Abstract
Aortic aneurysms are dilatations of the aorta that can rupture when left untreated. We used the aneurysmal Fibulin-4R/R mouse model to further unravel the underlying mechanisms of aneurysm formation. RNA sequencing of 3-month-old Fibulin-4R/R aortas revealed significant upregulation of senescence-associated secretory phenotype (SASP) factors and key senescence factors, indicating the involvement of senescence. Analysis of aorta histology and of vascular smooth muscle cells (VSMCs) in vitro confirmed the senescent phenotype of Fibulin-4R/R VSMCs by revealing increased SA-β-gal, p21, and p16 staining, increased IL-6 secretion, increased presence of DNA damage foci and increased nuclei size. Additionally, we found that p21 luminescence was increased in the dilated aorta of Fibulin-4R/R|p21-luciferase mice. Our studies identify a cellular aging cascade in Fibulin-4 aneurysmal disease, by revealing that Fibulin-4R/R aortic VSMCs have a pronounced SASP and a senescent phenotype that may underlie aortic wall degeneration. Additionally, we demonstrated the therapeutic effect of JAK/STAT and TGF-β pathway inhibition, as well as senolytic treatment on Fibulin-4R/R VSMCs in vitro. These findings can contribute to improved therapeutic options for aneurysmal disease aimed at reducing senescent cells.
Similar content being viewed by others
Introduction
Aortic aneurysms are pathological dilatations of the aorta to greater than 50% of its original size and can lead to dissections, which have a high mortality rate. Aortic aneurysms and dissections are responsible for 1–2% of all deaths in developed countries1. Next to advanced age, risk factors for developing aortic aneurysms include male gender and family history of aortic aneurysms2. Thoracic aortic aneurysms (TAA) are usually observed in inherited syndromes, such as Marfan syndrome, Loeys–Dietz syndrome, and cutis laxa syndrome. Heterozygous mutations in FBN1, the gene encoding for Fibrillin-1 protein involved in extracellular matrix (ECM) integrity, cause Marfan syndrome (MFS1; MIM 154700), a fibrous connective tissue disorder characterized by skeletal, ocular, and cardiovascular abnormalities including aneurysm development. Patients with mutations in the SMAD2/3 and TGFBR1/2 genes, encoding proteins involved in transforming growth factor beta (TGF-β) signaling, develop Loeys–Dietz syndrome (TGFBR1; LDS1; MIM 609192, TGFBR2; LDS2; MIM 61068, SMAD2; LDS6; MIM 619656, SMAD3; LDS3 (AOS); MIM 613795). This syndrome is characterized by aneurysms, dissections, and cardiac abnormalities, as well as early-onset osteoarthritis3,4. Homozygous or compound heterozygous mutations in EFEMP2, which encodes for the ECM protein Fibulin-4, have been found in patients with cutis laxa syndrome (ARCL1B; MIM 614437), which is characterized by loose skin, lung emphysema, bone fragility, vascular tortuosity and aneurysms5,6. Fibulin-4 is located in microfibril bundles that tether elastic fibers to vascular smooth muscle cells (VSMCs) and therefore plays an essential role in elastic fiber formation7. Fibulin-4 is particularly important in vascular maturation and the maintenance of structural integrity and elasticity of the aortic wall8,9.
Fibulin-4 deficiency has been mimicked in previously developed Fibulin-4R/R mutant mice with a systemic 4-fold decreased expression of Fibulin-48. Fibulin-4R/R mice (where R stands for reduced expression) develop TAAs and additionally present with increased arterial stiffness and impaired vascular contractility8. Fibulin-4R/R mice die at an early age of 3 months. Deregulation of the TGF-β signaling pathway, involved in the regulation of proliferation, differentiation, and development, underlies aortic aneurysm development in patients and mice with Fibulin-4 deficiency. In the aorta of Fibulin-4R/R mice, an increase in free TGF-β causes activation of downstream transcription of the TGF-β pathway, resulting in increased matrix metalloproteinase (MMP) activity and ECM remodeling10,11,12. Furthermore, mitochondrial dysfunction plays an important role in aneurysm formation both in Fibulin-4R/R mice and aneurysm patients9,13.
Aging is a major risk factor for developing aortic aneurysms2,14. With age, many structural and functional changes occur in the aorta such as increased stiffness, increased aortic diameter, inflammation, aberrant ECM, and endothelial dysfunction15. More specifically, VSMCs, which are the main cell type of the aortic media, can undergo phenotypic changes under the influence of pathological stimuli such as those that occur with aging16. Many of these aforementioned processes are also involved in aortic aneurysm formation. Therefore, we aimed to investigate these processes in our aneurysmal Fibulin-4R/R mouse model.
To further investigate the molecular mechanisms underlying aneurysm formation in Fibulin-4R/R mice, RNA sequencing of the aorta was performed. RNA expression analysis confirmed previously identified deregulated molecular pathways such as the TGF-β pathway and pathways involved in ECM remodeling and mechanosensing. Additionally, data analysis pointed to the involvement of genes and pathways involved in senescence and VSMC phenotypic switching. Furthermore, we identified markers for the senescent phenotype in aneurysmal Fibulin-4R/R aortic tissue and VSMCs and demonstrated the therapeutic effect of JAK/STAT and TGF-β pathway inhibition and senolytic treatment.
Results
RNAseq analysis confirms previously identified pathways involved in aneurysm formation in Fibulin-4 R/R mice
To further investigate the molecular mechanisms underlying aortic aneurysm formation in Fibulin-4R/R mice, RNAseq was performed on Fibulin-4R/R mouse aorta using the Illumina NextSeq500 platform. Differential expression analysis resulted in 3036 significant differentially expressed genes (p value ≤ 0.05; |fold change| ≥ 1.2) in the aortic arch (Fig. 1a and Supplementary Table 1) and 851 in the abdominal aorta (Fig. 1b and Supplementary Table 1) of Fibulin-4R/R mice compared to Fibulin-4+/+ mice. Fibulin-4R/R mice develop an aneurysm in the aortic arch, possibly resulting in more differentially expressed genes in the aortic arch compared to the abdominal aorta, which is less affected. Therefore, we focused on the aortic arch in this study. The differential expression data confirmed that expression of the gene for Fibulin-4, Efemp2, was significantly downregulated in the Fibulin-4R/R aorta (Fig. 1c).
a Volcano plot of genes differentially expressed between the Fibulin-4R/R aortic arch and the Fibulin-4+/+ aortic arch. b Volcano plot of genes differentially expressed between the Fibulin-4R/R abdominal aorta and the Fibulin-4+/+ abdominal aorta. The top 20 differentially expressed genes are highlighted (ranked based on fold change). Significantly upregulated genes are colored red and significantly downregulated genes are colored blue (p value ≤ 0.05; |fold change| ≥ 1.2). c Expression of the Efemp2 gene (rlog normalized counts), encoding Fibulin-4 protein, in the Fibulin-4R/R aortic arch compared to the Fibulin-4+/+ aortic arch (****p < 0.0001, FC = −2.421). The mean (±SD) is plotted. d Heatmap of significantly differentially expressed genes in the TGF-β pathway (p ≤ 0.05). e Heatmap of significantly differentially expressed genes downstream of the TGF-β pathway (p ≤ 0.05). f Heatmap of significantly differentially expressed genes involved in mechanosensing (p ≤ 0.05).
Since the Fibulin-4R/R mouse model was developed to study aneurysm formation, several pathways have been uncovered to play a role in disease development in these mice. The TGF-β signaling pathway is the main pathway known to be involved in aneurysm formation in patients and mice with decreased Fibulin-4 expression6,10,11. Therefore, we investigated whether RNA expression analysis of Fibulin-4R/R mouse aorta would also reveal an increase in TGF-β pathway activity using Ingenuity Pathway Analysis (IPA). Our data showed significant deregulation of the TGF-β signaling pathway (−log (p value) > 1.3) in the Fibulin-4R/R aortic arch without clear directionality (z score ≤ 2)(Fig. 1d and Supplementary Fig. 1a). Nonetheless, upregulation of downstream genes Gsc, Smad6, Smad7, Irf7, and Nkx2.5 was observed. Additionally, analysis of 26 target genes of the TGF-β pathway (a list previously generated to determine pathway activity based on downstream gene expression17) revealed that 46.2% of analyzed target genes (12/26 genes) were upregulated in the Fibulin-4R/R aortic arch, whereas only one gene was downregulated (Fig. 1e and Supplementary Fig. 1b). Thus, our RNA expression data from 3-month-old animals was in line with previous findings in 10-day-old animals and suggested increased TGF-β signaling in the aortic arch of Fibulin-4R/R mice8.
Our RNAseq analysis revealed that in the Fibulin-4R/R aortic arch, integrin signaling, integrin-like kinase (ILK) signaling, calcium signaling, RhoA mediated signaling, regulation of actin-based motility by Rho, actin cytoskeleton signaling and focal adhesion kinase (FAK) signaling were significantly changed (Supplementary Table 2). More specifically, the expression of important mechanosensing protein-encoding genes such as cadherins, integrins, and paxillin was significantly changed in the Fibulin-4R/R aortic arch (Fig. 1f). These results confirmed previous findings in Fibulin-4SMKO mice (SMKO = smooth muscle cell knockout), revealing that alterations in the structure and mechanical properties of the aortic wall are associated with dysregulated mechanosensing in Fibulin-4SMKO VSMCs18. These results suggest that mechanosensing is dysregulated in the aorta of mice with decreased Fibulin-4 expression.
Cytoskeleton reorganization and ECM remodeling in aortas of Fibulin-4 R/R mice
Previous research showed that Fibulin-4R/R VSMCs present with excessive ECM production and aberrations in actin cytoskeleton structure and dynamics10. The formation and proper functioning of the cytoskeleton is essential for VSMC contractility. Additionally, VSMCs are highly sensitive to changes of the surrounding ECM, which can therefore also influence their state. Our RNA expression analysis also revealed several significantly changed genes and pathways that are associated with cytoskeleton reorganization and ECM production (Fig. 2a). The actin cytoskeleton pathway was the fourth most significantly changed pathway in the Fibulin-4R/R aortic arch and was predicted to be upregulated with a z score of 2.32 (Fig. 2b and Supplementary Fig. 1c). The top 20 of upregulated genes contained several genes encoding structural proteins, such as collagen type VIII alpha 1 chain (Col8a1), reelin (Reln), thrombospondin 1 (Thbs1), fibrillin 2 (Fbn2), and tubulin beta 3 class III (Tubb3) (Fig. 1a). Additionally, expression of 15 other collagen chains, 3 laminin subunits and fibronectin 1 (Fn1) was significantly changed, all of which are ECM proteins (Fig. 2c). The genes encoding for myosin light chain kinase 2 (Mylk2) and actin gamma 2 (Actg2), proteins involved in skeletal muscle contraction, were in the top 20 downregulated genes in the Fibulin-4R/R aortic arch (Fig. 1a). These results suggested that dysregulation of mechanisms involved in the maintenance of proper aortic wall structure and function is reflected at the RNA expression level.
a Significantly changed canonical pathways involved in ECM remodeling from IPA analysis (−log (p value) > 1.3). Percentages written in the bars represent the ratio ((number of genes in the pathway that meet cutoff criteria/total number of genes in the pathway)* 100%). b Heatmap of significantly differentially expressed genes in the actin cytoskeleton pathway (p ≤ 0.05). Note: not all gene names were plotted. c Heatmap of significantly differentially expressed ECM proteins in the Fibulin-4R/R aortic arch compared to Fibulin-4+/+controls (p ≤ 0.05). d HE staining of Fibulin-4R/R and Fibulin-4+/+ mouse aortic arch. Bar = 500 µm. e Quantification of the lumen size, media size, and media:lumen ratio. In all graphs, the mean (±SD) is plotted (n = 5–6 per group, *p < 0.05, **p < 0.01, unpaired t test). Arch aortic arch, Thor thoracic aorta, Abd abdominal aorta. f Movat’s pentachrome staining (left) of Fibulin-4R/R mouse aorta identifying collagen (yellow) and fibrin (bright red) deposition, elastin fiber (black) disorganization and proteoglycan (blue) deposition at sites of smooth muscle cell (red) loss compared to Fibulin-4+/+ controls (n = 3 per group). Resorcin Fuchsin staining (middle) of Fibulin-4R/R and Fibulin-4+/+ mouse aortas (n = 5–6 per group). Alcian blue staining (right) of Fibulin-4R/R and Fibulin-4+/+ mouse aortas (n = 5–6 per group). Bar = 100 mm, m media, a adventitia, lu lumen. g Quantification of Alcian blue staining in the Fibulin-4R/R and Fibulin-4+/+ aortic wall. The mean percentage (±SD) of Alcian blue staining of the total aortic media surface area is plotted (n = 5–6 per group, ****p < 0.0001, unpaired t test).
To validate findings from the RNAseq data analysis, histochemical stainings for several (extra) cellular structures were performed on the mouse aorta. In Fibulin-4R/R mice, media and lumen size were significantly increased in the aortic arch (Fig. 2d, e). No significant change was observed in the ratio between media and lumen, indicating that the increase in media size is equivalent to the increase in lumen size in the Fibulin-4R/R mouse aorta (Fig. 2e). Furthermore, Movat’s staining revealed fibrotic changes portrayed by increased collagen (yellow) and fibrin (bright red) deposition, as well as disorganization of elastin fibers (black) in the medial layer of the Fibulin-4R/R aorta (Fig. 2f). Additionally, proteoglycan (blue) deposition was visible particularly at sites with loss of smooth muscle cells (red). RNAseq data analysis revealed significantly changed genes and pathways involved in ECM remodeling in the Fibulin-4R/R aortic arch. Elastin is one of the main components of the ECM involved in pathological remodeling of the aortic wall upon aneurysm formation. Resorcin-fuchsin (RF) staining confirmed altered elastin structures in the Fibulin-4R/R aorta (Fig. 2f). Accumulation but also disorganization of elastic laminae was visible in the media of the Fibulin-4R/R aorta. Additionally, flattening of the elastic laminae was observed and further reflected stiffening of the aortic wall19,20. Alcian blue staining showed a significantly increased accumulation of proteoglycans, another major component of the ECM, in the aortic wall of Fibulin-4R/R mice (Fig. 2f, g). These findings together confirmed our RNAseq data, showing significant ECM remodeling in the aortic wall of Fibulin-4R/R mice.
Significantly altered expression of markers for the contractile and synthetic VSMC phenotype in Fibulin-4 R/R aortas
A phenotypic switch of VSMCs from a contractile to a synthetic phenotype often appears to be involved in aortic dysfunction16. Therefore, we investigated the expression of genes that are markers for either a contractile or a synthetic VSMC phenotype in our RNA expression data based on literature (Supplementary Table 3)21. Of the 17 investigated markers for a contractile phenotype, 12 were downregulated in the Fibulin-4R/R aortic arch (Fig. 3a and Supplementary Fig. 2). The two most suitable marker proteins for a contractile phenotype are smoothelin (encoded by Smtn) and SM-MHC (encoded by Myh11) and both were significantly downregulated21. Of the 12 investigated markers for a synthetic phenotype, five were upregulated and one was downregulated (Fig. 3a and Supplementary Fig. 2). These findings suggested a phenotypic switch of VSMCs in the Fibulin-4R/R aortic arch from a contractile to a synthetic phenotype.
a Heatmap of significantly differentially expressed genes that are markers for the contractile VMSC phenotype and the synthetic VSMC phenotype (p ≤ 0.05). b Staining (left) and quantification (right) for contractile marker αSMA and synthetic marker vimentin on Fibulin-4R/R and Fibulin-4+/+ mouse aortas. The mean percentage (±SD) of DAB staining of the total aortic media surface area is plotted (n = 5–6 per group, *p < 0.05, ****p < 0.0001, unpaired t test). Bar = 100 mm, m media, a adventitia, lu lumen. c Staining (left) and quantification (right) of DAPI staining on Fibulin-4R/R and Fibulin-4+/+ mouse aortas to determine the number of nuclei, with an αSMA staining as reference for the aortic media. The mean number (±SD) of nuclei per total media area in pixels is plotted (n = 5–6 per group, p > 0.05, unpaired t test).
To validate the RNAseq findings suggesting a less contractile, more synthetic VSMC phenotype in the Fibulin-4R/R aortic arch, immunohistochemical staining was performed on mouse aorta for contractile marker αSMA and synthetic marker vimentin (Fig. 3b). Both αSMA and vimentin staining were significantly decreased in the Fibulin-4R/R compared to the Fibulin-4+/+ aorta after correction for total media area. To assess whether this is caused by a loss of VSMCs in the medial layer rather than by phenotypic switching, a staining for DNA (DAPI) and a counterstain with αSMA, to indicate the aortic media, was performed (Fig. 3c). After correction for total aortic media area, no significant change was observed in the number of VSMCs in the Fibulin-4R/R aortic arch compared to the Fibulin-4+/+ aortic arch. This suggested that the observed decreased expression of αSMA and vimentin in the Fibulin-4R/R aortic arch was due to phenotypic changes of VSMCs, but did not necessarily support a switch from a contractile to a synthetic phenotype.
Increased expression of SASP factors, senescence markers p21, p16, and senescence-associated β-galactosidase (SA-β-gal) in the Fibulin-4 R/R aortic arch
We further investigated the RNAseq data for an alternative explanation for the altered phenotypic state of VSMCs in the Fibulin-4R/R aorta. Several indications exist for the involvement of senescence in aneurysm development. A main feature of senescent cells is the secretion of senescence-associated secretory phenotype (SASP) factors, including pro-inflammatory cytokines, chemokines, MMPs, and ECM proteins. Therefore, we investigated the expression of 71 (groups of) genes that belong to the SASP phenotype based on literature (Fig. 4a and Supplementary Table 4)22. Of the investigated SASP factors, 39.4% was significantly changed in the Fibulin-4R/R aortic arch compared to the Fibulin-4+/+ aortic arch (Fig. 4a, Supplementary Fig. 3, and Supplementary Table 5). Most factors were upregulated, which suggested the presence of senescent cells secreting SASP factors. In the Fibulin-4R/R aortic arch, expression of MMP3, MMP12, MMP14, and tissue inhibitor of metalloproteinase-1 (TIMP1) was upregulated, and 16 genes encoding for different types of collagen chains were differentially expressed (Figs. 2c and 4a). This indicated that MMP expression and ECM remodeling were increased, which has been previously demonstrated in the Fibulin-4R/R aorta8,23. Furthermore, expression of inflammatory factors interleukin-1B (IL-1B), interleukin-6 (IL-6), interleukin-7 (IL-7), C-X-C motif chemokine ligand 3 (CXCL3) and 13 (CXCL13) was upregulated in the Fibulin-4R/R aortic arch, of which the pro-inflammatory cytokine IL-6 is the most prominent cytokine of the SASP22. Additionally, expression of CDKN2A (p16INK4A) and CDKN2B (p15INK4B), both cyclin-dependent kinase inhibitors involved in establishing cell cycle arrest in senescence, was significantly increased in the Fibulin-4R/R aorta. To confirm these RNAseq findings, qPCR was performed which confirmed significantly increased expression of Igfbp2, Timp1, Mmp3, Fn1, Col8a1, Col2a1, Il6, Il1b, Cdkn1a, Cdkn2a, and Cdkn2b in the Fibulin-4R/R aorta (Supplementary Fig. 4). Altogether, these findings suggested the presence of senescent cells in the Fibulin-4R/R aortic arch.
a Heatmap of significantly differentially expressed SASP factors and senescence markers p21 (Cdkn1a)*, p16 (Cdkn2a) and p15 (Cdkn2b) in the Fibulin-4R/R aortic arch compared to Fibulin-4+/+controls (p ≤ 0.05). *Note: Cdkn1a was not significantly changed (p = 0.302), all other displayed genes were. b Immunohistochemical staining for senescence markers p21 (left), p16 (middle), and Ki67 (right) on Fibulin-4R/R and Fibulin-4+/+ mouse aorta. Bar = 100 mm. c Quantification of staining for p21, p16, and Ki67 on Fibulin-4R/R and Fibulin-4+/+ mouse aortas (for p21; n = 6–7 per group, **p < 0.01, unpaired t test, for p16; n = 3–4 per group, **p < 0.01, unpaired t test and for Ki67; n = 6–8 per group, p > 0.05, unpaired t test). The mean number (±SD) of positive cells per 0.1 mm2 aortic media surface area is plotted per mouse. d Staining (left) and quantification (right) of senescence-associated β-galactosidase (SA-β-gal) on Fibulin-4R/R and Fibulin-4+/+ mouse aortas. The mean percentage (±SD) SA-β-gal of the total area is plotted (n = 4 per group, *p < 0.05, unpaired t test). e SA-β-gal staining on Fibulin-4R/R and Fibulin-4+/+ mouse aortas showing localization of SA-β-gal positive cells. Bar = 100 mm, m media, a adventitia, lu lumen.
To validate findings from our RNAseq data, immunohistochemical staining for senescence markers p21 and p16 was performed on Fibulin-4R/R and Fibulin-4+/+ aortas. A significant increase was observed in p21 and p16 positive cells in the Fibulin-4R/R aortic arch (Fig. 4b, c). These p21 and p16 positive cells were mainly present in the media, suggesting senescence of VSMCs. Ki67 staining revealed some proliferating cells in the Fibulin-4R/R aorta, however, there was no significant increase compared to Fibulin-4+/+ mice. Additionally, a SA-β-gal staining on mouse aorta revealed increased staining of this marker in the Fibulin-4R/R aorta, mainly visible in VSMCs residing in the medial layer (Fig. 4d, e). Increased staining for senescence markers p21, p16, and SA-β-gal in the Fibulin-4R/R aorta, as well as no increase in Ki67 staining, confirmed the findings from our RNAseq data and suggested senescence of Fibulin-4R/R VSMCs. Although cells that were positive for most of these markers were also visible in the thoracic and abdominal part of the aorta, their presence was most pronounced in the aneurysmal aortic arch (Supplementary Fig. 5).
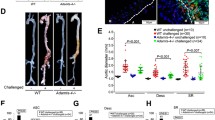
Increased p21 luminescence in the Fibulin-4 R/R aortic arch
An important factor in the response to different stress stimuli, including DNA damage, that can trigger the induction of cellular senescence, is p2124. We generated Fibulin-4R/R|p21-Luciferase mice in which expression of firefly luciferase was placed under the control of the endogenous p21 promoter. Fibulin-4R/R|p21-Luciferase aortas showed increased luminescence compared to Fibulin-4+/+|p21-Luciferase aortas (Fig. 5). As a control, both Fibulin-4R/R and Fibulin-4+/+ mice without p21 luciferase showed no luminescence. These results confirmed the presence of increased p21 expression in the aortic arch of Fibulin-4R/R|p21-Luciferase mice.
Fibulin-4 R/R VMSCs exhibit increased senescence markers in vitro
Additionally, the senescent phenotype of Fibulin-4R/R VSMCs was investigated in vitro. After isolation from the mouse aorta, VSMC phenotype was confirmed by immunofluorescent staining for the contractile markers SM22 and αSMA and synthetic markers vimentin and collagen I (Supplementary Fig. 6). Next, the expression of several senescence markers was assessed. After 7 days in culture, immunofluorescent staining was performed for the stress and senescence marker p21 (Fig. 6a). The percentage of p21 positive cells was significantly increased in Fibulin-4R/R VSMCs compared to Fibulin-4+/+ VSMCs (Fig. 6b). Since senescent cells show chronic accumulation of DNA damage repair proteins (senescence-associated DNA damage foci), staining for γH2Ax and 53BP1 was performed (Fig. 6c). A significant increase of both γH2Ax and 53BP1 foci was observed in Fibulin-4R/R VSMCs compared to Fibulin-4+/+ VSMCs (Fig. 6c and Supplementary Fig. 7a). Additionally, the correlation between foci number and nuclear size was investigated in Fibulin-4R/R VSMCs, since increased nuclear size is another characteristic of the senescent phenotype. The nucleus area of Fibulin-4R/R VSMCs was significantly increased compared to Fibulin-4+/+ VSMCs (Fig. 6d, e and Supplementary Fig. 7b). Correlation analysis revealed a significant correlation between nucleus area and γH2Ax and 53BP1 foci number in Fibulin-4R/R VSMCs (Fig. 6d, e). Furthermore, SA-β-gal staining revealed significantly increased SA-β-gal activity in Fibulin-4R/R VSMCs compared to Fibulin-4+/+ VSMCs (Fig. 6f). Lastly, Fibulin-4R/R VSMCs showed increased secretion of SASP factor IL-6 in the culture medium in vitro (Fig. 6g). Altogether, these markers indicated that Fibulin-4R/R VSMCs also have a senescent phenotype in vitro. The Fibulin-4R/R mouse model was generated through transcriptional interference by placing a TKneo-targeted construct in the downstream gene Mus81. Mus81 is involved in the homologous recombination DNA repair pathway, therefore knockout of this gene could result in an increased presence of DNA damage foci and induction of cellular senescence due to impaired DNA repair capacities. To confirm that the senescent phenotype (and especially the accumulation of DNA damage repair proteins) was not due to the knockout of Mus81, staining for SA-β-gal, γH2Ax, and 53BP1 was performed on Fibulin-4SMKO VSMCs (Supplementary Fig. 8). These VSMCs express no Fibulin-4 protein and do not harbor the Mus81 mutation. Similar to Fibulin-4R/R VSMCs, Fibulin-4SMKO VSMCs showed increased SA-β-gal staining and an increased number of γH2Ax and 53BP1 foci compared to their wildtype controls, confirming that this was caused by aberrant Fibulin-4 expression.
a Immunofluorescent staining for senescence marker p21 on Fibulin-4R/R and Fibulin-4+/+ VSMCs. Bar = 50 µm. b Quantification of p21 positive cells in Fibulin-4R/R VSMCs and Fibulin-4+/+ controls. The percentage (mean ± SD) of p21 positive cells is plotted per cell line (n = 3, *p < 0.05, unpaired t test). c Immunofluorescent staining for DNA damage markers γH2Ax and 53BP1 on Fibulin-4R/R and Fibulin-4+/+ VSMCs. Bar = 50 µm. d Plot showing the correlation between the number of γH2Ax foci per nucleus and nucleus area (μm2). Each data point represents an individual nucleus (n = 2–3 cell lines per group, 37–81 nuclei analyzed per cell line). There is a significant correlation between foci number and nucleus area in the Fibulin-4R/R VSMC group (p < 0.0001, Spearman nonparametric correlation test). e Plot showing the correlation between the number of 53BP1 foci per nucleus and nucleus area (μm2). Each data point represents an individual nucleus (n = 2–3 cell lines per group, 37–81 nuclei analyzed per cell line). There is a significant correlation between foci number and nucleus area in the Fibulin-4R/R VSMC group (p < 0.0001, Spearman nonparametric correlation test). f Staining (left) and quantification (right) of SA-β-gal in Fibulin-4R/R and Fibulin-4+/+ VSMCs. The percentage (mean ± SD) of SA-β-gal positive cells is plotted per cell line (n = 3–4 per group, **p < 0.01, unpaired t test). Bar = 500 µm. g Plot showing the level of IL-6 (mean ± SD) secreted into the medium by Fibulin-4+/+ controls (n = 4) and two separate Fibulin-4R/R VSMC lines. Experiments were performed in triplo, separate replicates were plotted (*p < 0.05, ***p < 0.001, unpaired t test).
Treatment of Fibulin-4 R/R VMSCs with AG490, TGF-β nAb, or navitoclax reduces senescence markers in vitro
To investigate therapeutic compounds that could alleviate the Fibulin-4R/R phenotype, an upstream regulator analysis was performed in IPA. AG490, an inhibitor of the JAK/STAT pathway, was predicted to be inhibited as an upstream regulator (activation z score = −4.253). Additionally, IPA analysis revealed upregulation of signaling through STATs resulting in increased expression of downstream IL-6 and SOCS genes (Supplementary Fig. 9). The JAK/STAT pathway is activated in senescent cells, and inhibition of this pathway suppresses the SASP25. These findings suggest that the JAK/STAT pathway could be a possible therapeutic target. Furthermore, TGFB1 and TGFB3, activators of the TGF-β pathway, were both predicted to be activated as upstream regulators (activation z score = 3.938 and 2.643, respectively). As previously mentioned, increased TGF-β signaling was observed in the aortic arch of Fibulin-4R/R mice (Fig. 1d, e). Additionally, a previous study revealed that treatment of Fibulin-4R/R VSMCs with TGF-β neutralizing antibodies (nAb) reversed their reduced growth, suggesting that this could also alleviate the senescent VSMC phenotype11. Lastly, different senolytic compounds including quercetin, fisetin, curcumin, resveratrol, and metformin were predicted to be inhibited as upstream regulators (activation z score = −2.103, −2.051, −4.168, −3.039, and −2.897, respectively)26. Together with the observation of increased expression of senescence markers in Fibulin-4R/R VSMCs, these findings suggest the therapeutic potential of senolytics. In conclusion, upstream regulator analysis uncovered multiple possible therapeutic targets.
To investigate whether possible therapeutic compounds could reduce senescence in vitro, Fibulin-4R/R VSMCs were treated with AG490, TGF-β nAb or navitoclax. AG490 treatment significantly reduced the percentage of SA-β-gal positive cells and the percentage of p21 positive cells in Fibulin-4R/R VSMCs in vitro (Fig. 7a, b, d, and e). Furthermore, the therapeutic effect of TGF-β nAb was assessed in vitro. TGF-β nAb treatment significantly reduced the percentage of SA-β-gal positive cells and the percentage of p21 positive cells in Fibulin-4R/R VSMCs in vitro (Fig. 7a, b, d, and e). Additionally, although not significant (p = 0.0796, unpaired t test), a trend of increased cell number was observed after treatment, suggesting increased proliferation of VSMCs (Fig. 7c). Lastly, the therapeutic effect of the senolytic compound navitoclax was assessed. Navitoclax treatment significantly reduced the percentage of SA-β-gal positive cells, as well as the total cell number in Fibulin-4R/R VSMCs in vitro (Fig. 7a–c). However, navitoclax treatment did not affect the percentage of p21 positive cells, suggesting that the remaining VSMCs stay in cell cycle arrest (Fig. 7d, e). Altogether, these results demonstrated the therapeutic effect of AG490, TGF-β nAb, and navitoclax on Fibulin-4R/R VSMCs in vitro and indicated the involvement of the JAK/STAT and TGF-β signaling pathway in establishing the senescent phenotype.
a SA-β-gal staining on Fibulin-4R/R VSMCs treated with AG490, TGF-β nAb, or navitoclax for 48 h (or untreated). Bar = 500 µm. b Quantification of SA-β-gal staining on Fibulin-4R/R VSMCs. The percentage (mean ± SD) of SA-β-gal positive cells is plotted (*p < 0.05, **p < 0.01, unpaired t test). c Quantification of the amount of nuclei per mm2 (mean ± SD, ***p < 0.001, unpaired t test). d Immunofluorescent staining for p21 on Fibulin-4R/R VSMCs treated with AG490, TGF-β nAb, or navitoclax for 48 h (or untreated). Bar = 50 µm. e Quantification of the percentage (mean ± SD) of p21 positive cells (*p < 0.05, unpaired t test). All experiments were performed in triplo, separate replicates were plotted. Note: SA-β-gal analysis after navitoclax treatment was performed in duplo.
Discussion
Aortic aneurysms are a life-threatening condition that can be fatal without timely surgical repair. Up to now, pharmacological treatment is lacking, mostly because there are still gaps of knowledge regarding the molecular mechanism underlying aneurysm formation. Mutations in the Fibulin-4 gene result in the development of cutis laxa syndrome in human patients, characterized by aortic aneurysm formation. To investigate the underlying molecular mechanisms of aneurysm disease development, this deficiency was mimicked in Fibulin-4R/R mice. Previous research showed alterations in the TGF-β signaling pathway, ECM remodeling, aberrations of the cytoskeleton, and mitochondrial dysfunction in this mouse model8,9,10,12. We aimed to further identify the effect of Fibulin-4 mutation in the mouse aorta at the molecular level to explain the underlying mechanism of aneurysm formation using an RNA sequencing approach.
Several findings from our RNA expression analysis confirm data presented in previous research on Fibulin-4R/R mice. Similar to previous findings, our data shows that downstream TGF-β signaling in the Fibulin-4R/R aortic arch is increased. Additionally, previous proteomics and microarray analyses of 3-month-old Fibulin-4R/R mouse aorta revealed significant changes in pathways related to mitochondrial function and metabolism9. We compared our RNA sequencing results with these previously published microarray data. Although our current study was different in platform, reference analysis, and amount of significantly differentially expressed molecules, the identified shared pathways included metabolic pathways, PPAR, RAS, and MAPK/ERK signaling. Additionally, a comparison analysis of the top 20 inhibited and top 20 activated upstream regulators predicted to be significantly regulated in both datasets showed an overlap of 20 molecules, including Pparg and Il1b. Furthermore, IPA analysis reveals a change in genes and pathways involved in the cytoskeleton and ECM remodeling in Fibulin-4R/R mice. Accumulation and disorganization of elastin fibers and accumulation of proteoglycans in the medial layer of the Fibulin-4R/R aortic arch confirm these findings. This shows that analysis of the current RNAseq data properly uncovered important processes at play in the Fibulin-4R/R aneurysmal aorta, supporting the fact that the other pathways and molecules identified are equally valid.
Analysis of the Fibulin-4R/R aortic media shows increased medial wall thickness, but no significant change in the number of VSMCs (after correction for surface area) in the Fibulin-4R/R aortic arch compared to the Fibulin-4+/+ aortic arch. Additionally, Ki67 staining reveals some proliferating cells in the Fibulin-4R/R aorta, but no significant increase compared to Fibulin-4+/+ mice. As the proliferation of VSMCs in the aortic media of 10-day-old Fibulin-4R/R mice is increased, it is possible that VSMCs contribute to the observed increased wall thickness in an earlier stage of aneurysm growth, but may be less involved in later stages8.
VSMCs show high phenotypic plasticity and can switch from a functional, contractile phenotype to a proliferative, synthetic phenotype upon stress signals to enable vascular remodeling. Phenotypic switching of VSMCs is thought to play a role early in aneurysm formation, causing pathological remodeling of the vessel wall16,27. IPA analysis reveals the downregulation of markers for the contractile VSMC phenotype and upregulation of synthetic markers in the Fibulin-4R/R aortic arch. Interestingly, Fibulin-4R/R aortas show decreased expression of both contractile marker αSMA and synthetic marker vimentin. Previous research showed increased αSMA and SM22 protein levels in Fibulin-4R/R VSMCs in vitro, which would indicate a more contractile phenotype10. However, Fibulin-4R/R VSMCs exhibit aberrant cytoskeletal structures and ECM production, which impair contractility, still causing an aberrant contractile phenotype. As previously mentioned, downstream signaling in the TGF-β pathway is increased in Fibulin-4R/R mice28. TGF-β signaling is known to regulate the differentiation of VSMCs by targeting VSMC markers such as αSMA, SM22 and calponin 1, and abnormal TGF-β signaling could therefore be responsible for the observed phenotypic changes29,30. Even though the aortic staining for vimentin does not indicate a switch to a synthetic phenotype, the RNAseq data shows significant upregulation of several other synthetic markers. This suggests that Fibulin-4R/R VSMCs undergo a phenotypical change that might be involved in aneurysm formation, such as the adaptation of a senescent phenotype, in which VSMCs change from the contractile to the synthetic phenotype31,32.
Differential expression data suggests the presence of senescent cells in the Fibulin-4R/R aortic arch. The presence of senescent cells in the Fibulin-4R/R aortic arch is confirmed ex vivo in aortic tissue and in vitro in VSMCs, by investigation of senescence markers p21, p16, SA-β-gal, DNA damage foci, and SASP factor IL-6. Senescence of VSMCs has been previously observed in several cardiovascular diseases, amongst which aortic aneurysms33,34,35,36. We now further provide evidence that suggests the involvement of VSMC senescence in aortic aneurysm development. TGF-β can induce the expression of cell-cycle inhibitors p21, p16INK4A, and p15INK4B and thereby induce senescence37,38. A previous study already showed reduced proliferation of Fibulin-4R/R VSMCs in vitro, indicating cell cycle arrest which is also characteristic of the senescent phenotype11,39. Additionally, TGF-β was shown to induce the production of reactive oxygen species (ROS) in the mitochondria of several cell types40. ROS can induce DNA damage which eventually can trigger senescence41,42,43,44. Mitochondrial dysfunction and increased ROS production were previously observed in Fibulin-4R/R VSMCs, and we now also observe increased DNA damage in these cells9. Furthermore, previous research showed increased senescence of VSMCs from patients with Marfan syndrome45. Much like in patients and mice with Fibulin-4 mutations, TGF-β signaling is increased in Marfan syndrome and it is thought that TGF-β might trigger the observed senescence of these VSMCs33. Our results show that inhibition of the TGF-β pathway using TGF-β neutralizing antibodies reduces senescence markers SA-β-gal and p21 in Fibulin-4R/R VSMCs in vitro. Therefore, our findings further evidence the involvement of the TGF-β pathway in establishing the senescence phenotype and indicate that targeting of the TGF-β pathway is effective in alleviating the senescent phenotype.
Another proposed mechanism of senescence induction is mechanical stress. The structure of the vessel wall of Fibulin-4R/R mice is already compromised, making it less resistant to mechanical strain caused by cyclic pulsation of the blood46,47. Furthermore, stiffening of the aorta reduces arterial compliance, causing pulse pressure (the difference between systolic and diastolic blood pressure) to increase48. Indeed, our RNAseq data analysis reveals that mechanosensing is disturbed in the Fibulin-4R/R aortic arch. The ECM is important in the transduction of mechanical stimuli from the surrounding environment and in the Fibulin-4R/R aorta we observe an aberrant ECM, which could explain why mechanosensing is disturbed. Abnormal or excessive mechanical stimuli can induce pathological phenotypical changes in VSMCs, and therefore possibly also trigger senescence. Additionally, compositional changes of the ECM can trigger phenotypic modulation of VSMCs, also contributing to this process49.
VSMC senescence has been previously associated with thoracic aortic aneurysm formation in patients33,50. In mice, senescence of VSMCs has only been observed in models for abdominal aortic aneurysms51,52. Senescence of VSMCs is associated with persistent DNA damage, as we also observe, and the presence of a SASP induces degradation of the ECM, as well as triggers an inflammatory response22. Both ECM degradation and inflammation impair vessel wall structure and function, further promoting aneurysm formation53. Furthermore, the SASP includes secreted molecules, amongst which TGF-β, that can induce paracrine senescence and maintain the senescent phenotype40. Altogether, the senescence of VSMCs in the aortic wall potentially contributes to aortic aneurysm formation.
The JAK/STAT signaling pathway is activated in senescent cells and inhibition of this pathway has been shown to suppress SASP secretion and attenuate senescence25,54. Additionally, JAK/STAT signaling contributes to the maintenance of the cytokine profile in aneurysmal aorta55. Our results show that treatment of Fibulin-4R/R VSMCs with the JAK/STAT inhibitor AG490 reduces senescence markers SA-β-gal and p21 in vitro. This further evidences that the JAK/STAT pathway plays a role in the induction of the senescent phenotype.
Treatment with the senolytic compound navitoclax reduces senescence marker SA-β-gal in Fibulin-4R/R VSMCs in vitro. These results indicate that it would be of interest to specifically target senescent cells as a form of therapy. Treatment with senolytic agents dasatinib and quercetin already showed effectivity against abdominal aortic aneurysm growth in aged mice infused with angiotensin II, where reduced expression of Mmp2 and Mmp9 was observed upon treatment56. Additionally, navitoclax treatment of Ang II-induced premature senescent VSMCs in vitro also reduced the expression of Mmp2 and Mmp9. These studies indicate that senescence could be targeted to prevent or reduce aneurysm development57. Importantly, specifically targeting senescent cells as a form of therapy also shows potential for translation to the clinic, as senescence of VSMCs has been identified in the aortas of patients with Marfan syndrome. However, it should be noted that there are also risks to using senolytic treatment. Apoptosis of a large number of senescent VSMCs in the aneurysmal aorta could promote wall instability if they are not directly replaced by healthy VSMCs. Therefore, the use of senomorphics, compounds that block or regulate the secretion of SASP factors, could also be considered58. Lastly, since targeting the JAK/STAT and TGF-β pathway reduces senescence markers in vitro, the treatment that reduces JAK/STAT and TGF-β pathway activity could also be therapeutic in preventing the senescent phenotype.
In conclusion, these results further reveal underlying molecular mechanisms of aortic aneurysm development in Fibulin-4R/R mice. Our studies demonstrate that Fibulin-4R/R VSMCs show a senescent phenotype which might contribute to impaired functioning of the aortic wall. Furthermore, our results indicate the involvement of the JAK/STAT and TGF-β pathway in establishing this senescent phenotype. Notably, the Fibulin-4R/R mouse model is representative of thoracic aneurysm formation in patients and is a suitable model to investigate its underlying mechanisms. These findings form interesting leads for further investigation and can contribute to improved therapeutic options to alleviate or even prevent aortic aneurysm formation. Importantly, by generating Fibulin-4R/R|p21-Luciferase mice, we can now study the effect of therapeutic treatment on both senescence and aneurysm development simultaneously, as well as longitudinally, providing a tool to study potential new anti-senescence treatment options.
Methods
Experimental animals
Fibulin-4+/R mice were bred into a C57BL6 background to obtain Fibulin-4R/R and wild-type (Fibulin-4+/+) experimental animals. Fibulin-4SMKO animals, with a VSMC-specific deletion of Fibulin-4, were kindly provided by Hiromi Yanagisawa and bred into the same C57BL6 background. Mice were fed a normal chow diet. Animals were housed at the Animal Resource Centre (Erasmus University Medical Centre), which operates in compliance with the “Animal Welfare Act” of the Dutch government, using the “Guide for the Care and Use of Laboratory Animals” as its standard. As required by Dutch law, formal permission to generate and use genetically modified animals was obtained from the responsible local and national authorities. An independent Animal Ethics Committee consulted by Erasmus Medical Center (CCD) approved these studies (permit number AVD1010020186886), in accordance with national and international guidelines. For the described experiments mice were sacrificed by CO2 inhalation.
RNA sequencing
Fibulin-4R/R and Fibulin-4+/+ littermate control mice were sacrificed at the age of 3 months (n = 7 per group, 3 male and 4 female). Total RNA and µRNA were obtained from the aortic arch and abdominal aortas using the miRNeasy Mini Kit (Qiagen). Library preparation, sequencing, and primary data analysis were performed at GenomeScan B.V. (Genomescan B.V., Leiden, The Netherlands). Libraries were single-end sequenced in an Illumina NextSeq500 platform at a sequencing depth of 12 million reads and 75 bp read length. Reads were mapped to the reference sequence Mus_Musculus_GRCm38.p3 using a short read aligner based on Burrows-Wheeler Transform with a default matching rate of 2%. Read counts were generated using htseq-count. Differential expression analysis was performed with DESeq2 v1.10.1, a statistical package within the R platform v2.15.3, to compare gene expression in the aortic arch and abdominal aorta of mutant mice to gene expression in control mice with an adjusted p value threshold of 0.0559,60. The sequencing data were uploaded to the Galaxy web platform61. The Volcano Plot tool was used to create volcano plots, in which the top 20 most upregulated genes and the top 20 most downregulated genes were highlighted (ranked based on fold change). The heatmap2 tool was used to create heatmaps of pathways and processes of interest. The expression of each gene was scaled individually based on the rlog normalized counts.
IPA
For the comparison of Fibulin-4R/R with Fibulin-4+/+ sample groups (aortic arch and abdominal aorta), normalized expression values of 22,779 genes were uploaded into IPA (IPA© software version 107193442, QIAGEN, Redwood City, California, USA). An IPA core analysis was performed on significantly differentially expressed genes with a fold change of −1.2 ≤ FC ≥ 1.2 and a p value ≤ 0.05, of which the numbers are indicated per sample group in Supplementary Table 1. The top 20 up- and downregulated genes, canonical pathways, and upstream regulators were investigated (see Supplementary Fig. 10 for an analysis overview). The top up- and downregulated genes were identified by ranking based on fold change. The top canonical pathways were ranked based on significance (p value ≤ 0.05, −log (p value) ≥ 1.3). A z score ≥ 2 indicates prediction of activation of the pathway and a z score ≤ −2.0 indicates prediction of inhibition of the pathway. Upstream regulators were selected based on z score significance cut-offs set to −2.0 ≤ z score ≥ 2.0 and p value ≤ 0.05. For the targeted analysis, canonical pathways of interest were searched manually in IPA. For the TGF-β and JAK/STAT pathway, an overlay of the dataset on the pathway was created to visualize which genes were significantly changed. For analysis of contractile and synthetic VSMC markers and SASP factors, separate gene lists were manually created in IPA based on literature (Supplementary Tables 3 and 4, respectively)21,22.
RT-qPCR
Total RNA was reverse transcribed into cDNA using the iScript cDNA Synthesis Kit (#1708890, Bio-Rad) and by running the following program on the Arktik Thermal Cycler (Thermo Scientific): 1) 5 min at 25 °C, 2) 30 min at 42 oC and 3) 5 min at 85 °C. Real-time qPCR was performed on 5 ng cDNA per sample using the iQ SYBR Green Supermix (#1708886, Bio-Rad) and by running the following program on the Bio-Rad CFX384 real-time system: (1) 3 min at 95 °C, 2) 40 cycles of 15 s at 95 °C and 30 s at 55 or 60 °C (depending on primers) and 3) 5 s at 55 or 60 °C. Primer sequences are listed in Supplementary Table 6. Each sample was run in duplicate and samples with duplicates deviating > 6% were excluded from the analysis. Genes were normalized to their respective housekeeping gene; Hprt for genes with 55 °C primers and Ppia for genes with 60 °C primers. The relative expression of genes was compared between Fibulin-4R/R and Fibulin-4+/+ samples.
Immunohistochemical staining of mouse aortic tissue
For histological analysis, Fibulin-4+/+ (n = 11, 8 male and 3 female) and Fibulin-4R/R (n = 9, 4 male and 5 female) mice were sacrificed at an age of 3–4 months. Mice were perfused through the left ventricle with phosphate-buffered saline (PBS). Aortas were fixed in formalin, dehydrated through the histokinette processor (Microm), and subsequently paraffin-embedded, after which 4 µm sections were prepared and placed on slides. Hematoxylin and eosin (HE) staining was performed to assess general pathology. Media and lumen size were quantified by manual selection of the media and lumen using the freehand region tool in NDP view.2 (Hamamatsu Photonics K.K., U12388-01), which calculates the surface area in mm2 (Supplementary Fig. 11a). A Movat’s pentachrome staining was performed for simultaneous visualization of different components of the vessel wall62, Resorcin-Fuchsin (RF) staining was performed to visualize the elastin structure and Alcian blue (AB) staining was performed to assess the ECM (stain for proteoglycans). AB staining was quantified using Fiji (ImageJ)63 (Supplementary Fig. 11b).
For immunohistochemical analysis, aorta sections were first deparaffinized and boiled in antigen retrieval buffer (10 mM Tris base, 1 mM EDTA solution, pH 9.0) at 300 W for 15 min. Slides were incubated in 3% H2O2 in methanol for 10 min to block endogenous peroxidase activity and subsequently blocked with 5% Protifar in PBS with 0.025% Triton X-100 for 1 h. Slides were incubated overnight at 4 °C with the following primary antibodies diluted in 1% Protifar in PBS with 0.025% Triton X-100; anti-αSMA (1:500 mouse monoclonal, ab7818, Abcam), anti-vimentin (1:500, ab2547, Abcam), anti-p21 (1:100 rat monoclonal [Hugo291], ab107099, Abcam), anti-p16 (1:200 rabbit monoclonal, ab211542, Abcam), and anti-Ki67 (1:200 rat monoclonal, Clone TEC-3, DAKO). The following day, slides were incubated with biotinylated secondary antibody (1:200, DAKO) for 30 min and subsequently with avidin-biotinylated complex (Vectastain Universal Elite ABC kit Vector Laboratories) for 30 min. DAB chromogen (DAKO Liquid Dab substrate-chromogen system) was used as a substrate and slides were counterstained with Eosin. Quantification of p21, p16, and Ki67 staining was performed by manually counting the number of positive cells and correcting for media size (Supplementary Fig. 11c). Quantification of αSMA and vimentin staining was performed using Fiji (ImageJ)63 (Supplementary Fig. 11d).
Immunofluorescent staining of mouse aortic tissue
For immunofluorescent analysis, aorta sections were first deparaffinized and boiled in antigen retrieval buffer (10 mM Sodium Citrate, 0.05% Tween-20 solution, pH 6.0) at 300 W for 15 min. Tissue was permeabilized in TBS with 0.05% Triton X-100 for 10 min. Slides were blocked with 5% normal goat serum (NGS) + 0.3 M glycine in TBS with 0.05% Tween-20 for 1 h. Slides were incubated overnight at 4 °C with primary antibody (anti-αSMA (1:500 mouse monoclonal, ab7818, Abcam)) diluted in 1% NGS in TBS with 0.05% Tween-20. The following day, slides were incubated for 1 h at room temperature with a secondary antibody (anti-mouse Alexa 594, 1:1000) diluted in 1% NGS in TBS with 0.05% Tween-20. Slides were mounted with Vectashield containing DAPI (H-1200, Vector laboratories) and sealed with nail polish. Images were recorded on a near-infrared wide-field microscope (Axio Imager D2, Zeiss) using a 10× objective lens. Quantification of the number of nuclei in αSMA-positive area was performed using Fiji (ImageJ)63.
Isolation of VSMCs from mouse aorta
To investigate VSMCs derived from the mutant mouse aorta in vitro, VSMCs were isolated from the aorta of Fibulin-4R/R (n = 3), Fibulin-4+/+ (n = 4), Fibulin-4SMKO (n = 3), and Fibulin-4SMWT (n = 2) mice according to the protocol in Supplementary Table 7. In short, mice were sacrificed and perfused through the left ventricle with PBS. Aortas were isolated and incubated in 2 mg/ml collagenase type II (LS004176, Worthington) at 37 °C, 5% CO2 for 1–6 h until the tissue was dissolved. Primary VSMCs were centrifuged, and the cell pellet was resuspended in Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco) supplemented with 10% fetal calf serum (FCS) and 1% penicillin-streptomycin (PS) and cells were cultured on gelatinized dishes (0.1% gelatin) at 37 °C, 5% CO2.
Immunofluorescent staining of VSMCs
To investigate phenotype and senescence markers, VSMCs were seeded on 0.1% gelatin-coated coverslips. VSMCs were incubated in culture for 7 days to allow the formation of ECM, and to ensure a better representation of in vivo conditions. After 7 days, VSMCs were fixed with 2% paraformaldehyde in PBS for 15 min. Fixated cells were washed with PBS + 0.1% Triton X-100 and blocked with PBS+ (PBS supplemented with 0.5% bovine serum albumin and 0.15% glycine) for 20 min. Coverslips were incubated overnight at 4 °C with the primary antibodies diluted in PBS+ as mentioned in Supplementary Table 8. Coverslips were washed with PBS + 0.1% Triton X-100 and shortly washed with PBS+ before incubation with the corresponding secondary antibodies diluted in PBS+ for 1 h at room temperature (see Supplementary Table 8). Additionally, SiR-Actin (1:1000, SC001, Tebu Bio) was added to visualize the cytoskeleton. After incubation, the coverslips were washed with PBS + 0.1% Triton X-100 and shortly washed with PBS, after which they were mounted on glass slides with Vectashield containing DAPI (H-1200, Vector laboratories) and sealed with nail polish. Images were recorded on a near-infrared wide-field microscope (Axio Imager D2, Zeiss) using a 20× objective lens. Images for quantification of the staining for 53BP1 and γH2AX were recorded on a TCS SP5 confocal microscope (Leica) using a 40× objective lens. The brightness and/or contrast of representative images shown in Figs. 6a, c and 7d was adjusted. This was performed equally for all images of each individual staining. Quantifications were performed on raw, unedited images using Fiji (ImageJ)63.
SA-β-galactosidase (SA-β-gal) staining of VSMCs
To investigate SA-β-gal activity, VSMCs were seeded on 0.1% gelatin-coated coverslips. After 7 days, VSMCs were fixed and stained for SA-β-gal activity using the Senescence β-Galactosidase Staining Kit (#9860, Cell Signaling Technology) according to the protocol provided by the manufacturer. In short, VSMCs were fixed with 1× Fixative solution for 15 min at room temperature, after which they were washed with PBS and incubated with the β-galactosidase staining solution at 37 °C overnight in a dry incubator. Without removing the staining solution, images were recorded with an Olympus IX70 microscope using a Digital Color Camera (DFC300 FX, Leica). After washing with PBS, coverslips were mounted on glass slides with Vectashield containing DAPI (H-1200, vector laboratories) and sealed with nail polish. Images for quantification were recorded with a Leica DM4000 B microscope using a Digital Color Camera (DFC300 FX, Leica). Quantification was performed using Fiji (ImageJ)63 by counting the number of SA-β-gal positive cells in the bright-field channel and counting the total number of nuclei in the DAPI channel.
Elisa
Fibulin-4R/R and Fibulin-4+/+ VSMCs (n = 2–4) were seeded on 0.1% gelatin-coated 6 cm dishes. After 7 days, VSMCs were put on starvation medium (DMEM with 1% PS, without FBS). After 24 h, the medium was harvested and cells were counted. A sandwich ELISA was performed with the starvation medium to measure IL-6 secretion by VSMCs using the Mouse IL-6 Quantikine ELISA kit (M6000B, R&D Systems). Cell counts were used to correct the IL-6 levels to pg/100,000 cells. Experiments were performed in triplo.
Treatment of VSMCs
Fibulin-4R/R VSMCs were seeded in 12-well plates on 0.1% gelatin-coated coverslips. The medium was refreshed after 4 days. After 7 days, cells were treated with either 15 µg/ml TGF-β neutralizing antibodies (TGF-β 1,2,3 monoclonal antibody, MAB1835, R&D Systems), 25 µM AG-490 (S1143, Selleckchem) or 5 µM navitoclax (ABT-263, HY-10087, MedChemExpress) for 48 h. Immunofluorescent staining for p21 and SA-β-gal staining was performed according to the previously mentioned methods. Experiments were performed in triplo.
P21 reporter assay
In order to evaluate p21 expression levels in vivo in mice, Fibulin-4 mice were crossed with p21-luciferase reporter mice. The reporter construct was directed to the end of exon 3 of the endogenous p21 locus where a sequence, which encodes for T2A-β-gal-T2A-luciferase, was inserted64. This insertion led expression of p21, β-gal, and luciferase from the one engineered allele. Fibulin-4+/+ and Fibulin-4R/R mice were crossed with p21-luciferase reporter mice, which led to both hetero- and homozygous animals for the reporter loci. Next, 3-month-old animals that were homozygous for the p21 reporter loci were selected (n = 2 for both Fibulin-4+/+|p21-Luciferase and Fibulin-4R/R|p21-Luciferase mice). First, mice were imaged for background levels. Then mice were injected with 150 mg/kg luciferin intraperitoneal and p21-luciferin activity was imaged 10–20 min after injection using the IVIS® spectrum in vivo imaging system (Perkin Elmer). As a control for background luminescence, we used Fibulin-4+/+ and Fibulin-4R/R mice without any reporter loci, which showed no bioluminescent signal upon injection of luciferin.
SA-β-galactosidase staining of mouse aorta
To investigate SA-β-gal activity in aortas ex vivo, Fibulin-4R/R (n = 4) and Fibulin-4+/+ (n = 4) mice were sacrificed and subsequently perfused through the left ventricle with PBS. Aortas were removed and placed in fixative buffer (1% formaldehyde, 0.1% glutaraldehyde, 2 mM MgCl2, 5 mM EGTA, 0.1 M sodium phosphate buffer pH 7.8) overnight at 4 °C. Aortas were washed (2 mM MgCl2, 0.01% sodium deoxycholate, 0.02% NP-40, 0.1 M sodium phosphate buffer pH 7.8) and stained with X-gal staining solution (5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2% X-gal in DMF) overnight at 37 °C.
Statistics
Statistical analysis is embedded in the core analysis of IPA. The p value is calculated using a right-tailed Fisher’s exact test and reflects the probability that the overlap of significant differentially expressed genes in the dataset with a certain process or pathway is due to random chance. The z score is calculated to provide predictions on processes that are upstream or downstream of differentially expressed genes.
Statistical analysis of the quantification of lumen and media size, AB staining, and immunohistochemical staining on aorta sections, as well as immunofluorescent staining and SA-β-gal staining on VSMCs was performed in GraphPad Prism 9.3.0 (GraphPad Software Inc., La Jolla, California, USA). The Shapiro–Wilk test was used to test for normal distribution of the data. An unpaired two-sided t test was performed to determine significant differences between two groups. For not normally distributed data, a Mann–Whitney test was performed to determine significance. For the calculation of the correlation between two parameters, the Spearman nonparametric correlation test was performed. For each statistical test, a p value < 0.05 was considered significant. All results are expressed as mean ± standard deviation (SD). In figures, one asterisk (*) indicates a p value < 0.05, two asterisks (**) indicate a p value < 0.01, three asterisks (***) indicate a p value < 0.001 and four asterisks (****) indicate a p value < 0.0001.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
References
Lindsay, M. E. & Dietz, H. C. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature 473, 308–316 (2011).
Kent, K. C. et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J. Vasc. Surg. 52, 539–548 (2010).
van de Laar, I. M. et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat. Genet. 43, 121–126 (2011).
Loeys, B. L. et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 37, 275–281 (2005).
Hoyer, J., Kraus, C., Hammersen, G., Geppert, J. P. & Rauch, A. Lethal cutis laxa with contractural arachnodactyly, overgrowth and soft tissue bleeding due to a novel homozygous fibulin-4 gene mutation. Clin. Genet. 76, 276–281 (2009).
Renard, M. et al. Altered TGFbeta signaling and cardiovascular manifestations in patients with autosomal recessive cutis laxa type I caused by fibulin-4 deficiency. Eur. J. Hum. Genet. 18, 895–901 (2010).
Papke, C. L. & Yanagisawa, H. Fibulin-4 and fibulin-5 in elastogenesis and beyond: insights from mouse and human studies. Matrix Biol. 37, 142–149 (2014).
Hanada, K. et al. Perturbations of vascular homeostasis and aortic valve abnormalities in fibulin-4 deficient mice. Circ. Res. 100, 738–746 (2007).
van der Pluijm, I. et al. Decreased mitochondrial respiration in aneurysmal aortas of fibulin-4 mutant mice is linked to PGC1A regulation. Cardiovasc. Res. 114, 1776–1793 (2018).
Burger, J. et al. Fibulin-4 deficiency differentially affects cytoskeleton structure and dynamics as well as TGFbeta signaling. Cell. Signal. 58, 65–78 (2019).
Ramnath, N. W. et al. Fibulin-4 deficiency increases TGF-beta signalling in aortic smooth muscle cells due to elevated TGF-beta2 levels. Sci. Rep. 5, 16872 (2015).
Ramnath, N. W. et al. Extracellular matrix defects in aneurysmal Fibulin-4 mice predispose to lung emphysema. PLoS One 9, e106054 (2014).
Li, Y. et al. Single-cell transcriptome analysis reveals dynamic cell populations and differential gene expression patterns in control and aneurysmal human aortic tissue. Circulation 142, 1374–1388 (2020).
Howard, D. P. et al. Age-specific incidence, risk factors and outcome of acute abdominal aortic aneurysms in a defined population. Br. J. Surg. 102, 907–915 (2015).
Mikael, Ld. R. et al. Vascular aging and arterial stiffness. Arq. Bras. Cardiol. 109, 253–258 (2017).
Cao, G. et al. How vascular smooth muscle cell phenotype switching contributes to vascular disease. Cell Commun. Signal. 20, 180 (2022).
van de Stolpe, A., Holtzer, L., van Ooijen, H., Inda, M. A. & Verhaegh, W. Enabling precision medicine by unravelling disease pathophysiology: quantifying signal transduction pathway activity across cell and tissue types. Sci. Rep. 9, 1603 (2019).
Yamashiro, Y. et al. Abnormal mechanosensing and cofilin activation promote the progression of ascending aortic aneurysms in mice. Sci. Signal. 8, ra105 (2015).
Yanagisawa, H. & Wagenseil, J. Elastic fibers and biomechanics of the aorta: Insights from mouse studies. Matrix Biol. 85-86, 160–172 (2020).
Cavalcante, J. L., Lima, J. A. C., Redheuil, A. & Al-Mallah, M. H. Aortic stiffness: current understanding and future directions. J. Am. Coll. Cardiol. 57, 1511–1522 (2011).
Rensen, S. S., Doevendans, P. A. & van Eys, G. J. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth. Heart J. 15, 100–108 (2007).
Coppe, J. P., Desprez, P. Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Moltzer, E. et al. Impaired vascular contractility and aortic wall degeneration in fibulin-4 deficient mice: effect of angiotensin II type 1 (AT1) receptor blockade. PLoS One 6, e23411 (2011).
Althubiti, M. et al. Characterization of novel markers of senescence and their prognostic potential in cancer. Cell Death Dis. 5, e1528 (2014).
Xu, M., Tchkonia, T. & Kirkland, J. L. Perspective: targeting the JAK/STAT pathway to fight age-related dysfunction. Pharm. Res. 111, 152–154 (2016).
Zhang, L., Pitcher, L. E., Prahalad, V., Niedernhofer, L. J. & Robbins, P. D. Targeting cellular senescence with senotherapeutics: senolytics and senomorphics. FEBS J. 290, 1362–1383 (2023).
Ailawadi, G. et al. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J. Thorac. Cardiovasc. Surg. 138, 1392–1399 (2009).
van der Pluijm, I. et al. Defective connective tissue remodeling in smad3 mice leads to accelerated aneurysmal growth through disturbed downstream TGF-beta signaling. EBioMedicine 12, 280–294 (2016).
Iyemere, V. P., Proudfoot, D., Weissberg, P. L. & Shanahan, C. M. Vascular smooth muscle cell phenotypic plasticity and the regulation of vascular calcification. J. Intern. Med. 260, 192–210 (2006).
Tang, Y., Yang, X., Friesel, R. E., Vary, C. P. & Liaw, L. Mechanisms of TGF-beta-induced differentiation in human vascular smooth muscle cells. J. Vasc. Res. 48, 485–494 (2011).
Chi, C. et al. Vascular smooth muscle cell senescence and age-related diseases: state of the art. Biochimi. Biophys. Acta Mol. Basis Dis. 1865, 1810–1821 (2019).
Rubio-Ruiz, M. E., Perez-Torres, I., Soto, M. E., Pastelin, G. & Guarner-Lans, V. Aging in blood vessels. medicinal agents FOR systemic arterial hypertension in the elderly. Ageing Res. Rev. 18, 132–147 (2014).
You, W. et al. TGF-beta mediates aortic smooth muscle cell senescence in Marfan syndrome. Aging (Albany NY) 11, 3574–3584 (2019).
Aschacher, T. et al. Impacts of telomeric length, chronic hypoxia, senescence, and senescence-associated secretory phenotype on the development of thoracic aortic aneurysm. Int J. Mol. Sci. 23, 15498 (2022).
Hou-Zao, C. et al. Age-associated sirtuin 1 reduction in vascular smooth muscle links vascular senescence and inflammation to abdominal aortic aneurysm. Cir. Res. 119, 1076–1088 (2016).
Peng, G. et al. Caloric restriction exacerbates angiotensin ii–induced abdominal aortic aneurysm in the absence of p53. Hypertension 73, 547–560 (2019).
Zhang, Y., Alexander, P. B. & Wang, X. F. TGF-beta family signaling in the control of cell proliferation and survival. Cold Spring Harb. Perspect. Biol. 9, a022145 (2017).
Harmon, G. J. & Beach, D. pl5INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature 371, 257–261 (1994).
Kumari, R. & Jat, P. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front. Cell Dev. Biol. 9, 645593 (2021).
Tominaga, K. & Suzuki, H. I. TGF-beta signaling in cellular senescence and aging-related pathology. Int. J. Mol. Sci. 20, 5002 (2019).
Borodkina, A., Shatrova, A., Abushik, P., Nikolsky, N. & Burova, E. Interaction between ROS dependent DNA damage, mitochondria and p38 MAPK underlies senescence of human adult stem cells. Aging (Albany NY) 6, 481–495 (2014).
Chen, Q., Fischer, A., Reagan, J. D., Yan, L. J. & Ames, B. N. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc. Natl. Acad. Sci. USA 92, 4337–4341 (1995).
Passos, J. F. et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol. Syst. Biol. 6, 347 (2010).
Lu, T. & Finkel, T. Free radicals and senescence. Exp. Cell Res. 314, 1918–1922 (2008).
He, H. et al. Vascular progenitor cell senescence in patients with Marfan syndrome. J. Cell. Mol. Med. 23, 4139–4152 (2019).
Bäck, M., Gasser, T. C., Michel, J. B. & Caligiuri, G. Biomechanical factors in the biology of aortic wall and aortic valve diseases. Cardiovasc. Res. 99, 232–241 (2013).
Thorin-Trescases, N. & Thorin, E. Lifelong cyclic mechanical strain promotes large elastic artery stiffening: increased pulse pressure and old age-related organ failure. Can. J. Cardiol. 32, 624–633 (2016).
Homan, T. D., Bordes, S. J. & Cichowski, E. Physiology, pulse pressure. doi:NBK482408 [bookaccession] (2022).
Jensen, L. F., Bentzon, J. F. & Albarrán-Juárez, J. The phenotypic responses of vascular smooth muscle cells exposed to mechanical cues. Cells 10, 2209 (2021).
Balint, B. et al. Seno-destructive smooth muscle cells in the ascending aorta of patients with bicuspid aortic valve disease. EBioMedicine 43, 54–66 (2019).
Zhang, W. et al. Nucleolar stress induces a senescence-like phenotype in smooth muscle cells and promotes development of vascular degeneration. Aging (Albany NY) 12, 22174–22198 (2020).
Zhang, C. et al. Cyclic nucleotide phosphodiesterase 1C contributes to abdominal aortic aneurysm. Proc. Natl. Acad. Sci. USA 118, e2107898118 (2021).
Cho, M. J., Lee, M.-R. & Park, J.-G. Aortic aneurysms: current pathogenesis and therapeutic targets. Exp. Mol. Med. 55, 2519–2530 (2023).
Chen, M. et al. Inhibition of JAK-STAT signaling pathway alleviates age-related phenotypes in tendon stem/progenitor cells. Front. Cell Dev. Biol. 9, 650250 (2021).
Ohno, T. et al. Cytokine profile of human abdominal aortic aneurysm: involvement of JAK/STAT pathway. Ann. Vasc. Dis. 11, 84–90 (2018).
Parvizi, M. et al. Senolytic agents lessen the severity of abdominal aortic aneurysm in aged mice. Exp. Gerontol. 151, 111416 (2021).
Xie, J. et al. Clearance of stress-induced premature senescent cells alleviates the formation of abdominal aortic aneurysms. Aging Dis. 14, 1778–1798 (2023).
Nehlin, J. O. in Advances in Protein Chemistry and Structural Biology, Vol. 136 (eds Ufuk Çakatay & Mehmet Can Atayik) 217–247 (Academic Press, 2023).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
R Core Team R. A language and environment for statistical computing. R Foundation for Statistical Computing https://www.R-project.org (2021).
Afgan, E. et al. The galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 46, W537–W544 (2018).
Bruijn, L. E., van den Akker, B., van Rhijn, C. M., Hamming, J. F. & Lindeman, J. H. N. Extreme diversity of the human vascular mesenchymal cell landscape. J. Am. Heart Assoc. 9, e017094 (2020).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
McMahon, M., Frangova, T. G., Henderson, C. J. & Wolf, C. R. Olaparib, monotherapy or with ionizing radiation, exacerbates DNA damage in normal tissues: insights from a new p21 reporter mouse. Mol. Cancer Res. 14, 1195–1203 (2016).
Edgar, R., Domrachev, M. & Lash, A. E. Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002).
Acknowledgements
S.J.M. Stefens is funded by NWO-FAPESP joint call for Proposals Healthy Ageing, ‘The role of DNA damage and mitochondrial function in vascular, immune and neurological aging’—project number 457002001. The funder played no role in the study design, data collection, analysis and interpretation of data, or the writing of this manuscript. Fibulin-4SMKO animals were kindly provided by Hiromi Yanagisawa. The p21 reporter mice were a kind gift of Henderson and Wolf.
Author information
Authors and Affiliations
Contributions
S.J.M.S., R.K., J.E., and I.P. conceptualized and designed the project. S.J.M.S. performed the experiments, collected data, analyzed data, and prepared figures. A.I.J., Y.L., and S.J.M.S. analyzed RNA sequencing data. J.B. and P.M.H. isolated cell lines and isolated RNA from aorta tissue. N.V., J.B., and P.M.H. assisted with experiments. J.H.N.L. performed and analyzed MOVAT staining. P.M.H. performed experiments with p21-Luc mice. S.J.M.S., I.P., A.I.J., J.B., Y.L., J.H.N.L., D.M.K., H.J.M.V., R.K., and J.E. wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stefens, S.J.M., van Vliet, N., IJpma, A. et al. Increased vascular smooth muscle cell senescence in aneurysmal Fibulin-4 mutant mice. npj Aging 10, 31 (2024). https://doi.org/10.1038/s41514-024-00154-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41514-024-00154-4
- Springer Nature Limited