Abstract
It has been reported that schizophrenia (SCZ) and inflammatory bowel disease (IBD) are related. However, whether there is a bidirectional interaction between them remains unclear. The aim of this study was to conduct a bidirectional Mendelian randomization (MR) analysis to elucidate the causal relationship between SCZ and IBD and its subtypes, including Crohn’s disease (CD) and ulcerative colitis (UC). Single-nucleotide polymorphisms (SNPs) extracted from the summary data of genome-wide association studies were used as genetic instruments. MR was performed using the inverse-variance-weighted method. The MR-Egger and weighted median methods were used for sensitivity analyses. Analysis using 70 SNPs as genetic instruments showed that SCZ was associated with an increased risk of IBD (OR = 1.14, 95% CI: 1.09–1.20, P = 9.21 × 10−8), CD (OR = 1.16, 95% CI: 1.07–1.25, P = 1.42 × 10−4), and UC (OR = 1.14, 95% CI: 1.07–1.21, P = 2.72 × 10−5). The results of the sensitivity analyses were robust and no evidence of pleiotropy was observed. Bidirectional MR analyses showed no causal effects of IBD, CD, or UC on SCZ. This study suggests that SCZ has causal effects on IBD and its subtypes, whereas IBD has no effect on SCZ. Brain-gut axis interactions may help clarify the causal relationship between SCZ and IBD. However, further studies are needed to elucidate the biological mechanisms behind the brain-gut interactions.
Similar content being viewed by others
Introduction
Schizophrenia (SCZ) and inflammatory bowel disease (IBD) are responsible for a substantial proportion of cases of disability in the general population worldwide1. SCZ is a chronic psychiatric disorder that mainly manifests as cognitive and behavioral abnormalities2, whereas IBD is characterized by immune dysregulation and inflammation of the gut3. Observational epidemiological investigations have indicated that patients with SCZ have an increased risk of developing IBD4 and vice versa5,6. Emerging genetic evidence also shows that there is a genetic association between the two diseases7,8. However, whether there is a bidirectional interaction between SCZ and IBD remains unclear.
Mendelian randomization (MR) is an alternative tool for identifying causal associations between modifiable exposure and disease outcomes using genetic variants as instrumental variables9. The fundamental framework of the MR study design is that if genetic variants can predict the level or biological effect of a modifiable exposure to some extent, then they should also be causally associated with the exposure-related disease outcome to the same extent that they act on the exposure10. Utilizing the fact that genetic variants are randomly assigned before birth and fixed at conception, the MR design can prevent confounding, reverse causation, and various biases that are common in observational investigations11. In addition, the considerable increase in the number of publicly available genome-wide association studies (GWASs) has provided abundant data sources and increased the statistical power of MR. This makes the MR very popular for elucidating causality.
In this study, we conducted a bidirectional MR analysis to elucidate the causal relationship between SCZ and IBD and its subtypes, including ulcerative colitis (UC) and Crohn’s disease (CD). We hypothesized that there is a bidirectional causal interaction between SCZ and IBD.
Results
Using 70 single-nucleotide polymorphisms (SNPs) (R2 = 3.5%; F = 42.8; Supplementary Table 1) as genetic instruments, the inverse-variance weighted (IVW) MR analysis showed that SCZ was associated with an approximately 14% increased risk of developing IBD (odds ratio [OR] = 1.14, 95% confidence interval [CI]: 1.09–1.20, P = 9.21 × 10−8; Table 1; Fig. 1a). The causal inference of the sensitivity analysis conducted using the MR-Egger method (OR = 1.25, 95% CI: 1.03–1.50, P = 0.025), the weighted median method (OR = 1.13, 95% CI: 1.06–1.20, P = 1.25 × 10−4) and the MR-PRESSO method (OR = 1.14, 95% CI: 1.09–1.20, P = 1.12 × 10−6) was robust. The results of the MR-Egger intercept test suggested no evidence of horizontal pleiotropy (P = 0.350). Significant causal effects of SCZ on CD (IVW OR = 1.16, 95% CI: 1.07–1.25, P = 1.42 × 10−4; Table 1; Fig. 1c) and UC (IVW OR = 1.14, 95% CI: 1.07–1.21, P = 2.72 × 10−5; Table 1; Fig. 1e) were also observed. The MR-Egger intercept test showed no evidence of horizontal pleiotropy.
Scatter plots show the MR-derived associations between genetically predicted (a) SCZ on IBD; b IBD on SCZ; c SCZ on CD; d CD on SCZ; e SCZ on UC; f UC on SCZ. SCZ: schizophrenia. Associations are calculated using the inverse-variance weighted method. SCZ Schizophrenia, IBD Inflammatory bowel disease, CD Crohn’s disease, UC Ulcerative colitis.
Table 2 shows the results of the causal effects of IBD, CD, and UC on SCZ. Ninety-eight (R2 = 11.2%; F = 70.0; Supplementary Table 2), 75 (R2 = 15.0%; F = 94.3), and 50 (R2 = 7.7%; F = 76.4) SNPs were extracted for IBD, CD, and UC, respectively. However, based on these SNPs, no causal effects of IBD (IVW OR = 1.01, 95% CI: 0.98–1.04, P = 0.470; Fig. 1b), CD (IVW OR = 1.02, 95% CI: 0.99–1.05, P = 0.267; Fig. 1d) and UC (IVW OR = 1.02, 95% CI: 0.98–1.06, P = 0.254; Fig. 1f) on SCZ were observed. Sensitivity analyses also showed consistent results, suggesting that IBD and its subtypes have no causal effects on SCZ.
Discussion
In this study, we conducted a bidirectional MR analysis to assess the causal relationship between SCZ and IBD using GWAS summary-level data. Our findings provide genetic evidence that SCZ has causal effects on IBD and its subtypes, whereas IBD has no causal effect on SCZ. To the best of our knowledge, this is the first MR study to elucidate the causal relationship between SCZ and IBD.
The psychiatric comorbidities of IBD are well known. However, whether psychiatric factors cause IBD or whether IBD has an impact on psychiatric disorders has not yet been determined. The present study provides evidence that SCZ has causal effects on IBD and its subtypes, which supports the idea that psychiatric factors play a role in the development of IBD. Indeed, psychiatric symptoms, such as anxiety, have been reported to be associated with an increased risk of surgery and disease relapse, poorer quality of life, and increased likelihood of using immunomodulators in patients with IBD12,13,14,15. In addition, a nationwide study indicated that patients diagnosed with SCZ have an elevated incidence rate of IBD compared with those without SCZ4. Moreover, a growing body of evidence suggests that patients with IBD could benefit from psychological treatments16,17,18. Furthermore, some clinical experts have recommended including psychiatric symptom scales in the routine screening of IBD. These previous reports highlight the significance of psychiatric factors in the diagnosis and treatment of IBD19.
The findings of the present study do not support the idea that IBD has a causal effect on SCZ. This is inconsistent with the findings of several previous studies5,6. There may be several reasons for this discrepancy. First, although psychiatric comorbidities are common in IBD, psychiatric disorders in most cases of IBD are underdiagnosed. In addition, some psychiatric problems may have occurred years prior to the diagnosis of IBD20. Second, observational studies are easily biased by confounding factors. Some unknown factors, such as psychotropic substance abuse, infection, or psychological trauma, may be responsible for the elevated incidence of SCZ among patients with IBD21,22. Third, the present study demonstrated that genetic determinants of SCZ have a significant impact on IBD. However, some genetic mediators may lead to the development of IBD before the diagnosis of SCZ. A recent general population-based study showed no evidence of a correlation between IBD and an increased risk of SCZ, a result that supports the findings of our study23. Interestingly, a recent bidirectional MR study of depression and IBD also suggested that depression was associated with a higher risk of IBD. While in contrast, no causal effect was observed of IBD and depression, which was consistent with the findings of our study24.
The brain-gut axis is believed to play an essential role in elucidating the underlying biological mechanisms linking SCZ and IBD25,26,27. Psychological representations may influence gastrointestinal function through the generation of a stress response, activation of the neuroendocrine system, or stimulation of the autonomic nervous system28. The gut microbiome is also involved in a number of brain processes, such as stress hormone signaling, neural function, and neuroprotection29. Bidirectional brain-gut axis interaction is a plausible explanation for the occurrence of psychiatric comorbidities in cases of IBD26. However, the results of the present study do not support the idea of a bidirectional interaction between SCZ and IBD, suggesting that bidirectional communication along the brain-gut axis is not specifically reversible. The impact of SCZ on gastrointestinal function is more direct, whereas IBD cannot lead to SCZ through a specific brain-gut pathway.
This study has several strengths. First, the effects of unmeasured confounders and reverse causation were avoided through the use of MR design and data from large GWASs. Second, the large sample sizes of the GWASs utilized for this study strengthened the power of the causal inferences from the summary data-based MR analysis. Third, the sensitivity analyses and pleiotropy tests conducted using multiple MR methods provided a robustness evaluation of the MR estimates.
There were also limitations to this study. First, most of the participants in the included study samples were of European ancestry. This might limit the generalizability of the study findings to other populations. In fact, the genetic architecture of SCZ in East Asian populations is very different from that in European populations30. In contrast, the incidence and prevalence of IBD among Asians are significantly lower than those among Europeans31. Therefore, the causal relationship between SCZ and IBD needs to be studied in other populations. Second, although MR is an effective tool for elucidating causality without the interference of environmental confounders, it is known to be susceptible to horizontal pleiotropy. We adopted several methods of sensitivity analysis to control for horizontal pleiotropy. However, we could not completely exclude bias from pleiotropy, which could reduce the validity of the results. Third, although brain-gut interactions may help reveal the biological mechanisms underlying the relationship between SCZ and IBD, further studies are needed to determine the specific process or pathway of gut-brain axis interactions.
In conclusion, this study provides genetic evidence that SCZ has causal effects on IBD and its subtypes, whereas IBD has no effect on SCZ. Brain-gut axis interactions may aid the understanding of such associations. However, the specific biological mechanisms behind the interactions need to be explored further.
Methods
Study design
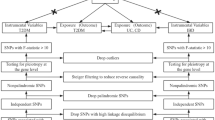
We employed a bidirectional MR study design to estimate the causal relationship between SCZ and IBD (Fig. 2. MR analysis was first performed in one direction to determine the causal effect of SCZ on IBD. Thereafter, the analysis was performed in the opposite direction. All analyses were performed using summary-level data from publicly available GWASs. Therefore, no ethical approval and consent were required for this study.
Data source and instruments
Data for schizophrenia
Information on the genetic associations with SCZ were obtained from the GWAS by the Psychiatric Genomics Consortium (PGC)32. The PGC conducted the most comprehensive GWAS on SCZ, including 36,989 cases and 113,075 controls selected from 46 European and three East Asian cohorts. Cases were diagnosed according to the criteria in the Diagnostic and Statistical Manual of Mental Disorders (DSM)-III or DSM-IV, after an interview by a psychiatrist and review of medical records. Genotypes were gathered from each cohort and were processed by the PGC using unified quality control procedures. Association meta-analysis was conducted using an inverse-weighted fixed-effects model, after adjusting for the first ten principal components.
Data for inflammatory bowel disease
Summary data for IBD, UC, and CD were derived from the study by the International Inflammatory Bowel Disease Genetics Consortium33. The study participants comprised 25,042 cases and 34,915 controls for IBD, 12,366 cases and 33,609 controls for UC, and 12,194 cases and 28,072 controls for CD. All the study participants were of European ancestry, and all cases were diagnosed using accepted endoscopic, histopathological, and radiological criteria. Association tests were performed using an additive frequentist model conditioned on the first ten principal components for each cohort, followed by a meta-analysis using the weighted standard error method.
Instrument selection criteria
Genetic instruments for SCZ and IBD were extracted using the same criteria. We selected all relevant SNPs at the genome-wide significance (P < 5 × 10−8) threshold from each GWAS, and pruned for independence using a clumping procedure in PLINK v1.9 (http://www.cog-genomics.org/plink/1.9/), setting a linkage disequilibrium threshold of r2 < 0.001 in a 10-Mb window. Datasets for exposure and outcome were then harmonized, and palindromic SNPs with intermediate allele frequencies were excluded. For SNPs absent in the outcome dataset, a proxy SNP (r2 > 0.8) was used or discarded if no proxy was available. Two parameters, the proportion of variance explained by the SNPs (R2) and F statistics, were used to evaluate the strength of the selected instrument34. Typically, an F statistic >10 is considered sufficiently informative for MR analyses35.
Power calculations
Power calculations were performed using the online tool mRnd (http://cnsgenomics.com/shiny/mRnd/). There were 87% power to detect a relative 10% difference (an OR of at least 1.10 or 0.90) in risk of SCZ on IBD, and 97% power to detect a 10% difference in risk of IBD on SCZ.
Statistical analysis
The MR analysis was conducted in two directions. The first was the causal effect of genetically predicted SCZ on IBD, whereas the second was the reverse effect of genetically predicted IBD on SCZ. The IVW method was used for the standard MR analysis, in which all genetic variants were assumed to be valid instruments. Therefore, the MR provided a true slope of SNP-outcome association on SNP-exposure association with the intercept constrained to zero36. However, the IVW method is vulnerable to horizontal pleiotropy37. Thus, for the sensitivity analysis, we utilized several additional methods, such as the weighted median, the MR-Egger, and the MR-PRESSO methods, which are insusceptible to horizontal pleiotropy. The weighted median method can provide a consistent estimate when up to 50% of the SNPs are invalid instruments38, whereas the MR-Egger method works even when all SNPs are invalid39. The MR-PRESSO is a newly developed method that has the ability to detect and correct for horizontal pleiotropic outliers40. Together, these methods provide a robustness test of the causal estimate derived from the MR analysis41. Besides, the MR-Egger intercept test was used to detect whether horizontal pleiotropy existed39. All analyses were performed using the “TwoSampleMR” and the “MR-PRESSO” packages in R v3.61 (https://www.r-project.org/). Statistical significance was set at P < 0.05.
Data availability
Publicly available datasets are analyzed in this study. GWAS summary data for SCZ are publicly available at the PGC website (https://www.med.unc.edu/pgc). GWAS datasets of IBD, CD, and UC can be downloaded from the GWAS Catalog (https://www.ebi.ac.uk/gwas/).
References
Hay, S. I. et al. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1260–1344 (2017).
Owen, M. J., Sawa, A. & Mortensen, P. B. Schizophrenia. Lancet 388, 86–97 (2016).
Zhang, Y. Z. & Li, Y. Y. Inflammatory bowel disease: pathogenesis. World J Gastroenterol 20, 91–99 (2014).
Benros, M. E. et al. A nationwide study on the risk of autoimmune diseases in individuals with a personal or a family history of schizophrenia and related psychosis. Am J Psychiatry 171, 218–226 (2014).
Benros, M. E. et al. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry 168, 1303–1310 (2011).
Eaton, W. W. et al. Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am J Psychiatry 163, 521–528 (2006).
Pouget, J. G. et al. Cross-disorder analysis of schizophrenia and 19 immune-mediated diseases identifies shared genetic risk. Hum Mol Genet 28, 3498–3513 (2019).
Duncan, L. et al. Genetic correlation profile of schizophrenia mirrors epidemiological results and suggests link between polygenic and rare variant (22q11.2) cases of schizophrenia. Schizophrenia Bulletin 44, 1350–1361 (2018).
Davies, N. M., Holmes, M. V. & Davey Smith, G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 362, k601 (2018).
Evans, D. M. & Davey Smith, G. Mendelian randomization: new applications in the coming age of hypothesis-free causality. Annu Rev Genomics Hum Genet 16, 327–350 (2015).
Smith, G. D. & Ebrahim, S. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol 33, 30–42 (2004).
Regueiro, M., Greer, J. B. & Szigethy, E. Etiology and treatment of pain and psychosocial issues in patients with inflammatory bowel diseases. Gastroenterology 152, 430–439 e434 (2017).
Ananthakrishnan, A. N. et al. Psychiatric co-morbidity is associated with increased risk of surgery in Crohn’s disease. Aliment Pharmacol Ther 37, 445–454 (2013).
Chan, W. et al. Symptoms of anxiety and depression are independently associated with inflammatory bowel disease-related disability. Dig Liver Dis 49, 1314–1319 (2017).
Graff, L. A., Walker, J. R. & Bernstein, C. N. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflamm Bowel Dis 15, 1105–1118 (2009).
Yanartas, O. et al. The effects of psychiatric treatment on depression, anxiety, quality of life, and sexual dysfunction in patients with inflammatory bowel disease. Neuropsychiatr Dis Treat 12, 673–683 (2016).
van den Brink, G. et al. Effectiveness of disease-specific cognitive-behavioural therapy on depression, anxiety, quality of life and the clinical course of disease in adolescents with inflammatory bowel disease: study protocol of a multicentre randomised controlled trial (HAPPY-IBD). BMJ Open Gastroenterol 3, e000071 (2016).
Alarhayem, A., Achebe, E. & Logue, A. J. Psychosocial support of the inflammatory bowel disease patient. Surg. Clin. N. Am. 95, 1281–1293 (2015). vii-viii.
Bernstein, C. N. et al. The validity and reliability of screening measures for depression and anxiety disorders in inflammatory bowel disease. Inflamm. Bowel Dis. 24, 1867–1875 (2018).
Marafini, I. et al. High frequency of undiagnosed psychiatric disorders in inflammatory bowel diseases. J. Clin. Med. 9, https://doi.org/10.3390/jcm9051387 (2020).
Tarricone, I. et al. Prevalence and effectiveness of psychiatric treatments for patients with IBD: A systematic literature review. J. Psychosom. Res. 101, 68–95 (2017).
Petruzzelli, M. G., Margari, L., Ivagnes, S., Palumbi, R. & Margari, F. Early onset first-episode psychosis during treatment with thalidomide for refractory ulcerative colitis: a case report. J. Med. Case Rep. 13, 175 (2019).
West, J., Logan, R. F., Hubbard, R. B. & Card, T. R. Risk of schizophrenia in people with coeliac disease, ulcerative colitis and Crohn’s disease: a general population-based study. Aliment. Pharmacol. Ther. 23, 71–74 (2006).
Luo, J., Xu, Z., Noordam, R., van Heemst, D. & Li-Gao, R. Depression and inflammatory bowel disease: a bidirectional two-sample mendelian randomization study. J Crohns Colitis, https://doi.org/10.1093/ecco-jcc/jjab191 (2021).
Bonaz, B. L. & Bernstein, C. N. Brain-gut interactions in inflammatory bowel disease. Gastroenterology 144, 36–49 (2013).
Gracie, D. J., Guthrie, E. A., Hamlin, P. J. & Ford, A. C. Bi-directionality of brain-gut interactions in patients with inflammatory bowel disease. Gastroenterology 154, 1635–1646 (2018).
Gunther, C., Rothhammer, V., Karow, M., Neurath, M. & Winner, B. The gut-brain axis in inflammatory bowel disease-current and future perspectives. Int J Mol Sci 22, https://doi.org/10.3390/ijms22168870 (2021).
Gracie, D. J., Hamlin, P. J. & Ford, A. C. The influence of the brain–gut axis in inflammatory bowel disease and possible implications for treatment. Lancet Gastroenterol. Hepatol. 4, 632–642 (2019).
Margolis, K. G., Cryan, J. F. & Mayer, E. A. The microbiota-gut-brain axis: from motility to mood. Gastroenterology 160, 1486–1501 (2021).
Lam, M. et al. Comparative genetic architectures of schizophrenia in East Asian and European populations. Nat Genet 51, 1670–1678 (2019).
Ng, S. C. et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390, 2769–2778 (2017).
Schizophrenia Working Group of the Psychiatric Genomics, C. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427 (2014).
de Lange, K. M. et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet 49, 256–261 (2017).
Burgess, S., Dudbridge, F. & Thompson, S. G. Combining information on multiple instrumental variables in Mendelian randomization: comparison of allele score and summarized data methods. Stat Med 35, 1880–1906 (2016).
Brion, M. J., Shakhbazov, K. & Visscher, P. M. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol 42, 1497–1501 (2013).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genetic Epidemiology 37, 658–665 (2013).
Bowden, J. et al. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med 36, 1783–1802 (2017).
Bowden, J., Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 40, 304–314 (2016).
Bowden, J., Smith, G. & Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44, 512–525 (2015).
Verbanck, M., Chen, C. Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 50, 693–698 (2018).
Bowden, J. et al. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol 48, 728–742 (2019).
Acknowledgements
We would like to thank the High-Performance Computing Cluster of the First Affiliated Hospital of Xi’an Jiaotong University for data processing. The study was supported by the Natural Science Basic Research Program of Shaanxi (2021JQ-390) and the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University (XJTU1AF-CRF-2017-025).
Author information
Authors and Affiliations
Contributions
L.Q. and J.Y. conceptualized and designed the study, conducted the data analysis, and drafted the manuscript. X.H., F.G., and Y.F. collected data and carried out the initial analyses. B.Z. and Q.M. contributed to the interpretation of results. B.Y. contributed to the study supervision. W.W., X.M., and J.Y. critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Ethical approval and consent were not thought because all data used here were downloaded from public domain.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Qian, L., He, X., Gao, F. et al. Estimation of the bidirectional relationship between schizophrenia and inflammatory bowel disease using the mendelian randomization approach. Schizophr 8, 31 (2022). https://doi.org/10.1038/s41537-022-00244-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-022-00244-w
- Springer Nature Limited