that include numerous conformational and path sampling methods, FE estimators, molecular minimization, dynamics, and analysis techniques, and model-building capabilities39,40,41. Initially, the energy is minimized by the steepest descent algorithm to remove undesirable contacts and interactions39. Then, the MD simulation run is performed for 75 ns, at T = 310 K and pressure = 1 bar via V-Rescale thermostat and Berendsen barostat, respectively42. For neutralizing the system, Na+, and Cl− (0.15 M) are added into the simulation box. All bond lengths are fixed with the LINCS (linear constraint solver) algorithm43. The leap-frog method with a time step of 2 fs is utilized to integrate Newton’s equations of motion44. The cut-off distance for van der Waals (vdW) interactions is considered at 1.4 nm. The coordinate images have been prepared using the Visual Molecular Dynamics tool before and after simulations45.
WTMD simulation
The WTMD is an FE calculation utilized for increasing sampling in MD simulations where the FE surface is investigated as a function of a few collective variables (CVs)46. The WTMD is a powerful, well-established technique for exploring the FE surface with enhanced sampling. The FE calculations for the adsorption of phenolic pollutants on the MOF-5 and functionalized MOF-5 are running via WTMD simulation by the sum-hills tools in the PLUMED plugin (ver 2.5.2) augmented to Gromacs software47 For this purpose, three boxes containing MOF-5/F-MOF-5/CH3-MOF-5 at a distance of 2 nm from TCP molecules are constructed with the dimensions of 8 × 8 × 8 nm3 (I, II, and III systems, respectively). The center of mass distances (dCOMs) of the pollutant molecules to MOF-5 and functionalized MOF-5 are taken as the reaction CVs in the designed systems. It is necessary to mention that the convergence of WTMD simulations depends on the sampling of all CV physical regions of interest and the number of transition events between states48. So, if the CV is not chosen properly, the convergence speed of WTMD is similar to that of conventional MD, making it not helpful. In conclusion, WTMD solves the metadynamics convergence problems and permits the computational effort to be focused on the physically relevant regions of the structural space49. Gaussian hills with an initial height of 1.2 kJ mol−1 and a width of 0.35 rad are deposited every 500-time steps with a bias factor of 15. In addition, the temperature is also set at 310 K. Generally, 75 ns WTMD simulations have been performed for the modeled systems. In the simplest possible implementation of a metadynamics calculation, the expense of a metadynamics calculation increases with the length of the simulation as one has to, at every step, evaluate the values of a larger and larger number of Gaussian kernels. Hence, the length of the simulation can be regarded as an indicator of the certainty of the accuracy of its result49.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
The codes that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Nazir, M. A. et al. Facile synthesis of Tri-metallic layered double hydroxides (NiZnAl-LDHs): adsorption of rhodamine-B and methyl orange from water. Inorg. Chem. Commun. 145, 110008 (2022).
Nazir, M. A. et al. Heterointerface engineering of water stable ZIF-8@ ZIF-67: adsorption of rhodamine B from water. Surf. Interface Anal. 34, 102324 (2022).
Ishfaq, M. et al. The in situ synthesis of sunlight-driven Chitosan/MnO2@ MOF-801 nanocomposites for photocatalytic reduction of Rhodamine-B. J. Mol. Struct. 1301, 137384 (2024).
Kamalesh, T. et al. An insights of organochlorine pesticides categories, properties, eco-toxicity and new developments in bioremediation process. Environ. Pollut. 333, 122114 (2023).
Malik, A. et al. Sources, distribution, associated health risks and remedial technologies for inorganic contamination in groundwater: a review in specific context of the state of Haryana, India. Environ. Res. 236, 116696 (2023).
Peng, J. et al. Removal of phenols by highly active periodate on carbon nanotubes: a mechanistic investigation. Environ. Sci. Technol. 57, 10804–10815 (2023).
Lu, C. et al. Nickel-catalyzed ring-opening of benzofurans for the divergent synthesis of ortho-functionalized phenol derivatives. ACS Catal. 13, 2432–2442 (2023).
Choudhary, P., Kumari, K., Sharma, D., Kumar, S. & Krishnan, V. Surface nanoarchitectonics of boron nitride nanosheets for highly efficient and sustainable ipso-hydroxylation of arylboronic acids. ACS Appl Mater. Interfaces 15, 9412–9420 (2023).
AlYammahi, J. et al. Molecular guide for selecting green deep eutectic solvents with high monosaccharide solubility for food applications. ACS Omega. 8, 26533–26547 (2023).
Krishnamurthy, M. & Swaminathan, M. Carbon allotropes in phenolic compounds removal. Carbon allotropes and composites: materials for environment protection and remediation. Mater. Environ. Prot. Rem. 155, 171 (2023).
Fseha, Y. H., Shaheen, J. & Sizirici, B. Phenol contaminated municipal wastewater treatment using date palm frond biochar: optimization using response surface methodology. Emerg. Contam. 9, 100202 (2023).
Pathiraja, D. et al. Water-soluble phenolic compounds and their putative antioxidant activities in the seed coats from different lentil (Lens culinaris) genotypes. Food Chem. 407, 135145 (2023).
Gómez-Urios, C. et al. Choline chloride-based natural deep eutectic solvents for the extraction and stability of phenolic compounds, ascorbic acid, and antioxidant capacity from Citrus sinensis peel. LWT 177, 114595 (2023).
Shahid, M. U. et al. Engineering of metal organic framework (MOF) membrane for waste water treatment: synthesis, applications and future challenges. J. Water Process Eng. 57, 104676 (2024).
Yu, R.-L., Li, Q.-F., Li, Z.-L., Wang, X.-Y. & Xia, L.-Z. Analysis of radioactive iodine trapping mechanism by zinc-based metal–organic frameworks with various N-containing carboxylate ligands. ACS Appl Mater. Interfaces 15, 35082–35091 (2023).
Luan, J. et al. Metal–organic framework as catalyst precursor of floating catalyst chemical vapor deposition for single-walled carbon nanotube manufacture. ACS Sustain. Chem. Eng. 11, 12423–12434 (2023).
Kaur, H. et al. Metal organic framework-based materials for wastewater treatment: superior adsorbent materials for the removal of hazardous pollutants. ACS Omega 8, 9004–9030 (2023).
Chen, R., Chen, X., Wang, Y. & Wang, B. Biomimetic metal-organic frameworks for biological applications. Trends Chem. 5, 460–473 (2023).
Chen, Z. et al. Application of covalent organic frameworks and metal–organic frameworks nanomaterials in organic/inorganic pollutants removal from solutions through sorption-catalysis strategies. Carbon Res. 2, 8 (2023).
Xu, W. et al. High-porosity metal-organic framework glasses. Angew. Chem. Int. Ed. 62, e202300003 (2023).
Shah, S. S. A. et al. Recent trends in wastewater treatment by using metal-organic frameworks (MOFs) and their composites: a critical view-point. Chemosphere. 349, 140729 (2023).
Haso, H. W., Dubale, A. A., Chimdesa, M. A. & Atlabachew, M. High performance copper based metal organic framework for removal of heavy metals from wastewater. Front. Mater. Sci. 9, 840806 (2022).
Chen, B., Eddaoudi, M., Hyde, S. T., O’Keeffe, M. & Yaghi, O. M. Interwoven metal-organic framework on a periodic minimal surface with extra-large pores. J. Sci. 291, 1021–1023 (2001).
Yazdi, M. N., Dadfarnia, S. & Shabani, A. M. H. Synthesis of stable S-functionalized metal-organic framework using MoS42-and its application for selective and efficient removal of toxic heavy metal ions in wastewater treatment. J. Environ. Chem. Eng. 9, 104696 (2021).
Xie, K. et al. Study of adsorptive removal of phenol by MOF-5. Desalin. Water Treat. 54, 654–659 (2015).
Younis, S. A., Ali, T. A. & Serp, P. Potential applicability of Zn0. 05TiOxNy@ MOF-5 nanocomposite for adsorption and electrochemical detection of Zn (II) in saline wastewater. J. Environ. Chem. Eng. 9, 106186 (2021).
Razavi, S. A. A. & Morsali, A. Linker functionalized metal-organic frameworks. Coord. Chem. Rev. 399, 213023 (2019).
Yuan, N., Gong, X., Sun, W. & Yu, C. Advanced applications of Zr-based MOFs in the removal of water pollutants. Chemosphere 267, 128863 (2021).
Nath, K., Wright, K. R., Ahmed, A., Siegel, D. J. & Matzger, A. J. Adsorption of natural gas in metal-organic frameworks: selectivity, cyclability, and comparison to methane adsorption. Am. Chem. Soc. 146, 10517–10523 (2024).
Cho, K., Li, Z. & MacLachlan, M. J. Growth of metal organic frameworks on the surface of individual cellulose nanocrystals. Cryst. Growth Des. 24, 1792–1797 (2024).
Razavi, L., Raissi, H., Hashemzadeh, H. & Farzad, F. Strategy to improve Cu-BTC metal-organic frameworks performance in removal of Rhodamine B: MD and WT-MtD simulations assessment. npj Clean. Water 5, 1–8 (2022).
Yildiz, E. A. et al. Effect of group electronegativity on spectroscopic, biological, chromogenic sensing and optical properties of 2-formyl-benzene sulfonic acid sodium salt-based Schiff bases. J. Mol. Struct. 1286, 135611 (2023).
Tekin, H. O. et al. Molecular polar surface area, total solvent accessible surface area (SASA), heat of formation, and gamma-ray attenuation properties of some flavonoids. Front. Phys. 10, 104 (2022).
Amaro-Estrada, J. I., Wang, Y. & Torres-Verdín, C. Structural and nuclear magnetic resonance relaxation properties of shale condensate within organic nanopores via molecular dynamics simulations. Energy Fuels 37, 10318–10328 (2023).
Kumar, G. & Masram, D. T. Sustainable synthesis of MOF-5@ GO nanocomposites for efficient removal of rhodamine B from water. ACS Omega 6, 9587–9599 (2021).
Bahru, R., Zamri, M. F. M. A., Shamsuddin, A. H. & Mohamed, M. A. Simulation design for thermal model from various materials in electronic devices: a review. Numer. Heat. Transf. A 82, 640–665 (2022).
Hu, Z., Chen, Y. & Jiang, J. Liquid chromatographic separation in metal–organic framework MIL-101: a molecular simulation study. Langmuir 29, 1650–1656 (2013).
Meng, T. et al. MOF-Derived nanoarchitectured carbons in wood sponge enable solar-driven pumping for high-efficiency soil water extraction. J. Chem. Eng. 452, 139193 (2023).
Brooks, B. R. et al. CHARMM: a program for macromolecular energy, minimization, and dynamics calculations. J. Comput. Chem. 4, 187–217 (1983).
Bristow, J. K., Skelton, J. M., Svane, K. L., Walsh, A. & Gale, J. D. A general forcefield for accurate phonon properties of metal organic frameworks. Phys. Chem. Chem. Phys. 18, 29316–29329 (2016).
Brooks, B. R. et al. CHARMM: the biomolecular simulation program. J. Comput. Chem. 30, 1545–1614 (2009).
Bazaikin, V. Y., Komolkin, A. V. & Markelov, D. A. Molecular dynamics simulation of poly-m-phenylene isophthalamide polymer membrane modified by UiO-66 (NH2) MOF for separation of toluene/methanol mixture. J. Mol. Liq. 383, 122188 (2023).
Kollias, L., Rousseau, R., Glezakou, V.-A. & Salvalaglio, M. Understanding metal–organic framework nucleation from a solution with evolving graphs. Am. Chem. Soc. 144, 11099–11109 (2022).
Razavi, L., Raissi, H. & Farzad, F. Validation of an MD simulation approach for electrical field responsive micelles and their application in drug delivery. Sci. Rep. 13, 2665 (2023).
Spivak, M. et al. VMD as a platform for interactive small molecule preparation and visualization in quantum and classical simulations. J. Chem. Inf. Model. 63, 4664–4678 (2023).
Prajapati, J. D., Onuchic, J. N. & Sanbonmatsu, K. Y. Exploring the energy landscape of riboswitches using collective variables based on tertiary contacts. J. Mol. Biol. 434, 167788 (2022).
Sabbih, G. O., Wijesinghe, K. M., Algama, C., Dhakal, S. & Danquah, M. K. Computational generation and characterization of IsdA-binding aptamers with single-molecule FRET analysis. J. Biotechnol. 18, 2300076 (2023).
Barducci, A., Bussi, G. & Parrinello, M. Well-tempered metadynamics: a smoothly converging and tunable free-energy method. Phys. Rev. 100, 20603 (2008).
Lu, H. & Martí, J. Long-lasting salt bridges provide the anchoring mechanism of oncogenic kirsten rat sarcoma proteins at cell membranes. J. Phys. Chem. Lett. 11, 9938–9945 (2020).
Acknowledgements
The authors are grateful to the University of Birjand for financial support.
Author information
Authors and Affiliations
Contributions
Leila Razavi: writing—design simulation systems. Heidar Raissi: supervision, review & editing. Ozra Hashemzehi: review & editing. Farzaneh Farzad: review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Razavi, L., Raissi, H., Hashemzehi, O. et al. Significantly enhanced performance for phenol compounds removal by MOF-5 nano-composite via its surface modification. npj Clean Water 7, 44 (2024). https://doi.org/10.1038/s41545-024-00338-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-024-00338-1
- Springer Nature Limited