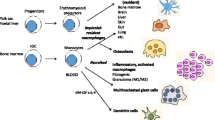
Abstract
Spaceflight and terrestrial spaceflight analogs can alter immune phenotypes. Macrophages are important immune cells that bridge the innate and adaptive immune systems and participate in immunoregulatory processes of homeostasis. Furthermore, macrophages are critically involved in initiating immunity, defending against injury and infection, and are also involved in immune resolution and wound healing. Heterogeneous populations of macrophage-type cells reside in many tissues and cause a variety of tissue-specific effects through direct or indirect interactions with other physiological systems, including the nervous and endocrine systems. It is vital to understand how macrophages respond to the unique environment of space to safeguard crew members with appropriate countermeasures for future missions in low Earth orbit and beyond. This review highlights current literature on macrophage responses to spaceflight and spaceflight analogs.
Similar content being viewed by others
Introduction
Space exploration and habitation will expose crew members to unique risk factors, including cosmic radiation, altered gravity forces, social isolation, and enclosed/hostile environments. In addition, these stressors will be experienced at a substantial distance from Earth, where emergency medical intervention will be limited. Multiple studies on crew health and model organisms have determined spaceflight-associated risks can impact nervous, musculoskeletal, and cardiovascular systems (reviewed in1). Tissue-resident macrophages, circulating blood monocytes, and lymphatic system responses to spaceflight-associated risk factors can influence physiological outcomes in biological systems. Studies performed on space exploration vehicles and on the International Space Station (ISS) have yielded varied findings on immunological patterns, which may be in part due to limitations to onboard sampling and experimental procedures. In general, crew members who have flown in space display altered leukocyte counts and function, as well as chronic low-grade inflammation2,3,4,5,6,7,8,9. Chronic inflammation experienced in-flight may be in response to oxidative damage10 (reviewed in11), which may also contribute to accelerated aging described post-flight12,13. However, due the dynamic nature of the immune system, contributions of circadian cycling, epigenetics, and differential response kinetics are only now beginning to be evaluated. Thus, understanding the impact of spaceflight-associated risks on macrophage phenotypes and functions is paramount for astronaut health monitoring and mitigation programs.
Macrophages are key elements of the innate immune response and play an important role in antigen removal via phagocytosis. They also direct the adaptive immune response through antigen presentation and lymphocyte immunological synapse formation. In addition, many non-genetic, multisystem terrestrial diseases are caused by aberrant inflammation, which is in part regulated by macrophages (reviewed in14,15).
There is a significant knowledge gap in the characterization of macrophage heterogeneity involved in spaceflight-associated dysfunctions, in addition to identifying homeostatic resolution processes. Consequently, teasing apart cell type-specific responses may help characterize distinct immunological processes observed in the spaceflight environment.
Macrophage immunobiology in spaceflight
Immune cell phenotypes and functions are altered in spaceflight, with known consequences for monocytes and macrophages (Table 1). For instance, elevated monocyte counts were described in crew at 1-day post-flight (>142-day mission)8. In line with this, ISS crew monocyte counts are not significantly altered at early- (14-days), mid- (2–4-months) and late-(6-months) timepoints in-flight, however, are slightly increased immediately upon return to 1 g and at 30-days post-flight7. Collectively, these findings indicate elevated monocyte counts during post-flight may be a factor of immune resolution and recovery. In rodents, on the other hand, absolute monocyte counts isolated from splenocytes were reduced immediately post-flight16. Similarly, other studies in rats report reduced post-flight monocyte counts upon return from a 14-day mission17.
Immune discrepancies between rodent and human mammalian systems are not entirely uncommon and may be due to multiple variables, including the strain of experimental mouse, multi-physiological system influence, age, sex, duration of spaceflight exposure, environmental microbial influence, methods or markers of analysis, and/or landing experiences. Moreover, rodent quadrupedal models do not recapitulate the bipedal human with an upright, stacked vertebrae spinal column. Therefore, simulated gravity models impact rodents and humans differently (reviewed in18), and may have important implications for monocyte (and other immune cell) functions. The majority of simulated microgravity studies in rodents utilize the hindlimb unloading (HU) model, which lifts rodent hindquarters to cause a cephalad fluid shift similar to what humans experience in spaceflight (reviewed in19). Immunological consequences observed following HU and spaceflight are somewhat similar, such as thymus involution and induced cytokine-type phenotypes (reviewed in20). Additionally, leukocyte differentials are also generally similar between HU rodent models and humans post-flight, including elevated production of inflammatory mediators (reviewed in21). However, careful consideration of spaceflight analog details and a thorough elucidation of all descriptive metadata from spaceflight missions are important for immunological interpretation.
In-flight changes of known macrophage lineage mediators have also been reported in crew members. For example, IL12-p406, CXCL-8/IL8, and CXCL-55 cytokines are elevated throughout multiple timepoints, indicating the potential for some degree of macrophage activation under microgravity conditions. In addition, multipaneled measurements of mediators indirectly involved in macrophage activation are elevated in-flight, including IL-1α, IL-1β, and IL-1RA3, and TNF-α, IL-17F, and IL-62. In line with this, the production of immune mediators CCL-2, IL-10, CRP, IL-6, and IL-1RA are substantially elevated immediately at landing (340-day mission) in the NASA Twins study (n = 1) that were quickly reversed in post-flight recovery22. Induction of TNF-α and IL-1β were also described following in-flight stimulation of the murine bone marrow-derived cell line, B6MP102 cells, with lipopolysaccharide (LPS, 12- and 24-hours) on-board STS-37 (6-day mission duration)23. In addition, although macrophage polarization profiles are beginning to be identified in simulated microgravity models and spaceflight24,25 (reviewed in26,27), more studies on these unique phenotypes may better assist with unraveling functional consequences of macrophage alterations in spaceflight (Fig. 1). Recently, Lv et al. discussed a negative role for microgravity on hematopoietic progenitor cells (HPC) differentiation and polarization processes, including non-polarized (M0), pro-inflammatory (M1), and anti-inflammatory (M2) subtypes. Under microgravity and simulated microgravity conditions, differentiation of HPC into macrophages and the polarization of macrophages into M1 or M2 types were mutually impaired24,25 (reviewed in26,27). Thus, spaceflight risk factors may influence macrophage differentiation/polarization processes in flight.
Representative timeline of the observable phenotypes produced in-flight from Table 1. Each line/color represents a mission experiment with the in-flight days listed above/below vertical nodes. Collective observable phenotypes display increased (above centerline) or decreased (below centerline) activity are depicted visually, including increased production of IL12-p40, CXCL-8, CXCL-5, TNF-α, IL-1β, IL-RA, IL-1α, IL-17F, and IL-6 and decreased macrophage differentiation processes from hematopoietic progenitor cells (HPC). Created with BioRender.com.
In a post-flight study, human monocytes displayed reduced phagocytic activity immediately following short- (8–10 days) and long- (125–195 days) duration spaceflight missions28. Another study found that monocyte phagocytic function was depressed immediately upon return to Earth (within 3 h) and at day 3 post-flight29. Murine splenocyte phagocytosis was also impaired immediately post-flight (within 5 h) after a 13-day spaceflight mission16. The inability to remove cellular debris, apoptotic cells, or pathogens can impede tissue regeneration, nutrient recycling, and can cause tissue damage, which is also a notable dysfunction in the elderly (reviewed in30). Concordantly, astronauts display accelerated aging phenotypes in peripheral blood mononuclear cells (PBMC) post-one-year missions via telomere shortening mechanics2,12, suggesting post-flight macrophages may also have an accelerated age phenotype, that is possibly inflammatory12 (reviewed in31). However, previous work described reduced monocyte expression levels of pro-inflammatory TNF-α, IL-6, and immunoregulatory IL-10 upon LPS ex vivo stimulation, post-short duration spaceflight shuttle missions (13–16 day missions, STS-124, STS-125, and STS-126)32. Differences in study outcomes may be a due to variables including duration in spaceflight and types of mission exposures (i.e., shuttle versus ISS). Additionally, while recovery to baseline macrophage function may be achieved post-flight on Earth, the time frame to recovery is an important consideration for future exploration missions on lunar and Martian surfaces.
Macrophages are also critically involved in directing type-specific adaptive immunity via antigen presentation and the production of lymphocyte differentiation cytokines (reviewed in33). Spaceflight can alter adaptive immunity through impaired T cell function7 and impaired lymphocyte maturation (reviewed in34), both of which can cause immune deficiency. As macrophages play an important role in antigen presentation and can shape lymphocyte effector cell phenotypes, we hypothesize that altered macrophages may significantly alter adaptive immune phenotypes observed during spaceflight. However, due to the complexity of performing in-flight studies to characterize immunological synapses or macrophage-lymphocyte communication processes, these studies have been lacking to date. Nonetheless, one study has characterized the potential for peripheral immune tolerance processes being disrupted in space-flown mice (15-day mission), whereby antigen-specific tolerance, mediated by antigen-presenting cells, including macrophages, was compromised and elevated inflammation was recorded post-flight35. This study highlights the potential for fundamental antigen presentation/processing mechanisms being compromised in-flight. Thus, deficits in antigen processing and presentation can influence inappropriate effector lymphocyte establishment, which may be the basis for reported adaptive immune dysfunctions in flight7, although additional studies are required (Fig. 2).
Representative timeline of the observable phenotypes produced post-flight analysis from Table 1. Each line/color represents a mission experiment with the in-flight days listed above horizontal nodes, and post-flight collection time points vertical lines. Collective observable phenotypes that display increased (above centerline) or decreased (below centerline) activity are depicted visually, including monocytic count, phagocytic function, cytokine production, and T cell tolerance outcomes. Each phenotype is color-coordinated with the experimental timeline of missions and collections. Created with BioRender.com.
Most studies that characterize macrophage lineage biology and function in response to the spaceflight environment are limited to post-flight outcomes, although some have characterized in-flight cell counts and cytokine/chemokine profiles. As such, additional experimental evidence is necessary to fully characterize unique phenotypes of monocytes and macrophages in spaceflight, including functional outcomes on lymphocyte populations.
Tissue-specific macrophage lineages in spaceflight
As described above, macrophages can undergo alterations in the spaceflight environment, which may in part contribute to spaceflight-induced conditions. Specialized tissue-specific macrophage lineages in the spaceflight environment are less characterized, such as microglia of the CNS or bone osteoclasts (reviewed in36). Yet, there are some intriguing spaceflight environmental consequences on these specialized tissue macrophage lineages that have been identified, displaying similarities to known terrestrial disorders. For instance, the spaceflight environment (12-day mission murine OSTEO payload) has known effects on osteoclasts resulting in osteoclastogenesis and increased bone resorption37, which may play an important role in exacerbating bone loss in spaceflight, resembling osteoporosis (reviewed in38,39). Furthermore, microglia are brain-resident macrophages that are responsible for the maintenance of brain homeostasis by surveying the microenvironment in a resting state. However, when activated, microglia can play a contributing role in neuroinflammation causing bystander tissue damage if unresolved (reviewed in40). Similar to features resembling Alzheimer’s disease41, microglia can be activated by space-relevant galactic cosmic rays (GCR) doses both in vitro and in vivo models of cosmic radiation (reviewed in42,43), leading to cognitive deficits and neuroinflammation in mice44. Interestingly, the removal of microglial populations through cellular depletion in mice can prevent sex-specific GCR effects such as learning deficits and phagocytic activation45, and indicates a potential target for neuroinflammatory regulation in spaceflight-associated radiation models (reviewed in46). Table 2 summarizes some of the similarities identified between various specialized macrophage lineages in spaceflight and associated terrestrial disorders.
There are several research gaps characterizing macrophage lineages in the spaceflight environment and are of interest for future spaceflight studies (Table 2). For example, Kupffer cells of the liver are part of the hepatic macrophage system and play an important role in liver immunological tolerance, producing IL-10 and high PD-L1 expression (reviewed in47). Reduced rat Kupffer cell populations have been previously reported in hepatic tissues post-spaceflight (14-day mission)48, suggesting possible disruption in the maintenance of liver homeostasis and may play a significant role in immunological resolution during inflammatory-induced conditions in spaceflight (reviewed in49), however, more studies are necessary. Similarly, macrophages within other less characterized tissues, such as the pancreas, kidneys, and adrenals also may play important roles, due to parallel terrestrial disorder overlaps with spaceflight-associated conditions of insulin resistance, metabolic disorders (reviewed in50) and renal stone formation risk51,52. However more studies elucidating the role of specialized tissue-resident macrophages lineages are required.
Influence of microgravity on macrophage lineages
Ground-based, terrestrial analogs of microgravity that assess cells in culture include rotating wall vessel (RWV) bioreactors or rotary cell culture systems (RCCS), and clinostats (2D/3D) or random positioning machines. Other possible analogs that are less utilized include, drop towers and parabolic flight (reviewed in53). As described above, HU and partial weight-bearing suspension are standard rodent models used to simulate microgravity, while head-down tilt bead rest and wet/dry immersions are used in humans (reviewed in53,54). However, analogs can produce disparate responses to true microgravity experienced in spaceflight on the ISS, therefore highlighting the importance of utilizing the ISS for future experiments in this field. For example, 2D clinostats can possibly induce spurious fluid motion and shear stress55, which can have negative immune cell consequences. Further, unless otherwise designed, ground-based microgravity analogs do not include other risk factors that are experienced in the spaceflight environment, such as ionizing radiation and elevated carbon dioxide levels, for example1. Therefore, accurately modeling immune function in simulated microgravity analogs that is similar to spaceflight requires careful attention to experimental design.
Considering mechanical and intercellular communication between heterogeneous populations of cells and the hypothesis that the cellular cytoskeleton has built-in mechanisms for sensing mechanical stress (reviewed in56,57), it is possible that macrophages may also be sensitive to mechanical stress. Mechanical stress includes stretch and compressive forces, and hydrostatic shear pressures that are experienced within multiple tissue types (reviewed in58), where macrophages reside. Mechanical unloading, or decreased mechanical stress on cells and tissues can simulate microgravity (reviewed in59). At the cellular level, mechanical unloading causes pathological phenotypes via altered cellular mechanotransduction pathways, which may reflect cellular changes in astronauts (reviewed in57). Although mechanosensitivity of macrophages in vivo has not been characterized in spaceflight, several human spaceflight missions report altered cytokine expression profiles2,4,8,28,32,60, which may be a response of altered mechanotransductive signaling cascades in macrophages (reviewed in26,57). These findings underscore complex and dynamic responses in the spaceflight environment and highlight a gap in knowledge that requires further studies.
Microgravity analogs can cause actin reorganization and changes in cytoskeletal and nuclear morphology61, which may impact transcriptional or replicative programs. In addition, the plasma membrane physically couples to cytoskeletal anchoring proteins and contains Piezo mechanosensitive channels that transduce mechanical stimuli into electrical signals (reviewed in62), which may be an important target for future microgravity research. In line with this, the FAK-ERK1/2 signaling pathway involved in actin polymerization and multiple other signal transduction cascades, is downregulated in HPC concomitantly differentiating into macrophages (12-day differentiation process) both in simulated microgravity conditions (12-days) and spaceflight (12-day mission)24, indicating aberrant cell signaling, impaired macrophage maturation, and cytoskeletal disturbances are influenced by the microgravity environment. In line with this, signaling pathways such as STAT3, P38, JNK, FAK-ERK, Rho/ROCK, AKT, CREB, NF-κB, RAC-WAVE-Arp2/3 (reviewed in63,64) and MRTF-A/SRF (reviewed in64,65), may all be engaged in microgravity, which also requires further studies.
Monocyte/macrophage migratory behavior is also altered in both simulated microgravity and spaceflight conditions. For example, integrin intracellular adhesion molecule-1 (ICAM-1), which regulates cellular migration and extravasation, is induced in differentiated U937 macrophage-like cells and BV-2 microglia cells, following rotating wall vessel and parabolic flight (reviewed in66). ICAM-1 is also elevated at 120 h of spaceflight67, which may result in enhanced extravasation processes and immune activity. Thus, microgravity poses a unique and varied environmental change that may require quantitative, comparative, and multifactorial approaches to tease out pathological macrophage mechanisms at the cellular, tissue, and physiological scales.
Cosmic ionizing radiation and macrophage destabilization
Exposure to photon and particle cosmic radiation, including gamma, X-ray, solar particle events (SPE) and GCR, may further influence macrophage dysfunction during deep space missions. The complex space radiation environment is nearly impossible to simulate on Earth, thus research on space-related ionizing radiation has historically utilized gamma rays and single ion particles68. With the introduction of the newly designed simulated GCR (33-ion sequential beam and simplified 5-ion beam) and SPE dosing schemes (including protracted and acute exposures) developed by the NASA Space Radiation Laboratory (NSRL) at Brookhaven National Laboratory, more opportunities are available to simulate some aspects of the deep space radiation environment69,70.
GCRs are composed of high-energy charged particles, primarily protons, helium ions, and high mass/high energy (HZE) particles68,70, all of which can have detrimental effects on biology (reviewed in71). Characterization of monocyte and macrophage responses to GCRs or their components currently remains inadequately defined. On the one hand, via the metric of apoptosis, macrophages are more radioresistant compared to monocytes, while the lymphoid lineage is even more radiosensitive72. Mice exposed to simulated 5-ion simplified GCR (0.5 Gy) and SPE (1 Gy) irradiation with and without HU displayed no differences in monocyte counts, although reduction in lymphocytes were observed 24-hours post-exposure3. Other studies have reported elevated blood monocyte counts seven-days post-GCR irradiation at comparable doses (0.5 Gy), which positively correlated with impaired spatial learning at five months post-exposure in mice45. This suggests that radiation-mediated damage during myelopoiesis may impact cognitive performance post-irradiation. This effect might be primarily mediated by brain-resident macrophages (microglia), as depleting microglia also improved cognitive outcomes after irradiation45(reviewed in73,74).
Although not a comprehensive analog to space radiation, cancer radiotherapy studies have provided some insight into immune phenotypes following ionizing radiation. Indeed, macrophages play an important role in both regulation and resolution of inflammation, for instance certain polarized types of macrophages are prominent in the progression of cancer, while radiotherapy may promote antitumor phenotypes (reviewed in75). Many characteristic inflammatory profiles produced by macrophages depend on cellular abundance and response to danger-associated molecular patterns (DAMPs), produced from radiation-induced DNA damage76. Thus, in spaceflight, damaged DNA may trigger DAMP receptor pathways inducing inflammatory mediator production2,3,4,8.
Notably, space mission-relevant doses of X-ray irradiation (0.1, 0.5, 1, and 2 Gy) did not alter macrophage phenotypes77. Interestingly, dose-dependence did alter macrophage phenotypes when cells were cultured in the presence of radiation-conditioned fibroblast supernatant, suggesting physiological mediators, received from other cells were necessary to generate unique cellular phenotypes in response to radiation77. Thus, the complex nature of the space radiation environment and its energies, doses, and dose rates make assessing macrophages function a difficult task. Future studies on this topic will require better characterization of macrophage polarity and responses following acute and protracted doses, combination spaceflight exposures, and longitudinal immune sampling.
Isolation, confinement, and extreme environmental stressors
Isolation, confinement, and extreme (ICE) environments also pose unique risks to crewmembers on exploration missions78. Operational on-board stressors that fall into this category include circadian misalignment, social isolation, and closed/hostile environments, all of which may impact macrophage biology. Spaceflight and ground analogs indicate that ICE may disrupt circadian rhythms critical to immune functions (reviewed in79,80). More specifically, ICE may impact circadian misalignment by manifesting disruptions in monocyte and macrophage molecular clocks (reviewed in81). Indeed, light (in the form of visible sunlight) is a dominant environmental cue for many biological clock physiological processes, such as sleep/wake, endocrine hormone release, metabolic processes, and temperature regulation (reviewed in82). Circadian misalignment, as a result from irregular light cues in spaceflight, can cause deviations from normal cycling and may have profound impacts on multiple physiological systems83. In line with this, circadian misalignment may innately impact immune function. Indeed, there have been reports describing cell-autonomous clock gene expressions in rat natural killer cells84 and mouse peritoneal macrophages85. Notably, in the absence of the core clock component protein cryptochrome (CRY), elevated proinflammatory cytokine expression mediated through NF-κB activation has been reported86. Further as mentioned above, neuroendocrine hormone release is regulated by the circadian system. For example, glucocorticoid release peaks in the early morning in response to light (diurnal), which integrates with cyclic systemic immunity (reviewed in87), suggesting neuroendocrine system crosstalk with immunity. Indeed, glucocorticoids can also directly impact macrophage polarization phenotypes, indirectly linking immune regulation to circadian cycling88. Therefore, modulation of glucocorticoid production that aligns with circadian cycle regulation in astronauts on deep space missions should be considered in countermeasure designs. Furthermore, diurnal sample collection metadata should also be evaluated during immune response analysis and considered for future experimental design.
Social isolation in mice in combination with HU caused population shifting in leukocytes, including neutrophils and lymphocytes, while monocyte populations were unaltered89. These studies further support crosstalk with the neuroendocrine hypothalamus-pituitary-adrenal (HPA) axis is involved in immune regulation. In addition, immune differentials reported in the Mars500 isolation project in humans indicate the effects of extreme isolation on gut microbiome maintenance and immunity, a site populated by macrophages90. In line with this, multiple other isolation analogs, including NEEMO, Antarctica, and MOON-2015 have assessed the influence of isolation on macrophage lineage phenotypes (primarily monocytes), collectively indicating differential outcomes are observed during- and post-study collections (reviewed in91). Differential outcomes may be due to the unique microbial environment present in each analog, further adding to the complexity of macrophage phenotype characterizations. Thus, an important future research direction would be to determine the extent of spaceflight-caused macrophage dysfunction and the mechanistic underpinnings involved in macrophage function as a result of ICE-related operational stressors, along with considering environmental microbial influence.
Hypercapnia and macrophage function
Increased partial pressure of CO2 aboard the ISS might also contribute to spaceflight-induced immune dysfunction. It is well-accepted that elevated levels of environmental CO2 can cause hypercapnia (elevated blood/tissue CO2 levels). Elevated levels of CO2 impair macrophage ability to defend against foreign invaders by inhibiting the production of cytokines critical for antimicrobial host defense, such as TNF and IL-692. Hypercapnia also downregulates genes associated with innate immunity, antiviral response, and cytokine signaling in both human and mouse macrophages93, while on the cellular level, hypercapnia has been shown to cause macrophage apoptosis and decreased phagocytic activity92,94. For example, hypercapnia can increase influenza A virus replication and inhibit antiviral gene and protein expression in mouse macrophages, which is dependent on the activity of Akt95. Indeed, Akt isoforms can modulate Akt activity levels in macrophages and their polarization phenotypes96; therefore, Akt may be a potential therapeutic target to enhance macrophage host defense. Collectively, these findings suggest deficits in macrophage immunity can be caused by hypercapnic conditions. This emphasizes a critical risk to consider for long-duration ( > 1 year) exploration missions, where exposures to elevated CO2 levels may be experienced for prolonged periods of time.
Macrophages and future lunar mission considerations
Lunar dust poses a serious challenge for exploration missions to the moon, but the limited availability of authentic lunar dust samples makes it difficult to conduct biological experiments on Earth. Studies of Apollo 14 lunar dust exposure in rats demonstrated lung toxicity when inhaled (reviewed in97). Further, an in vitro study assessed the behavior of a transformed macrophage cell line in the presence of SiO2 and Al2O3 (analogs of lunar dust). In the presence of these mineral particles, phagocytosis was impaired98 and inducible nitric oxide synthase (iNOS) was increased99. In addition, mouse alveolar macrophage counts decrease following lunar dust exposure, along with neutrophil aggregation100, suggesting increased cell death pathways and inflammation in the lung microenvironment may be involved in the pathophysiology of lunar dust exposure. Since macrophages play a major role in phagocytosis and defense against toxins, further studies on macrophage function following lunar dust and other celestial dust exposures are essential for mitigation agendas on future lunar missions.
Future outlook and summary
A growing body of experimental evidence, reviewed in this monograph, indicates that monocytes and macrophages are altered by the spaceflight environment. These findings have implications for a wide range of physiological processes, including innate immunity, acquired immunity, host defense, and tissue remodeling. Aside from spaceflight, the studies described in this review involve the examination of a single aspect of the space environment (such as weightlessness, space radiation or elevated CO2 levels). Future experiments involving combinations of spaceflight stressors, such as elevated CO2 levels combined with simulated space radiation and gravitational changes, would enable a more comprehensive understanding of the effects of the spaceflight environment on macrophage function. Further investigations of cell- and tissue-specific macrophage responses and phenotypes are also necessary to assess tissue-specific pathologies that connect cellular studies to human disease processes on long-duration missions in deep space. Considering the immune system “computes” the state of health throughout the body (reviewed in96) additional assessment of other immune cell types in the hematopoietic tree is also necessary. In brief, fundamental studies on macrophages in space have begun to lay the groundwork for the development of targeted countermeasures that optimize macrophage function and are needed to address clinical challenges that arise as space exploration moves beyond low Earth orbit.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
References
Afshinnekoo, E. et al. Fundamental Biological Features of Spaceflight: Advancing the Field to Enable Deep-Space Exploration. Cell 183, 1162–1184 (2020).
Garrett-Bakelman, F. E. et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 364, aau8650 (2019).
Paul, A. M. et al. Beyond Low-Earth Orbit: Characterizing Immune and microRNA Differentials following Simulated Deep Spaceflight Conditions in Mice. iScience 23, 101747 (2020).
Malkani, S. et al. Circulating miRNA Spaceflight Signature Reveals Targets for Countermeasure Development. Cell Rep. 33, 108448 (2020).
Crucian, B. E. et al. Plasma cytokine concentrations indicate that in vivo hormonal regulation of immunity is altered during long-duration spaceflight. J. Interferon. Cytokine Res 34, 778–786 (2014).
Krieger, S. S. et al. Alterations in Saliva and Plasma Cytokine Concentrations During Long-Duration Spaceflight. Front. Immunol. 12, 725748 (2021).
Crucian, B. et al. Alterations in adaptive immunity persist during long-duration spaceflight. NPJ Microgravity 1, 15013 (2015).
Buchheim, J. I. et al. Stress Related Shift Toward Inflammaging in Cosmonauts After Long-Duration Space Flight. Front. Physiol. 10, 85 (2019).
Crucian, B. E., Stowe, R. P., Pierson, D. L. & Sams, C. F. Immune system dysregulation following short- vs long-duration spaceflight. Aviat. Space Environ. Med 79, 835–843 (2008).
da Silveira, W. A. et al. Comprehensive Multi-omics Analysis Reveals Mitochondrial Stress as a Central Biological Hub for Spaceflight Impact. Cell 183, 1185–1201.e1120 (2020).
Hussain, T. et al. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med Cell Longev. 2016, 7432797 (2016).
Luxton, J. J. et al. Telomere Length Dynamics and DNA Damage Responses Associated with Long-Duration Spaceflight. Cell Rep. 33, 108457 (2020).
Luxton, J. J. et al. Temporal Telomere and DNA Damage Responses in the Space Radiation Environment. Cell Rep. 33, 108435 (2020).
Bennett, J. M., Reeves, G., Billman, G. E. & Sturmberg, J. P. Inflammation-Nature’s Way to Efficiently Respond to All Types of Challenges: Implications for Understanding and Managing “the Epidemic” of Chronic Diseases. Front. Med. (Lausanne) 5, 316 (2018).
Hunter, P. The inflammation theory of disease. The growing realization that chronic inflammation is crucial in many diseases opens new avenues for treatment. EMBO Rep. 13, 968–970 (2012).
Pecaut, M. J. et al. Is spaceflight-induced immune dysfunction linked to systemic changes in metabolism? PLoS One 12, e0174174 (2017).
Ichiki, A. T. et al. Effects of spaceflight on rat peripheral blood leukocytes and bone marrow progenitor cells. J. Leukoc. Biol. 60, 37–43 (1996).
Mortreux, M. & Rosa-Caldwell, M. E. Approaching Gravity as a Continuum Using the Rat Partial Weight-Bearing Model. Life (Basel) 10, 100235 (2020).
Globus, R. K. & Morey-Holton, E. Hindlimb unloading: rodent analog for microgravity. J. Appl Physiol. 120, 1196–1206 (2016).
Sonnenfeld, G., Butel, J. S. & Shearer, W. T. Effects of the Space Flight Environment on the Immune System. Rev. Environ. Health 18, 14 (2003).
Crucian, B. et al. Terrestrial stress analogs for spaceflight associated immune system dysregulation. Brain Behav. Immun. 39, 23–32 (2014).
Gertz, M. L. et al. Multi-omic, Single-Cell, and Biochemical Profiles of Astronauts Guide Pharmacological Strategies for Returning to Gravity. Cell Rep. 33, 108429 (2020).
Chapes, S. K., Morrison, D. R., Guikema, J. A., Lewis, M. L. & Spooner, B. S. Production and action of cytokines in space. Adv. Space Res 14, 5–9 (1994).
Shi, L. et al. Spaceflight and simulated microgravity suppresses macrophage development via altered RAS/ERK/NFκB and metabolic pathways. Cell Mol. Immunol. 18, 1489–1502 (2021).
Ludtka, C., Moore, E. & Allen, J. B. The Effects of Simulated Microgravity on Macrophage Phenotype. Biomedicines 9 https://doi.org/10.3390/biomedicines9091205 (2021).
Ludtka, C., Silberman, J., Moore, E. & Allen, J. B. Macrophages in microgravity: the impact of space on immune cells. NPJ Microgravity 7, 13 (2021).
Lv, H. et al. Microgravity and immune cells. J. R. Soc. Interface 20, 20220869 (2023).
Rykova, M., Antropova, E., Larina, I. & Morukov, B. Humoral and cellular immunity in cosmonauts after the ISS missions. Acta Astronautica 63, 697–705 (2008).
Kaur, I., Simons, E. R., Castro, V. A., Ott, C. M. & Pierson, D. L. Changes in monocyte functions of astronauts. Brain Behav. Immun. 19, 547–554 (2005).
Li, W. Phagocyte dysfunction, tissue aging and degeneration. Ageing Res Rev. 12, 1005–1012 (2013).
Zhang, J. et al. Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Res Rev. 25, 55–69 (2016).
Crucian, B., Stowe, R., Quiriarte, H., Pierson, D. & Sams, C. Monocyte phenotype and cytokine production profiles are dysregulated by short-duration spaceflight. Aviat. Space Environ. Med 82, 857–862 (2011).
Varol, C., Mildner, A. & Jung, S. Macrophages: development and tissue specialization. Annu Rev. Immunol. 33, 643–675 (2015).
Akiyama, T. et al. How does spaceflight affect the acquired immune system? NPJ Microgravity 6, 14 (2020).
Chang, T. T., Spurlock, S. M., Candelario, T. L., Grenon, S. M. & Hughes-Fulford, M. Spaceflight impairs antigen-specific tolerance induction in vivo and increases inflammatory cytokines. FASEB J. 29, 4122–4132 (2015).
Lee, J. W., Lee, I. H., Iimura, T. & Kong, S. W. Two macrophages, osteoclasts and microglia: from development to pleiotropy. Bone Res 9, 11 (2021).
Tamma, R. et al. Microgravity during spaceflight directly affects in vitro osteoclastogenesis and bone resorption. FASEB J. 23, 2549–2554 (2009).
Stavnichuk, M., Mikolajewicz, N., Corlett, T., Morris, M. & Komarova, S. V. A systematic review and meta-analysis of bone loss in space travelers. NPJ Microgravity 6, 13 (2020).
Kuo, T. R. & Chen, C. H. Bone biomarker for the clinical assessment of osteoporosis: recent developments and future perspectives. Biomark. Res 5, 18 (2017).
Saxena, S., Kruys, V., Vamecq, J. & Maze, M. The Role of Microglia in Perioperative Neuroinflammation and Neurocognitive Disorders. Front Aging Neurosci. 13, 671499 (2021).
Cherry, J. D. et al. Galactic cosmic radiation leads to cognitive impairment and increased aβ plaque accumulation in a mouse model of Alzheimer’s disease. PLoS One 7, e53275 (2012).
Cekanaviciute, E., Rosi, S. & Costes, S. V. Central Nervous System Responses to Simulated Galactic Cosmic Rays. Int J Mol Sci 19 https://doi.org/10.3390/ijms19113669 (2018).
Rienecker, K. D. A., Paladini, M. S., Grue, K., Krukowski, K. & Rosi, S. Microglia: Ally and Enemy in Deep Space. Neurosci. Biobehav Rev. 126, 509–514 (2021).
Alaghband, Y. et al. Galactic cosmic radiation exposure causes multifaceted neurocognitive impairments. Cell Mol. Life Sci. 80, 29 (2023).
Krukowski, K. et al. The impact of deep space radiation on cognitive performance: From biological sex to biomarkers to countermeasures. Sci. Adv. 7, eabg6702 (2021).
Mhatre, S. D. et al. Neuro-consequences of the spaceflight environment. Neurosci. Biobehav Rev. 132, 908–935 (2022).
Ju, C. & Tacke, F. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol. Immunol. 13, 316–327 (2016).
Racine, R. N. & Cormier, S. M. Effect of spaceflight on rat hepatocytes: a morphometric study. J. Appl Physiol. (1985) 73, 136S–141S (1992).
Vinken, M. Hepatology in space: Effects of spaceflight and simulated microgravity on the liver. Liver Int 42, 2599–2606 (2022).
Strollo, F. et al. Space Flight-Promoted Insulin Resistance as a Possible Disruptor of Wound Healing. Front Bioeng. Biotechnol. 10, 868999 (2022).
Pietrzyk, R. A., Jones, J. A., Sams, C. F. & Whitson, P. A. Renal stone formation among astronauts. Aviat. Space Environ. Med 78, A9–A13 (2007).
Smith, S. M. et al. Bone metabolism and renal stone risk during International Space Station missions. Bone 81, 712–720 (2015).
ElGindi, M. et al. May the Force Be with You (Or Not): The Immune System under Microgravity. Cells 10 https://doi.org/10.3390/cells10081941 (2021)
Herranz, R. et al. Ground-based facilities for simulation of microgravity: organism-specific recommendations for their use, and recommended terminology. Astrobiology 13, 1–17 (2013).
Mansour, J. et al. Simulated microgravity during clino-rotation is disturbed by spurious fluid motion. bioRxiv, 2023.2002.2010.527979 (2023).
Vorselen, D., Roos, W. H., MacKintosh, F. C., Wuite, G. J. & van Loon, J. J. The role of the cytoskeleton in sensing changes in gravity by nonspecialized. cells FASEB J. 28, 536–547 (2014).
Wu, X. T. et al. Cells respond to space microgravity through cytoskeleton reorganization. FASEB J. 36, e22114 (2022).
Maruyama, K., Nemoto, E. & Yamada, S. Mechanical regulation of macrophage function - cyclic tensile force inhibits NLRP3 inflammasome-dependent IL-1β secretion in murine macrophages. Inflamm. Regen. 39, 3 (2019).
Bradbury, P. et al. Modeling the Impact of Microgravity at the Cellular Level: Implications for Human Disease. Front Cell Dev. Biol. 8, 96 (2020).
Crucian, B. et al. Immune system dysregulation occurs during short duration spaceflight on board the space shuttle. J. Clin. Immunol. 33, 456–465 (2013).
Neelam, S. et al. Changes in Nuclear Shape and Gene Expression in Response to Simulated Microgravity Are LINC Complex-Dependent. Int J Mol Sci 21 https://doi.org/10.3390/ijms21186762 (2020).
Xiao, R. & Xu, X. Z. Mechanosensitive channels: in touch with Piezo. Curr. Biol. 20, R936–R938 (2010).
Murali, A. & Sarkar, R. R. Mechano-immunology in microgravity. Life Sci. Space Res. 37, 50–64 (2023).
An, R. MRTF may be the missing link in a multiscale mechanobiology approach toward macrophage dysfunction in space. Front Cell Dev. Biol. 10, 997365 (2022).
Kouznetsov, N. V. Cell Responses to Simulated Microgravity and Hydrodynamic Stress Can Be Distinguished by Comparative Transcriptomics. Int. J. Transl. Med. 2, 364–386 (2022).
Paulsen, K. et al. Regulation of ICAM-1 in cells of the monocyte/macrophage system in microgravity. Biomed. Res Int 2015, 538786 (2015).
Paulsen, K. et al. Severe disruption of the cytoskeleton and immunologically relevant surface molecules in a human macrophageal cell line in microgravity—Results of an in vitro experiment on board of the Shenzhou-8 space mission. Acta Astronautica 94, 277–292 (2014).
Nelson, G. A. Space Radiation and Human Exposures, A Primer. Radiat. Res 185, 349–358 (2016).
Norbury, J. W. et al. Galactic cosmic ray simulation at the NASA Space Radiation Laboratory. Life Sci. Space Res (Amst.) 8, 38–51 (2016).
Simonsen, L. C., Slaba, T. C., Guida, P. & Rusek, A. NASA’s first ground-based Galactic Cosmic Ray Simulator: Enabling a new era in space radiobiology research. PLoS Biol. 18, e3000669 (2020).
Chancellor, J. C. et al. Limitations in predicting the space radiation health risk for exploration astronauts. NPJ Microgravity 4, 8 (2018).
Heylmann, D., Ponath, V., Kindler, T. & Kaina, B. Comparison of DNA repair and radiosensitivity of different blood cell populations. Sci. Rep. 11, 2478 (2021).
Paladini, M. S., Feng, X., Krukowski, K. & Rosi, S. Microglia depletion and cognitive functions after brain injury: From trauma to galactic cosmic ray. Neurosci. Lett. 741, 135462 (2021).
Rosi, S. The final frontier: Transient microglia reduction after cosmic radiation exposure mitigates cognitive impairments and modulates phagocytic activity. Brain Circ. 4, 109–113 (2018).
Shi, X. & Shiao, S. L. The role of macrophage phenotype in regulating the response to radiation therapy. Transl. Res 191, 64–80 (2018).
Pariset, E. et al. DNA Damage Baseline Predicts Resilience to Space Radiation and Radiotherapy. Cell Rep. 33, 108434 (2020).
Deloch, L. et al. Low-Dose Irradiation Differentially Impacts Macrophage Phenotype in Dependence of Fibroblast-Like Synoviocytes and Radiation Dose. J. Immunol. Res 2019, 3161750 (2019).
Häuplik-Meusburger, S. & Bishop, S. in 50th International Conference on Environmental Systems (2021).
Haspel, J. A. et al. Perfect timing: circadian rhythms, sleep, and immunity - an NIH workshop summary. JCI Insight 5 https://doi.org/10.1172/jci.insight.131487 (2020).
Ponomarev, S. et al. Immunological Aspects of Isolation and Confinement. Front Immunol. 12, 697435 (2021).
Timmons, G. A., O’Siorain, J. R., Kennedy, O. D., Curtis, A. M. & Early, J. O. Innate Rhythms: Clocks at the Center of Monocyte and Macrophage Function. Front Immunol. 11, 1743 (2020).
Marcheva, B. et al. Circadian clocks and metabolism. Handb Exp Pharmacol 127–155 https://doi.org/10.1007/978-3-642-25950-0_6 (2013).
Flynn-Evans, E. E., Barger, L. K., Kubey, A. A., Sullivan, J. P. & Czeisler, C. A. Circadian misalignment affects sleep and medication use before and during spaceflight. NPJ Microgravity 2, 15019 (2016).
Arjona, A. & Sarkar, D. K. Circadian oscillations of clock genes, cytolytic factors, and cytokines in rat NK cells. J. Immunol. 174, 7618–7624 (2005).
Hayashi, M., Shimba, S. & Tezuka, M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol. Pharm. Bull. 30, 621–626 (2007).
Narasimamurthy, R. et al. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc. Natl Acad. Sci. USA 109, 12662–12667 (2012).
Shirato, K. & Sato, S. Macrophage Meets the Circadian Clock: Implication of the Circadian Clock in the Role of Macrophages in Acute Lower Respiratory Tract Infection. Front Cell Infect. Microbiol 12, 826738 (2022).
Lellupitiyage Don, S. S. et al. Macrophage circadian rhythms are differentially affected based on stimuli. Integr. Biol. (Camb.) 14, 62–75 (2022).
Tahimic, C. G. T. et al. Influence of Social Isolation During Prolonged Simulated Weightlessness by Hindlimb Unloading. Front Physiol. 10, 1147 (2019).
Brereton, N. J. B., Pitre, F. E. & Gonzalez, E. Reanalysis of the Mars500 experiment reveals common gut microbiome alterations in astronauts induced by long-duration confinement. Comput Struct. Biotechnol. J. 19, 2223–2235 (2021).
Ponomarev, S. et al. Changes in the cellular component of the human innate immunity system in short-term isolation. Acta Astronautica 166, 89–92 (2020).
Wang, N. et al. Elevated CO2 selectively inhibits interleukin-6 and tumor necrosis factor expression and decreases phagocytosis in the macrophage. FASEB J. 24, 2178–2190 (2010).
Casalino-Matsuda, S. M. et al. Hypercapnia Alters Expression of Immune Response, Nucleosome Assembly and Lipid Metabolism Genes in Differentiated Human Bronchial Epithelial Cells. Sci. Rep. 8, 13508 (2018).
Casalino-Matsuda, S. M., Nair, A., Beitel, G. J., Gates, K. L. & Sporn, P. H. Hypercapnia Inhibits Autophagy and Bacterial Killing in Human Macrophages by Increasing Expression of Bcl-2 and Bcl-xL. J. Immunol. 194, 5388–5396 (2015).
Casalino-Matsuda, S. M. et al. Hypercapnia Suppresses Macrophage Antiviral Activity and Increases Mortality of Influenza A Infection via Akt1. J. Immunol. 205, 489–501 (2020).
Linton, M. F., Moslehi, J. J. & Babaev, V. R. Akt Signaling in Macrophage Polarization, Survival, and Atherosclerosis. Int J Mol Sci 20 https://doi.org/10.3390/ijms20112703 (2019).
Linnarsson, D. et al. Toxicity of Lunar Dust. Planet. Space Sci. 74, 57–71 (2012).
Jordan, J. A., Verhoff, A. M., Morgan, J. E. & Fischer, D. G. Assessing the in vitro toxicity of the lunar dust environment using respiratory cells exposed to Al(2)O(3) or SiO(2) fine dust particles. Vitr. Cell Dev. Biol. Anim. 45, 602–613 (2009).
Chatterjee, A., Wang, A., Lera, M. & Bhattacharya, S. Lunar soil simulant uptake produces a concentration-dependent increase in inducible nitric oxide synthase expression in murine RAW 264.7 macrophage cells. J. Toxicol. Environ. Health A 73, 623–626 (2010).
Sun, Y. et al. Research on rat’s pulmonary acute injury induced by lunar soil simulant. J. Chin. Med. Assoc. 81, 133–140 (2018).
Acknowledgements
We would like to thank the 2021 NASA Ames Research Center Space Life Sciences Training Program (SLSTP) and Mentors for providing an avenue for student collaboration in space biosciences. SLSTP is funded by NASA’s Space Biology Program, part of the Biological and Physical Sciences Division of NASA. Publication of this review was supported by the Embry-Riddle Aeronautical University Start Up fund to AMP.
Author information
Authors and Affiliations
Contributions
R.A., V.K.B., B.H., A.C.G., O.S., I.I., and A.R. are co-first authors. R.A., V.K.B., B.H., A.C.G., O.S., I.I., A.R., N.C., K.S., T.S., and N.C.S. wrote the original draft. All authors revised and read the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
An, R., Blackwell, V.K., Harandi, B. et al. Influence of the spaceflight environment on macrophage lineages. npj Microgravity 10, 63 (2024). https://doi.org/10.1038/s41526-023-00293-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41526-023-00293-0
- Springer Nature Limited